
Case Report
Austin ENT Open Access. 2024; 4(2): 1018.
Uncommon Benign Disease of the Salivary Glands Necrotizing Sialo Metaplasia (NS): Review of the Literature and Serie of 2 CASES
N Belhaj1,2*; R Bencheikh1,2; MA Benbouzid1,2; LH Essakalli1,2
1Department of Otorhinolaryngology Head and Neck Surgery, CHU Ibn Sina Rabat, Morocco
2Department of Otorhinolaryngology Head and Neck Surgery, CHR Guelmim, Morocco
*Corresponding author: Najoua Belhaj Department of Otorhinolaryngology Head and Neck Surgery, CHU Ibn Sina Rabat, & CHR Guelmim, Morocco. Email: belhajnajwa1990@gmail.com
Received: April 25, 2024 Accepted: May 28, 2024 Published: June 04, 2024
Abstract
Necotizing Sialo metaplasia (NS) is an uncommon self-limiting commonly confused with malignant epithelial entities; the aim of this article is to report series of two cases of necrotizing sialo metaplasia: the first case is in 45-year-old women with clinical documentary; the second case is a 37-year-old man
Data from an extensive review of literature including clinical; Imagin logic and microscopic features are provided.
Information on diagnostic and prognostic factors is offered and comprehensibly discussed.
Keywords: Necrotizing sialo metaplasia; Salivary gland lesion; Oral squamous cell carcinoma
Introduction
Necrotizing sialo metaplasia is an uncommon self-limiting confused with malignant entities; the first was described by Dun lap and braker. Afterwards Branon et al using data for their own cases and review of the literature ;described extensively its clinicopathological features [1-3].
During the following years this entity has been found in different locations [4-8].
And since Abrams et al first description; the associated etiologic factors of NS have been extensively discussed [2-4,8].
The most accept theory suggested ischemia as the main etiologic factor.
Age range in NS patient varies from (1.5-83 years old) man is more commonly affected with male-female ratio 1.95; size from 0.5to 4 cm.
Series of Cases
Observation 1
A 43-year-old man, he denied alcohol-smoking habits; he was first viewed by his general physician and he told him that probably that was malignant lesion; because it is an ulcerated lesion and rapidly growing. The patient was viewed two days ago and the daytime clinical examination finds an ulcerates lesion with fibrinous deposits; the diameter’s lesion was 2cm in large; the patient report that the lesion appeared peering during the previous day; and reported that non traumatic episode had occurred recently in the involved area (Figure 1).
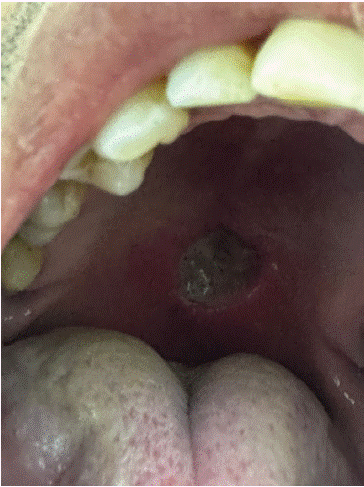
Figure 1: Ulcerated lesion with fibrinous deposits.
Panoramic and occlusally radio-graphs were taken and review confirmed that non bone involvement was present behind the ulcers.
Under regional anesthesia; an incisional biopsy measuring 1.1cm /1.1 cm was taken from the right ulcer and immersing in formalin for sent to pathological laboratory.
The patient was discharged by analgesic and antibiotics covered.
The presence of hyperplasic stratified squamous epithelium with long rete ridges and pseudoepitheliomatous hyperplasia (Figure 2); The bulk of lesion was composed of metaplastic squamous epithelial cells deriving from ductal epithelium; no neoplastic changes were observed; no tuberculosis lesions were observed.
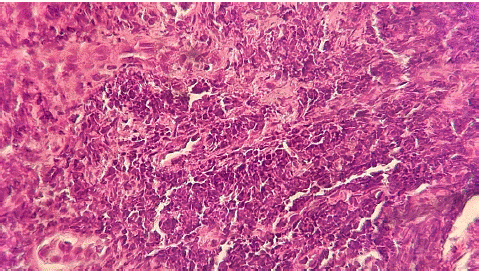
Figure 2: HE.40 metaplasic squamous epithelial cells deriving from ductual epithelium.
TPHA-VDRL and HIV serology were negative.
Two week later the lesion was discovered with fibrinoid pseudo-membranes; one month late the ulcers decreased in size to approximatively half of the origin size.
Observation 2
45-year-old women; she consulted his dentist for painful lesion located in the oral palate that bleeding upon food contact; the patient use oral-washes and buccal miconazole. She denied alcohol-smoking habits. This lesion appear 2 months
Non-improvement and height growth; the patient was addressed in our formation
The clinical examination found an ulcero-budding lesion 3cm in the large diameter (Figure 3)
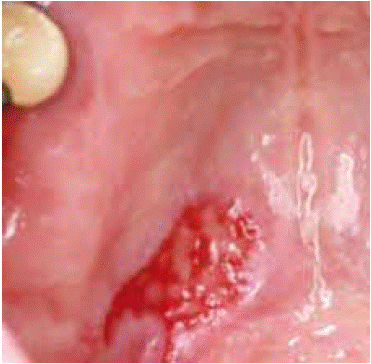
Figure 3: Ulcero-budding lesion 3 cm in the large diameter.
Under a local anesthesia; a biopsy was realized; after the patient was discharged by analgesic and antibiotiques covered. The result of the histopathological stud shows the same aspect of the first case (Figure 4 & 5).
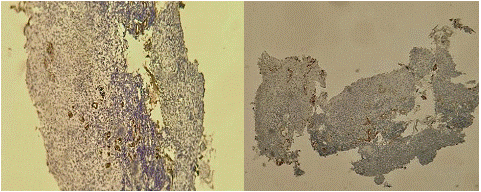
Figure 4/5: Microscopic sections of biopsy showing necrosis within salivary glands.
Discussion
NSM is an uncommon self-limiting lesion commonly confused with malignant epithelial neoplasms. Despite having been recognized by Abrams et al1 in 1973 and with numerous intraoral and extra oral cases reported,1-40 no agreement on the etiology for NSM development has been reached to date. Most authors sustain that some kind of physical-chemical or biological aggressions over the local blood vessels will produce ischemic changes, leading to infarction with posterior necrosis, and that NSM is the ductal proliferation in response to injury [2,8,20,21].
Many causes have been suggested as possible etiologic factors including: traumatic, chemical, surgical, and infectious events including alcohol, smoking, and drug abuse [3,10,12-16,22-24].
In other instances, some diseases characterized by vascular disturbances have been associated with NSM, sup- porting the theory that ischemic necrosis is the main etiologic factor [17-19].
In 1982, Anneroth and Hansen26 analyzed its histopathogenesis and separated NSM into five microscopic stages: infarction, sequestration, ulceration, reparative stage, and healing.
This entity is more frequently found in palate, although it has been also described in other intraoral locations: retro-molar area, labial glands, tongue, floor of the mouth, buccal mucosa, mucobuccal fold, sublingual region, and it was also found in parotid and sub- mandibular glands [3,4,8-10].
Also, NSM cases were reported in the head and neck area: nasal cavity, maxillary sinus, tonsillar fossa, incisive canal, larynx, trachea, and lymph nodes [3,5,6,9,15,27,31].
The most commonly observed clinical feature is a palatal, painful or painless, deep-seated ulcer with clean, sharp, and sometimes erythematous or rolled margins, measuring from 1 cm to 3.5 cm. Other symptoms are somewhat variable: fever, chills, malaise, anesthesia, and paresthesia, and rarely NSM is found as a nonulcerative, purulent, diffuse lesion with palpable lymph nodes [3,10,12-14,32].
Our case is unique since one of the lesions measured almost 6 cm. The presence of a unique lesion is the most frequent clinical manifestation of NSM, but as it is in the case presented here, sometimes two lesions are present [3,14].
Another three rare and unique cases have been reported: one with three ulcers, one with multiple palatal ulcerated lesions, and one with metachronous lesions [11,13,32]. Also, several cases with one or two palatal asymptomatic, painless, smooth-surfaced nodules of normal color were reported [8,11,14,18,23]. Duration of the lesion varies from 3 weeks to 3 months [33].
It is well known that almost all studied NSM cases did not display radiographic alterations, but saucerization of the subjacent bone was observed in four reported cases [1,3,11].
Some studies of the features of NSM using Magnetic Resonance (MR) analysis [34-36] showed this lesion was hyperintense on T2 and hypointense on T1 [34]. Lee et al [35] reported that under MRI scan, a NSM case associated with an adenoid cystic carcinoma presented a diffuse infiltrating image suggestive of a malignant lesion, and a multi slice computed tomographic study showed no bone changes [36]
It is essential that the pathologist should receive an adequate biopsy specimen in order to observe all the diagnostic microscopic changes. Under microscopic examination, the lesion is usually covered by hyperplastic squamous stratified epithelium showing elongated epithelial ridges (pseudoepitheliomatous hyperplasia). A very important finding is the acinar necrosis featured by disintegration of the mucus-secreting acinar cells with persistence of the acinar outlines accompanied by spaces filled with mucin, cellular debris, and inflammatory infiltrate of variable intensity composed by polymorphonuclear neutrophilic leukocytes, plasma cells, foamy macrophages, and numerous eosinophils.
The most prominent and important diagnostic feature is the presence of ductal squamous metaplasia. It is characterized by a squamous change in the cell lining of the gland ducts. These squamous cells proliferate, and this proliferating lining obliterates the lumen, transforming the ducts into solid masses or nidus of squamous stratified epithelial cells showing eosinophilic cytoplasm with uniform and bland nuclei. It is important to note that mitotic cells are scarce, and of normal appearance with no atypical features. These solid squamous epithelial masses are often observed close to or continuous with the enlarged rete pegs of the superficial epithelium. This finding is suggestive of malignancy and may lead to the common misdiagnosis of squamous cell carcinoma [25]. These epithelial nodular masses are present in variable sizes and number, show smooth outlines, and sometimes are complemented with areas of more or less extensive reactive fibrosis. Sometimes, microscopic features of NSM may lead to a diagnostic pitfall of chronic sialadenitis or degenerative age changes. Occasionally, lesions in poorly controlled diabetic patients with mucormycosis may mimic NSM and should not be confused with the so-called sialo- metaplasia.26,30 Microscopic differential diagnosis should be performed with subacute necrotizing sialadenitis, Sutton disease, syphilis, and tuberculosis [38].
The use of immunohistochemistry may help to distinguish mucoepidermoid carcinoma from NSM lesions [33]. Lesions with similar microscopic appearance have been reported in non-neoplastic breast parenchyma, bronchial mucosa, lung, and sweat glands, [27-30] and there are several reports on malignant and benign tumors associated to NSM lesions, [3,6,34,36,39,40] indicating that NSM could develop as a secondary phenomenon. Sufficient data on long-term follow-up of NSM lesions is not available to date. In this manuscript we present the clinical features observed during the medium- term follow-up in a patient.
Conclusion
Necrotizing Sialo metaplasia (NS) is a relatively uncommon benign disease of the salivary glands that most commonly occurs in the palate. It is often confused clinically and histopathological with malignancies, such as squamous cell carcinoma or mucoepidermoid carcinoma; This condition requires histological evidence so as not to miss a cancer.
References
- Abrams AM, Melrose RJ, Howell FV. Necrotizing sialometaplasia. A disease simulating malignancy. Cancer. 1973; 32: 130–135.
- Dunlap LL, Barker BF. Necrotizing sialometaplasia. Oral Surg. 1974; 37: 722–727.
- Brannon RB, Fowler CB, Hartman KS. Necrotizing sialometaplasia. A clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1991; 72: 317–325.
- Russo A, Dell’Aquila A, Prota V, Sica GS. Necrotizing sialometaplasia of the submandibular gland. Report of a case. Minerva Stomatol. 1998; 47: 273–277.
- Ravn T, Trolle W, Kiss K, Balle VH. Adenosquamous carcinoma of the larynx associated with necrotizing sialometaplasia: a diagnostic challenge. Auris Nasus Larynx. 2009; 36: 721–724.
- Poulson TC, Greer RO Jr, Ryser RW. Necrotizing sialometaplasia obscuring an underlying malignancy: report of a case. J Oral Maxillofac Surg. 1986; 44: 570–574.
- Arguelles MT, Viloria JB Jr, Talens MC, McCory TP. Necrotizing sialometaplasia. Oral Surg Oral Med Oral Pathol. 1976; 42: 86–90.
- Lima MA, Rocha LC, Siqueira LMS, Carmo LC, Filho JH. Cystic form of necrotizing sialometaplasia in sublingual salivary gland. Rev Bras Otorrinolaringol. 2002; 6: 276–279.
- Maisel RH, Johnston WH, Anderson HA, Cantrell RW. Necrotizing sialometaplasia involving the nasal cavity. Laryngoscope. 1977; 87: 429–424.
- Randhawa T, Varghese I, Shameena P, Sudha S, Nair RG. Necrotizing sialo- metaplasia of tongue. J Oral Maxillofac Pathol. 2009; 13: 35–37.
- Imbery TA, Edwards PA. Necrotizing sialometaplasia: literature review and case reports. J Am Dent Assoc. 1996; 127: 1087–1092.
- Santis HR, Kabani SP, Roderiques A, Driscoll JM. Necrotizing sialometaplasia: an early, nonulcerative presentation. Oral Surg Oral Med Oral Pathol. 1982; 53: 387–390.
- Speechley JA, Field EA, Scott J. Necrotizing sialometaplasia occurring during pregnancy: report of a case. J Oral Maxillofac Surg. 1988; 46: 696–699.
- Keogh PV, O’Regan E, Toner M, Flint S. Necrotizing sialometaplasia: an unusual bilateral presentation associated with antecedent anaesthesia and lack of response to intralesional steroids. Case report and review of the literature. Br Dent J. 2004; 196: 79–81.
- Romagosa V, Bella MR, Truchero C, Moya J. Necrotizing sialometaplasia (adenometaplasia) of the trachea. Histopathology. 1992; 21: 280–282.
- Batsakis JG, Manning JT. Necrotizing sialometaplasia of major salivary glands. J Laryngol Otol. 1987; 101: 962–966.
- Buller DL. Nodular and ulcerated lesions of the hard palate. J Am Dent Assoc. 1980; 101: 823–824.
- Lynch DP, Crago CA, Martinez MG Jr. Necrotizing sialometaplasia. A review of the literature and report of two additional cases. Oral Surg Oral Med Oral Pathol. 1979; 47: 63–69.
- Mandel L, Kaynar A, DeChiara S. Necrotizing sialometaplasia in a patient with sickle-cell anemia. J Oral Maxillofac Surg. 1991; 49: 757–759.
- Johnston WH. Necrotizing sialometaplasia involving the mucous glands of the nasal cavity. Hum Pathol. 1977; 8: 589–592.
- Suckiel JM, Davis WH, Patakas BM, Kaminishi RM. Early and late manifestations of necrotizing sialometaplasia. J Oral Surg. 1978; 36: 902–905.
- Solomon LW, Merzianu M, Sullivan M, Rigual NR. Necrotizing sialometaplasia associated with bulimia: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103: 39–42.
- Prabhakaran VC, Flora RS, Kendall C. Pressure-induced necrotizing sialometaplasia of the parotid gland. Histopathology. 2005; 48: 464–465.
- Fava M, Cherubini K, Yurgel L, Salum F, Figueiredo MA. Necrotizing sialometaplasia of the palate in a cocaine-using patient. A case report. Minerva Stomatol. 2008; 57: 199–202.
- Hurt MA, Díaz-Arias AA, Rosenholtz MJ, Havey AD, Stephenson HE Jr. Post- traumatic lobular squamous metaplasia of breast. An unusual pseudocarcinomatous metaplasia resembling squamous (necrotizing) sialometaplasia of the salivary gland. Mod Pathol. 1988; 1: 385–390.
- Anneroth G, HaHurt MA, Díaz-Arias AA, Rosenholtz MJ, Havey AD, Stephenson HE Jr. Post- traumatic lobular squamous metaplasia of breast. An unusual pseudocarcinomatous metaplasia resembling squamous (necrotizing) sialometaplasia of the salivary gland. Mod Pathol. 1988; 1: 385–390.
- Anneroth G, Hansen LS. Necrotizing sialometaplasia. The relationship of its pathogenesis to its clinical. characteristics. Int J Oral Surg. 1982; 11: 283–291.
- Goldman RL, Klein HZ. Proliferative sialometaplasia arising in an intraparotid lymph node. Am J Clin Pathol. 1986; 86: 116–119.
- Hurt MA, Díaz-Arias AA, Rosenholtz MJ, Havey AD, Stephenson HE Jr. Post- traumatic lobular squamous metaplasia of breast. An unusual pseudocarcinomatous metaplasia resembling squamous (necrotizing) sialometaplasia of the salivary gland. Mod Pathol. 1988; 1: 385–390.
- Pagni F, Zàrate AF, Urbanski SJ. Necrotizing sialometaplasia of bronchial mucosa. Int J Surg Pathol. 2010; 18: 64–65.
- Zschoch H. Mucus gland infarct with squamous epithelial metaplasia in the lung. A rare site of so-called necrotizing sialometaplasia. Pathologe. 1992; 13: 45–48.
- King DT, Barr RJ. Syringometaplasia: mucinous and squamous variants. J Cutan Pathol. 1979; 6: 284–291.
- Rossie KM, Allen CM, Burns RA. Necrotizing sialometaplasia: a case with metachronous lesions. J Oral Maxillofac Surg. 1986; 44: 1006–1008.
- Rizkalla H, Toner M. Necrotizing sialometaplasia versus invasive carcinoma of the head and neck: the use of myoepithelial markers and keratin subtypes as an adjunct to diagnosis. Histopathology. 2007; 51: 184–189.
- Farina D, Gavazzi E, Avigo C, Borghesi A, Maroldi R. Case report. MRI findings of necrotizing sialometaplasia. Br J Radiol. 2008; 81: 173–175.
- Lee DJ, Ahn HK, Koh ES, Rho YS, Chu HR. Necrotizing sialometaplasia accompanied by adenoid cystic carcinoma on the soft palate. Clin Exp Otorhinolaryngol. 2009; 2: 48–51.
- Suomalainen A, Törnwall J, Hagström J. CT findings of necrotizing sialometaplasia. Dentomaxillofacial Radiol. 2012; 41: 529–532.
- Gnepp DR. Warthin tumor exhibiting sebaceous differentiation and necrotizing sialometaplasia. Virchows Arch A Pathol Anat Histol. 1981; 391: 267–273.
- Libero-Femopase F, Hernandez SL, Gendelman H, Criscuolo MI, de Blanc SAL. Sialometaplasia necrotizante: Presentación de cinco casos. Clínicos. Med Oral Patol Oral Cir Bucal. 2004; 9: 304–308.
- Wenig BM. Necrotizing sialometaplasia of the larynx. A report of two cases and a review of the literature. Am J Clin Pathol. 1995; 103: 609–613.
- Franchi A, Gallo O, Santucci M. Pathologic quiz case 1. Necrotizing sialometaplasia obscuring recurrent well-differentiated squamous cell carcinoma of the maxillary sinus. Arch Otolaryngol Head Neck Surg. 1995; 121: 584–586.