Abstract
Biodiesel adulteration is normally performed seeking illicit enrichment and usually executed by adding cheaper miscible components to the fuel. In addition to harm the consumer, adulterated biodiesel not only damage car’s engine due to incomplete combustion, but also exacerbate environmental pollution by increasing pollutants emission. In concern with that, we propose an alternative protocol for monitoring bio fuel adulteration. The method hypothesized in this study aims to identify adulteration in biodiesel by detecting viscosity changes in the fuel. To understand if these changes would happen, we analyzed biodiesel viscous behaviour of four different sources, such as rice, soy, sunflower and corn oil, in four different adulterant ratios (0%, 20%, 40% and 60%). Raw soy oil was the adulterant source used in this work. We obtained an average correlation value between the proportion of adulterant and biodiesel’s viscosity of 0.9920. We concluded that the viscosity analysis has a great potential to be employed as a monitoring tool to detect adulteration in biodiesel.
Keywords: Bio combustible; Purity test; Physicochemical properties
Introduction
The civilization as we know it is greatly reliant on fossil sources of energy, such as coal and petroleum. These combustible are the most used energy on earth. Energy, also, has become an important factor for continue the economic growth and maintain high standard of living especially after the industrial revolution [1]. Globally, the transportation sector is the second largest energy consuming sector after the industrial sector. The world’s road transport is currently responsible for nearly 60% of world oil demand [2].
Studies have reported biodiesel’s good impact on the environment [3,4] when the fuel is in the right conditions, however fuels are commonly adulterated. Adulteration is normally performed by adding cheaper miscible components, normally raw vegetable oil or old frying oil [5] to the fuel seeking illicit enrichment [6]. The burning of adulterated fuels cause carbon deposits, injection blocking, and incomplete combustion, due to adulterated fuel high viscosity which reduces fuel volatility and increases gum formation in engines [6].
Adulterated combustible can also result in increased fuel consumption, engine over heat, and higher pollutants emissions, such as particulate material, hydrocarbons and exhaustion gases [7]. Based on that, the Brazilian National Agency of Petroleum, Natural Gas and Bio combustibles (ANP) has developed means to improve regulations and fuel quality in Brazil. ANP has defined specific tests for fuel quality and sales standards [8]; however the tests proposed to be used routinely by the distributors are based on the samples colour and density [9].
Although the trials proposed by the regulatory agencies are based on fuel characteristics, more sophisticated techniques have been used by scientists to understand fuel properties [10]. One of them is based on elucidating the bio fuel composition. In order to comprehend biodiesel content, the fatty acid profile is an important test for biodiesel analysis. Gas Chromatography (GC) and High- Performance Liquid Chromatography (HPLC) are the most common analytical methods for elucidating fatty acids and triglycerides in samples [11,12]. Chromatographic tests are precise and well accepted; however they require specialized hand-work, sophisticated instruments and high investments.
Our group hypothesized if the viscosity analysis can be used as an analytical tool to detect adulteration in biodiesel. In this context the viscosity test would fill the gap between unreliable tests, such as colour and density, and expensive and laborious methodologies, such as GC and HPLC. In concern with that we propose an alternative protocol for bio fuel blends adulteration monitoring: a qualitative analysis based on fuel viscosity.
Materials and Methods
Equipments
The heating sheets were performed under agitation by electric agitator (IKA, model C MAG-HS 7) at 75 °C, and, reflux condensers using H2O as means refrigerators utilized for sample synthesis. For the viscosity analysis was employed one viscometer (Quimis Q288SR, Brazil).
The chromatographic analysis of the profile of fatty acids of the samples were operated on gas chromatograph (GC-FID, Shimadzu QP2010, Japan) equipped with split/split less injector FID detector and capillary RTX- Wax column (30 m x 0.32 mm x 0.25 μm). The analyses were performed at the Laboratory of Chromatography and Forensic Chemistry of the Federal University of Pelotas.
Standards and chemicals
Solutions of Potassium Hydroxide 85% (KOH, Vetec, Brazil), Methanol (MeOH P.A., Vetec) and Sulfuric Acid (H2SO4, Sigma, Brazil) were the reagents and catalysers used in the transesterification. For acidity index, ether/ethanol solution (2:1 v/v), Phenolphthalein and Sodium Hydroxide (NaOH, Vetec) were employed. For saponification index Hydrochloric Acid (HCl, Proquimios, Brazil) was employed. The solution of cyclohexane (Synth, Brazil), solution of Iodine-Chlorine (Wijs, Synth), and Potassium Iodide solution (KI, Vetec) and Thiosulfate of sodium solution (Na2S2O3, Sytnh) were employed for iodine index determination.
Samples
Four samples of edible oil soy, sunflower, corn and rice from different brands were in bought in Pelotas, RS, Brazil, and employed as raw material for biodiesel obtention. The adulteration process of biodiesel was performed by adding soy edible oil in the samples.
Transesterification conditions
Biodiesel synthesis followed the chemical reaction scheme demonstrated in the Scheme 1.
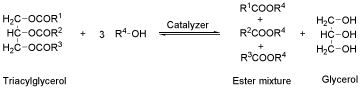
Scheme 1 Fatty acids transesterification general equation.
The synthesis procedure conditions were performed according to Oliveira et al., 2013 [13], as shown in Figure 1.
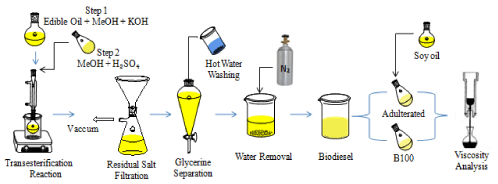
Figure 1: Protocol performed in this study for biodiesel production and
viscosity analysis.
Biodiesel purification protocol
Soon after the transesterification reaction, the mixtures were filtrated using a vacuum pump (to separate the salts formed when acid and base were mixed) and transferred to a decantation funnel (to separate the glycerine). Lastly, the biodiesel washed three times with 50 ml of water at 70 °C, taken to the rota evaporator (to eliminate the volatiles), and dried by bubbling Nitrogen (N2) for 15 minutes under a 0.4 ml min-1 flux [13].
Characterization of Biodiesel
Saponification index
Biodiesel samples (4.5 g) were added to 50 ml of KOH 4% in MeOH. This solution was carried under agitation for 60 minutes at 90 °C. After cooling, 1 ml of phenolphthalein (1%) was added to the mix and titration with HCl(aq) 0.5 mol L-1 was performed until the pink coloration disappearance [14].
Acidic Index
In an Erlenmeyer of 125 ml, 4 g of biodiesel were added in 25 ml of ether/ethanol (2:1) which was previously neutralized. After, two drops of phenolphthalein (1%) were added to the mix of ether/ ethanol and biodiesel. The mix was titillated with aqueous solution of NaOH 0.01 mol L-1 until disappearance of the pink coloration [14].
Iodine Index
Biodiesel (0.25g) were weighted and accumulated in a 500 ml Erlenmeyer with tip, after that, 10 ml of cyclohexane and 25 ml of Wijs solution were also added to the flaks. The Erlenmeyer containing the mix was sealed and agitated until complete homogenization and kept away from light for 30 minutes. Then, 10 ml of solution aqueous KI 15% and 100 ml of H2O were added to the solution and titration with Na2S2O3 0.1 mol L-1 was performed until the appearance of a light yellow colour. In sequence, 2 ml of starch indicator solution (1% w/v) was added and the titration with Na2S2O3 was continued until disappearance of the blue coloration [14].
Viscosity test
Biodiesel (60 ml) were added to the Say bolt (Q288SR) viscometer at 40°C, the tests were performed in triplicates and the data used in this study shows the mean and standard deviation [14].
Gas chromatography analysis
The conditions used for analyses of samples were the same the study of Rockembach et al., 2014 [15]. In this case hydrogen as carrier gas (flow rate of 1.2 ml min-1; split 1:50; sample volume of 1 μL. The programmed temperature of the oven was: initial temperature of 100 °C for 0.5 minutes, then a heating rate of 7 °C min-1 until 175 °C. After 175 °C the heating rate was reduced to 5 °C min-1 until reaching 190 °C. The samples were maintained at 190 °C for 1 minute, after that the heating rate was 1.20 °C min-1 until 230 °C and kept at that temperature for 11.45 minutes. The chromatographic analysis was 60 minutes long, and the injector and detector temperatures were 250 °C.
Statistical analysis
The Statistical analysis was performed using the software Graph Pad Instate v.3.0.1. In this study were implemented the correlation analysis, as well as One-way ANOVA (Confidence Interval 95%).
Results
Biodiesel’s physicochemical properties
Subsequently to biodiesel synthesis, our group studied biodiesel physicochemical properties, such as viscosity, acidic index, iodine index and saponification index (Table 1).
Source
h CO
(mm² s-1)
h BD
(mm² s-1)
II mg I2/100g
AI
mg KOH/g
SI
mg KOH/100g
ANP
NS*
3.0 – 6.0
103-128**
0.5
NS
Soy
18.5
2.8
145
0.06
218.5
Corn
30.2
3.9
147
0.04
179.6
Rice
37.0
4.5
111
0.08
182.7
Sunflower
33.8
3.8
142
0.06
185.3
*NS – Not specified ** National Health Surveillance Agency (ANVISA).
Biodiesel (BD), Commercial Oil (CO), Viscosity (h), Iodine Index (II), Acidity Index (AI), and Saponification Index (SI).
Table 1: Physicochemical Properties of Biodiesel Produced From Different Source and Commercial Oil, Viscosity, Iodine, Acidity, and Saponification Index.
Gas chromatography analysis
We evaluated biodiesel composition through Gas Chromatography analysis (Table 2).
Fatty Acid Composition
Fatty Acid Content (%)
C14:0-Miristic
0.05
C16:0- Palmitic
10.80
C16:1- Palmitoleic
0.03
C18:0- Estearic
3.90
C18:1- Oleic
24.30
C18:2- Linoleic
53.60
C18:3- Linolenic
5.80
C20:0- Araquidonic
0.17
C22:0- Beenic
0.35
Others
1.0
Table 2: Fatty acid proportion in soy biodiesel.
Adulterated biodiesel viscosimetric behaviour
After the physicochemical analysis, we evaluated the viscosimetric behaviour of the adulterated samples. Biodiesel adulteration was performed by adding commercial soybean oil. The viscosity results regarding biodiesel tempering are shown in Table 3.
Biodiesel Source
Ratio (Adulterant/Biodiesel)
r²
B100
20/80
40/60
60/40
Soy
2.8 ± 0.12
6.4 ± 0.08
8.7 ± 0.20
13.5 ± 0.40
0.9909
Corn
3.9 ± 0.13
6.4 ± 0.42
9.4 ± 0.14
12.4 ± 0.13
0.9988
Rice
4.5 ± 0.15
8.1 ± 0.12
12.3 ± 0.18
18.6 ± 0.15
0.9911
Sunflower
3.8 ± 0.18
6.1 ± 0.14
9.4 ± 0.46
14.4 ± 0.18
0.9874
Correlation coefficient (r²). One-way ANOVA between treatments P<0.001.
Table 3: Biodiesel viscosity when adulterated in different proportions followed by standard deviation and correlation coefficient between viscosity and adulterant ratio.
Discussion
Nowadays, scientists have dedicated many hours to investigate esters production using different raw materials and methodologies. The study which employed ultrasonic energy as promoter medium and sulfuric acid as catalyser is a good example of viable methodology to aliphatic esters production [16,17]. This procedure has shown advantages, such as quickness and low cost.
In this context, our group has recently researched biodiesel properties and production from algae biomass [18] and grape seed [15], both with satisfactory results. Although, the present study was to evaluate the viscosity analysis as an alternative protocol to analyze biodiesel samples regarding adulteration.
The commercial oils analyzed in this study presented viscosity between 18.5 and 37 mm²s-1, although the oleaginous based biodiesel examined in this study displayed viscosity between 2.8 and 4.5 ²s-1 (Table 1). The ANP regulation advocates viscosity between 3.0 e 6.0 ²s-1. Soy oil biodiesel was the only sample which is out of ANP regulation (viscosity of 2.8 ²s-1).
Based on the viscosity of the soybean commercial oil (18.5 mm²s-1), corn (30 mm² s-1), sunflower (34 mm² s-1) and rice (37 mm² s-1) we can observe a viscosity reduction of 6.0, 8.0, 9.0 and 8.0 times respectively after the transesterification reaction. The samples viscosity was satisfactory due to the fact that corn, rice, soy and sunflower based biodiesel can present viscosities between 4 e 6 mm² s-1 [19].
The iodine index (II) is related to the triglyceride source [20] and the number of double bonds along the carbon chain which are also correlated with biodiesel viscosity, density, oxidative stability (EN1403, 2003). In this work, The Wijs method was used to analyze the sample iodine index. The Wijs method is based on the addition of ICl (Wijs solution) on the samples. Part of the halogen in this solution is consumed by the double bonds. After reacting with KI, the excess is converted in I2 which is titrated with Na2S2O3, according to Staravache et al., 2007 [19]. The biodiesels investigated in this work showed II between 111 and 147 g I2/100 g of sample (Table 1). National Agency of Petroleum and Renewable Energy’s (ANP) does not regulate this range [8].
Despite iodine index and viscosity, another analysis which assesses fuel quality is the acid index [21]. Acid index can be used as a tool to monitor biodiesel degradation level, since AI tends to increase during stocking due to water and air interaction [20]. All the samples were according to ANP regulation, which predict 0.5 mg g-1 of KOH/g of sample. The acidic index determined in this study fluctuated between 0.06 e 0.08 mg of KOH/g of sample (Table 1). Based on that, the biodiesel produced exhibited great stability level.
We utilized commercial soybean oil as adulterant to investigate the samples viscosity behaviour because soy oil is abundant and the least viscous oil among the evaluated commercial oils (Table 3). Also, manufacturers looking for an adulterant source would consider a material which is relatively easy to find and would not modify drastically the product characteristics.
Similar results described in (Table 3) have been reported in the literature for biodiesel (B100) viscosity analysis. Soriano and co-authors, 2006 [22] obtained soy and sunflower based biodiesel showing viscosity of 4.12 e 4.3 mm² s-1, respectively. Lin et al., 2009 [23] have reported rice based biodiesel presenting viscosity of 4.12 mm² s-1. Higher viscosity values can be related to the presence of soap and glycerides, such as mono, di and triglycerides [24,25]. As stated by Martines et al., 2000 [26], these compounds when in excess can spoil combustion decreasing the atomization level in the engine and consequently increase the undesirable gases emissions.
As seen in (Table 3), pure soybean biodiesel had viscosity of 2.8 mm² s-1, however when tampered by 20% with commercial soy oil, the viscosity increased about 3.2 times. When tampering was carried out by adding 40 and 60% of commercial soybean oil, viscosity increased approximately 3.8 and 5.2 times, respectively, the value determined for the pure biodiesel.
The rice based biodiesel presented viscosity of 4.5 mm² s-1 (Table 3). However when tampered 20, 40 and 60% by the addition of commercial soy oil, the observed viscosity increased about 3, 5 and 6 times, respectively, in relation to the viscosity value determined for the pure biodiesel.
The obtained results for all the samples showed great statistical relevance (P<0.001) when compared the mean viscosity values of the three treatments utilizing the statistical analysis One-way ANOVA P<0.05.
Viscosity when chemically described can be understood as Van Dar Waals and intermolecular interactions between the triglycerides and methyl esters of same nature. Therefore, our group elucidated that the difference in viscosity showed in this study is due to adulterant higher molecular weight when compared to biodiesel’s methyl esters. In other words, triacylglyceride molecules (from the adulterant source) provide greater resistance to change the shape of these liquids due to more intense molecule interaction. This was demonstrated experimentally when evaluated adulterated biodiesel viscosity (Figure 2).
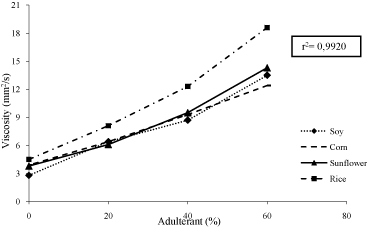
Figure 2: Biodiesel Viscosimetric Behavior when Pure (0%) and 20%, 40%
and 60% adulterated with commercial soy oil. Average r² presented.
Despite the molecular interaction, another property which is relevant when investigating viscosity is the activation energy. Regarding the monounsaturated and polyunsaturated fatty acids composition, showed in Table 2, as presented by Canciam, 2010 [27]. We can observe that the fatty acid profile can influence the action energy necessary for the reaction. Although as stated by Toralles et al., 2006 [28], in general a high activation energy implies that small temperatures variation can quickly modify the viscosity behaviour of the samples. Thus, the rice oil viscosity behaviour was higher than the others oils investigated in this work. Notwithstanding, after biodiesel synthesis and adulteration the rice oil based biodiesel presented higher viscosity values, as show in Table 3, due to the higher activation energy.
Regarding adulteration, in summary the more fatty acids added to the bio combustible the more viscous it gets. To comprehend the impact of added oil in biodiesel on viscosity samples we calculated the r². The r² for the samples fluctuated between 0.9874 and 0.9988 (Table 3). The mean r² observed was 0.9920 (Figure 2). The high correlation value between adulterant proportion and viscosity show us that the viscosity analysis has great potential to be used as purity test for biodiesel. Further investigation is needed to establish this alternative test.
In this study we inferred that the addition of 20% (v/v) adulterant to any of the biodiesel analyzed in this study is sufficient to detect adulteration based on viscosity. We also can observe on Figure 2 that even adulterant ratio lower than 20% might be detectable through viscosity analysis. However, more studies are needed to elucidate the minimal adulterant amount which can be detected by viscosimetric changes in biodiesel.
Conclusion
In spite of confirming that different raw materials result in biodiesel with different physicochemical properties, we also confirmed our hypothesis that the viscosity analysis is able to detect adulteration in biodiesel. Although, to put this protocol into practice, a database of different source biodiesel would be necessary, since we demonstrated that bio combustible fatty acid composition influence the product characteristics, such as viscosity. Further investigation is essential to comprehend the minimum adulterant content which can be detected by viscosity analysis.
Acknowledgment
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) for supporting this study.
References
- International Energy Agency (IEA). World Energy Outlook China and India Insights. France: STEDI MEDIA. 2007.
- Atabani AE, Silitonga AS, Badruddin IA, Mahlia TMI, Masjuki HH, Mekhilef S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. And Sust. Energy Rev. 2012; 16: 2070-2093.
- Granda CB, Zhu L, Holtzapple MT. Sustainable Liquid Biofuels and Their Enviromental Impact. Environ. Prog. Susntain. Energy. 2007; 26: 233-250.
- Talebi FA, Tabatabaei M. Chisti Y. Biodiesel Analyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J. 2014; 1: 55-57.
- Soranso AM, Filho GA, Lopes A, Souza EG, Dabdoub JD, Furlani CEA, et al. Dynamic performance of an agricultural tractor utilizing distilled biodiesel from spent oil. R. Bras. Eng. Agric. Ambiental. 2008; 12: 553-559.
- Pontes MJC, Pereira FC, Pimentel FM, Vasconcelos CVF, Silva AGA. Screening analysis to detect adulteration in diesel/biodiesel blends using near infrared spectrometry and multivariate classification. Talanta. 2011; 85: 2159-2165.
- Corgozinho CNC, Pasa VMD, Barbeira PJS. Determination of residual oil in diesel oil by spectrofluorimetric and chemometric analysis. Talanta. 2008; 76: 479-484.
- Brazilian National Agency of Petroleum, Natural Gas and Biocombustibles (ANP). 2016.
- Lobo I, Ferreira S, Cruz R. Biodiesel: quality parameters and analytical methods. Quim. Nova. 2009; 32: 1596-1608.
- Baptista P, Felizardo P, Menezes JC, Neiva MJ. Multivariate near infrared spectroscopy models for predicting the iodine value, CFPP, kinematic viscosity at 40 oC and density at 15oC of biodiesel. Talanta. 2008; 77: 144- 151.
- Santacesaria E, Vicente GM, Di Serio M, Tesser R. Main technologies in biodiesel production: State of the art and future challenges. Catalysis Today. 2012; 195: 2-13.
- Abbaszaadeh A, Ghobadian B, Omidkhah MR, Najafi G. Current biodiesel production technologies: A comparative review. Energy Convers. Manage. 2012; 63: 138-148.
- Oliveira DM, Ongaratto DP, Fontoura LAM, Naciuk FF, Santos VOB, Kunz JD, et al. Obtencao de biodiesel por transesterificacao em dois estagios e sua caracterizacao por cromatografia gasosa: oleos e gorduras em laboratorio de quimica organica. Quim. Nova. 2013; 36: 734-737.
- Sendzikiene E, Makareviciene V, Janulis P. Oxidation stability of biodiesel fuel produced from fatty wastes. Pol. J. Environ. Stud. 2005; 14: 335-339.
- Rockembach CT, Dias D, Vieira BM, Ritter M, Santos MAS, de Oliveira DM, et al. Sintese do Biodiesel Derivado do Oleo da Semente de Uva Promovida por Ultrassom. Rev. Virt. Quim. 2013; 6: 884-897.
- Hobuss CB, Venzke D, Pacheco BS, Souza AS, Santos MAS, Moura S, et al. Ultrasound-assisted synthesis of aliphatic acid esters at room temperature. Ultrason. Sonochem. 2012; 19: 387-389.
- Pacheco BS, Nunes CFP, Rockembach CT, Bertelli P, Mesko MF, Roeschely M, et al. Eco-friendly synthesis of esters under ultrasound with p -toluenesulfonic acid as catalyst. Green Chem. Lett. Rev. 2014; 7: 265-270.
- Hobuss CB, Rossales PF, Venzke D, Souza PO, Gobbi PC, Gouvea LP, et al. Cultivation of algae in photobioreator and obtention of biodiesel. Rev. Bras. Farmacogn. 2011; 21: 361-364.
- Stavarache C, Vinatoru M, Maeda Y. Aspects of ultrasonically assisted transesterification of various vegetable oils with methanol. Ultrason. Sonochem. 2007; 14: 380-386.
- Refaat AA. Correlation between the chemical structure of biodiesel and its physical properties. Int J Environ Sci Tech. 2009; 6: 677-694.
- Venzke D, Flores AFC, Quina FH, Pizzuti L, Pereira CMP. Ultrasound promoted greener synthesis of 2-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)-4- phenylthiazoles. Ultrason Sonochem. 2011; 18: 370-374.
- Soriano JNU, Migo VP, Matsumura M. Ozonized vegetable oil as pour point depressant for neat biodiesel. Fuel. 2006; 85: 25-31.
- Lin L, Ying D, Chaitep S, Vittayapadung S. Biodiesel production from crude rice oil and properties as fuel. Applied Energy. 2009; 86: 681-688.
- Instituto Adolfo Lutz. Normas analíticas do Instituto Adolfo Lutz: Metodos quimicos e fisicos para analises de alimentos. 4th Edn Brasilia. 2004.
- EN14103. Fatty Acid Methyl Esters (FAME) – Determination of ester and linolenic acid methyl ester contents. European Committee for Standardization: Brussels. 2003.
- Martines MAU, Davolos MR, Junior MJ. O efeito do ultrassom em reacoes quimicas. Quim. Nova. 2000; 23: 251-256.
- Canciam CA. Effect os Temperature on Viscosity of Refine Vegetable Oils. UEPG- Exact Earth Sci. Agr Sci Eng. 2010; 16: 7-12.
- Toralles RP, Vendruscolo JL, Vendruscolo CT. Reologia de Purê Homogeneizado de Pêssego: Efeito da Temperatura e Concentração. Braz J of Food Technol. 2006; 9: 1-8.
