Abstract
The study considered the special effects of Polycyclic Aromatic Hydrocarbons or PAHs present in the diesel infested Loco tank on the liver of three teleostean fishes namely, Labeobata,Labeorohita and Cirrhinusmrigala. The histopathological study on liver of these three fishes revealed the distortion of the hepatic cords, dilation of the sinusoids, degeneration of endothelial cells lining, shrinkage of acinar cells, bile stagnation and early signs of necrosis, prominent ones in L. bata. In L. rohita, the hepatic cords were disrupted. Apart from the deformed nucleus, nuclear megalocity, dilated sinusoids were noticed. In C. mrigala, vacuolation of the sinusoids, degeneration of the hepatic cords, bile stagnation, formation of small vacuoles, and mild necrosis in some places were the major symptoms of toxicity. The mean plasma ALPase activities (117.0±20.368, 412.0±128.550, and 160.6±9.074 IU/l) of all the fish, L. bata, L. rohita and C. mrigala amplified at 162.95%, 201.96% and 107.56% respectively, while SGOT (AST) activities (199.0±40.037, 232.0±39.661 and 276.3±31.086u/l) increased at 125.15%, 126.98% and 169.35% with significantly higher (p<0.05) in respect to control. Plasma SGPT (ALT) activity in fish illustrated a similar pattern of increment over control group. However, the modified histo-architechture phenomena and altered enzymatic activities of liver disclosed that the PHAs originated from diesel-fed aquatic system, may revolutionize physiological functions of these three fishes L. bata, L. rohita and C. mrigala of three different layers, surface, column and bottom, respectively.
Keywords: Polycyclic Aromatic Hydrocarbons (PAHs); Histopathology; Plasma enzymes; Fish
Introduction
Pollutants are continuously impairing the biological functioning of an organism to cause an undesirable change to ecosystem including humans even to non-target organisms, which are associated to that particular environmental condition [1]. At the present time, different aquatic systems exposed to pollutants due to unsystematic and spontaneous use of resources generating number of alarming responses to both on system as well as organisms. Petroleum is employed as the main source of energy; despite of its all importance, it also acts as a global environmental pollutant [2]. Petroleum, enter into the aquatic system through waste disposal and accidental spills is considered to be a complex mixture of many chemicals and its 1% is soluble in water having Water-Soluble Fraction (WSF) like Hydrocarbons (HCs) and heterocyclic compounds [3]. Some microorganisms can utilize petroleum and petroleum products as sole carbon sources for getting their energy and metabolic activities [4]. Multiplicity of sub-lethal effects of crude oil or diesel components have been accounted to a number of aquatic organisms, like fish [5] due to large quantities of PAHs originate form diesel in the course of microorganisms degradation [2]. Fish are suitable indicators of contaminants accumulation [6] and aquatic toxicity study of any contaminant because of their high sensitivity to environment. Its minimum modification can alter physiological and biochemical status of the respective organ, tissue or pathways related to that toxicant. Fishes are the good accumulator of soluble petroleum hydrocarbons [3]. Accumulation, excretion and biotransformation of most pollutants take place in liver, vital functions in essential metabolism, act in response specifically to the level and nature of contamination [7]. Histo-pathological changes in liver are used as biomarker as well as assessment of effect for the environmental pollution and its changes in fish livers exposed to number of organic compounds [8]. Like other, in fish multifunctional enzyme, alkaline phosphatase produces transphosphorylase in alkaline medium and mineralizes in skeleton [9]. Biochemical indicator of liver toxicity, ALT (Alanine aminotransferases) and AST (Aspartate aminotransferase) are aminotransferases, help gluconeogenesis from amino acids, and in hepatic cells provide aminotransferase activities [10].
AST and ALT, present in different organs plays a vital role in amino acid and carbohydrate metabolic pathway, can be hampered in several stress condition [11]. Alkaline phosphatase which involved in the hydrolysis of phosphomonoester substrates can be altered in cyanide exposure and thus affected on hepatic and renal tissues [12]. Transaminase enzymes act as a marker for the functions and integrity of the heart and liver. By examining blood physiological, nutritional and pathological status of an organism can be reveal. For the plasma membrane and endoplasmic reticulum ALP is act as a marker enzyme. It also can be use for assessing integrity of the plasma membrane. Increment of ALP level and ALP activity are the indication of the membrane and liver damage respectively. Hence, changes in enzyme kinetics in different organs reveal the presence/ exposure of toxic elements in the surrounding environment and thus enzyme studies facilitate to understand the toxicity in the organs [13].
The effects of PAHs are well documented in laboratory conditions, but its changes in liver for diesel and diesel derivative PAHs with other vital limnological factors in field conditions still are lacking. The present experiment has been conducted to show the effect of discharged diesel oil of the diesel-fed Loco tank in the liver of the fish collected from three different trophic levels of the pond. The histopathological studies and serum biochemical parameters like SGOT (AST), SGPT (ALT), Alkaline Phosphatase (ALPase) have been investigated to register the effect of diesel oil on the liver of target fish species viz.,Cirrhinusmrigala, Labeobata and Labeorohita which are abundantly cultured in the study site. According to the feeding habit Labeobata is a surface feeder, Labeorohita is a column feeder and Cirrhinusmrigala is a bottom feeder. So, these target species can cover the whole water column.
Methods
Study site
The Loco tank, which is adjacent to Barddhaman locomotives is used by the Rupali Cooperative Limited for aquaculture with a total area approximately 11.0 hectares with an yearly catch at an average of 22.72 metrictons. The main species cultured in this pond are tilapia, catla, rohu, mrigal, silver carp etc. Labeobata, Labeorohita and Cirrhinusmrigala were chosen during the experimental period and weight of the chosen fish species were 26.71±4.17, 35.53±2.97 and 41.32±3.49 cm while, weight were 0.24±0.14, 0.74±0.18, 1.40±0.23 Kg respectively. It is worth mentioning that total 12 number of fishes from each category were taken (n=12).This pond is located at 23014’42”N latitude and 87052’38”E longitude. As a consequence of residential and locomotive works, it is receiving an appreciable amount of domestic sewages also from the North and North-Western parts of the pond and diesel discharging points located at the Southern and South- Western parts. There are two main parts from where the diesel oils are directly discharged into the pond, although there is a small “effluent treatment plant” on the Southern part of the pond (Figure 1).
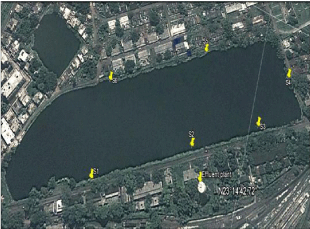
Figure 1: Sampling sites of study pond showing different sampling points
S1 (23°14’48.54”N, 87°52’24.41”E), S4 (23°14’41.39”N, 87°52’31.30”E), S5
(23°14’45.26”N, 87°52’30.4”E) and S6 (23°14’49.13”N, 87°52’27.32”E) are
domestic waste discharge point while S2 (23°14’44.91”N, 87°52’26.98”E)
is diesel effluent discharge point and S3 (23°14’42.31”N, 87°52’28.98”E) is
diesel discharge point.
Water samples were collected from epilimnion zone of six sampling sites of the Loco tank marked as S1, S2, S3, S4, S5 and S6. The S2 and S3 sites are the diesel discharge points from the Diesel shed, whereas, the S4 and S5 points usually receive domestic wastes. Samples were preserved in well plugged glass bottles, which were previously soaked in nitric acid (10 %) for 24 h followed by washing with millipore water. Prime physico-chemical features of water such as temperature, pH, Dissolved Oxygen (DO), phosphate, oil and grease, chloride and ammonia-nitrogen etc., were measured as per APHA (2005).
GC analysis of contaminated water
1 ml concentrated (1,000-fold) blanks were analyzed with Gas Shimadzu Class LC-1 (set of Auto-injector along with split/splitless injector and fused silica capillary B-5 (30m, 0.32mm, 0.17μm) 100 % dimethylpo-lysiloxane. 60 to 300 °C temperature with 5°C /min rate was programmed and then 290 °C for 25 min. The temperature of injector was 280°C and detector was 300 °C. 2 μL sample was injected in 10:1 ratio in split mode in purge time of 1min. Carrier gas (nitrogen) flow rate was 1.0m1min–1. Scanning range of spectrometer was 50– 350Da/ sec with the 70 eV electron energy. Based on the matching retention time with a mixture of PAH standards, identification and quantification were carried out of 16 PAH compounds. GC–MS (Hewlett-Packard 5889B MS “Engine”) with selected ion monitoring mode was done for confirmation. HP-1 column and helium as carrier gas were used while other programs were same as above.
Tissue preparation for histological examination
Liver tissues were dissected (0.5 ± 0.145 g) from control and treated fish species from three different trophic levels (surface, column and bottom). Physiological saline solution (0.75 % NaCl) was taken to rinse or clean the tissues. It was fixed in Bouin’s solution (75 ml saturated picric acid, 25 ml formaldehyde (37-40 %) and 5 ml glacial acetic acid) for 48 h. Consequently, dehydrated with graded alcohol series, followed by xylene, the clearing agent and embedded in paraffin. 3μm liver sections were taken from microtome (Leica-RM 2145). Hematoxylin-Eosin (H&E) staining method was performed and examined using an Olympus BX 51 optical microscope.
Plasma enzymes analysis
Blood samples from three sets of fishes like Labeobata, Labeorohita, Cirrhinusmrigala have been collected from caudal vain. The blood sample (1.3 ml) was taken midline just posterior of the anal fin, inserting the needle into the musculature perpendicular to the ventral surface of the fish until the blood enters the heparinized syringe (n=12).Both the activities of AST and ALT were estimated using Reitman and Frankel [14] method and activity of ALP was performed as phenolphthalein monophosphate method with Randox kits. Analysis was performed in Robonik Prietest Touch Biochemistry Analyser (Version 2.622A).
Statistical analysis
Observations were statically considered with ANOVA and least significance difference or LSD, which was mentioned as mean ± SEM. Significance of the differences between treated and control value was obtained (p< 0.05 and p< 0.01) using SPSS version 10.0.
Results and Discussion
Limnological study
Water quality indicates the physical, chemical and biological properties of aquatic system, determine the condition for utilize and facilitate to sustain the health of aquatic organisms farmed for commercial and economic growth. Dissolved or suspended constituents control or influence its properties. Primary physical properties like pH (av.8.35±0.31), temperature (av.20.39±0.74 0C), conductivity (av. 705.50±2.43 μS/cm), total dissolved solids or TDS (av. 463.27 ± 14.16 mg/l) and total suspended solids or TSS (11.00±6.07 mg/l) had a minimum range in alteration over seasons, but difference with the site was prominent. The average value of physico-chemical parameters like dissolved oxygen, total alkalinity, chloride, total hardness, biochemical oxygen demand or BOD, ammonia-nitrogen or NH3-N and orthrophosphate or PO4-P are 7.885±0.556, 260.94 ± 17.53, 72.782±4.528, 238.5±9.50, 16.6±17.73, 0.131±0.013, 0.111±0.104 mg/L respectively. pH, conductivity, TDS, total alkalinity, chloride and total hardness concentration were highest in S2 while in the case of TSS and BOD S3 showed the highest value. The study revealed that most of the factors were remain within the standard aquaculture water quality index for tropical climate except alkalinity. Changes in water quality parameters with sampling site has mentioned in Table 1. However, osmoregulation in high ion concentrations associated with alkalinity [15], but effects on liver and its function less documented. The average oil and grease concentration was 369.65±241.58 mg/l, but the values showed a wide range from 87.98 to 746.23 mg/l. A wide range between 560.12 and 746.23 mg/l was obtained from diesel effluent discharge point (S2) and diesel discharge point (S3) respectively in respect to others.
Parameters
S1
S2
S3
S4
S5
S6
pH
8.25
8.96
8.32
8.15
8.1
8.34
Temp (0C)
19.21
20.23
20.7
20.12
20.62
21.45
Conductivity (μS/cm)
702
708
707
707
706
703
TDS (mg/l)
470.34
474.36
473.69
445.41
444.78
471.01
TSS (mg/l)
7
9
22
14
8
6
DO (mg/l)
8.5
7.45
8.23
7.11
7.67
8.35
Total Alkalinity (mg/l)
272.89
287.16
266.13
245.25
242.09
252.12
Chloride (mg/l)
75.37
79.22
70.64
69.12
75.12
67.22
Total Hardness (mg/l)
241
247
231
246
243
223
BOD (mg/l)
6.5
37.4
41.5
4.2
4.9
5.1
PO4-P (mg/l)
0.07
0.01
0.1
0.06
0.12
0.31
Table 1: Changes in water quality parameters of different sampling sites.
Ara et al., [16] found the less pH content in the diesel oil contaminated water than that of control water, where the control water had the pH of 7.5 before the treatment and after the contamination, the pH showed the value of 6.3. Ara et al., [16] and Achudume (2009) which opined that the increasing oil concentration in water body could lower concentration of dissolved oxygen; it may be due to the subsequent release of free CO2, which supports the degradation of hydrocarbons as the end product of CO2. Alkalinities of Loco tank water was greater in cold season or winter, which supports the greater capability microbial growth and survivalists of aquatic organisms [17], which relates the present observations depicting the huge microbial growth in the pond.
Polycyclic aromatic hydrocarbons (PAHs)
According to the GCMS study of the pond water, the total concentration of PAH in S2 was 349.64 ng/l, in S3 489.92 ng/l, in S4 106.62 ng/l and in S5 it was 39.71 ng/l, where, the S2 and S3 sites are the main discharge points of diesel into the pond. The total concentration of the alkyl PAH has been estimated, where the alkyl PAH of S2 was 54.05 ng/l, in S3 was 377.04 ng/l, in S4 was 45.05 ng/l and S5 showed 20.23 ng/l. Among the PAHs, the concentration of naphthalene and phenanthrene were found to be much higher in S2 and S3 than the other sites S4 and S5 (288.59 and 21.49 ng/l, respectively). S3 also contained the higher concentrations of naphthalene, fluorine, phenanthrene, pyrene, benzo[a]anthracene, benzo[a]pyrene, anthracene, perylene, benzo[a]fluoranthene (192.27, 27.41, 11.24, 124.82, 15.27, 42.04, 13.93, 7.24 and 16.91 ng/l, respectively). Among the alkyl PAHs, the concentrations of 1-methyl naphthalene, 2-methyl naphthalene, 1-methyl fluorine, 1,2-dimethyl naphthalene, 1-methyl pyrene, 2,3,5-trimethyl naphthalene, 4,6-dimethyl dibenzothiophene, 2-methyl phenanthrene, 2,3-dimethyl anthracene, 2-methyl fluoranthene and 4-methyl dibenzothiophene were 17.06, 24.29, 9.29, 78.35, 34.44, 98.63, 16.68, 11.60, 19.89, 11.97, 50.48 ng/l, respectively, which were higher in S3, the main discharge points of diesel. But in case of the other sites, the concentrations of alkyl PAHs had been found in much lower amount except 2-methyl naphthalene, which showed their presence in the sites such as S2, S4 and S5 in adequate amount, 21.29, 10.83 and 4.91 ng/l, respectively (Figure 2 and Table 2).
PAH(ng/l)
No of rings
Abbr
S2
S3
S4
S5
Naphthalene
2
Nap
288.59
192.27
19.97
65.42
Acenaphthylene
3
Acy
2.93
6.69
0.92
2.99
Acenaphthene
3
Ace
4.91
4.64
0
4.33
Fluorene
3
Flu
9.55
27.41
1.94
5.98
Dibenzothiophene
3
DB
2.76
4.12
1.36
2.95
Phenanthrene
3
Phe
21.49
11.24
5.52
10.99
Anthracene
3
Ant
1.21
42.04
0.77
1.48
Fluoranthene
4
Flut
5.08
16.91
2.26
3.3
Pyrene
4
Pyr
5.44
124.82
3.54
2.93
Benzo[a]anthracene
4
BaA
0.64
13.93
0.52
0.78
Chrysene
4
Chr
0.45
5.58
0.26
0.56
Benzo[b]fluoranthene
5
BbF
0.96
7.24
0.3
0.7
Benzo[k]fluoranthene
5
BkF
0.27
1.17
0.1
0.16
Benzo[a]pyrene
5
BaP
0.82
8.83
0.39
0.61
Perylene
5
Per
2.86
15.27
1.54
2.52
Benzo[g.h.i]perylene
6
BP
0.71
2.51
0.14
0.42
Dibenzo[a.h]anthracene
5
DA
0.16
0.33
0
0
Indeno[1.2.3-c.d]pyrene
6
IP
0.81
4.92
0.18
0.5
∑PAHs
349.64
489.92
39.71
106.62
Table 2: Concentration of PAH in diesel fed pond water (ng/l).
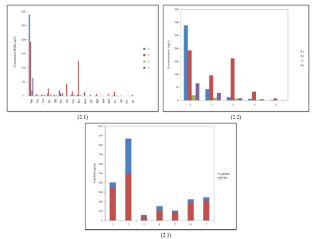
Figure 2: PAHs concentration (2.1), alkaline PAHs (2.2) and total PAHs (2.3)
in the diesel-fed pond water.
Among the 16 PAHs which are considered to be carcinogenic, all were obtained in the S2 and S3 sites of the diesel-fed pond in a considerable amount. The ranking of the PAHs levels revealed that the diesel-fed pond contained a larger number of Polynuclear Aromatic Hydrocarbons (PAHs). A number of PAHs are considered as potential human carcinogens; they are like, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[a] pyrene and benzo[ghi] perylene [18]. The Agency for Toxic Substances and Disease Registry (ATSDR) find a study, shows the limits of concentrations less than 0.2 mg/m3 of benzo(a)pyrene in the drinking water and within the work place of individuals work for 8 h per day [19] and in drinking water, it should not exceed 0.0002 mg/l (USEPA, 2009). Here, this pond contained less amount of benzo(a)pyrene (0.00012 mg/l) in S2 site than the standard limit.
Oxidative stress is mainly caused by the imbalance of pro- and antioxidants ratios and thus leads to the generation of Reactive Oxygen Species (ROS). Presences of some contaminants (PAHs, pesticides, metals etc.,) are responsible for the alteration of the antioxidant defense system and thus make oxidative damage through ROS formation. When ROS reacts with the biological macromolecules, it inactivates the enzymes, oxidize membrane lipids and proteins, make damage in DNA and occurs cell death [20,21]. In fish, antioxidant defense system pathways contain enzymatic antioxidants (Catalase (CAT), Glutathione Peroxidase (GPx) and Glutathione-S-Transferase (GST)) and non-enzymatic antioxidants, reduced glutathione (GSH) activity and other thiols (NP-SH)) [22,23].
Increased cellular vacuolization, RER proliferation and glycogen depletion have also been recorded in the liver of fish, which has been exposed to petroleum hydrocarbons [24,25]. Sindermann [26] have noticed the increased vacuolization in liver cells, which reflects the increased hepatic lipid content in fish exposed to petroleum hydrocarbons.
Liver histology
The liver is considered as a good indicator of nutritional pathology because of its function in metabolism. The histology of fish liver has been depicted by the absence of portal triads and liver lobules, which are the basic morphological unit of liver structure of mammals. The fish liver structurally contains the liver cells-hepatocytes, bile ducts and blood vessels, which are differently organized as compared with mammals. Liver is a bilobed gland. The hepatocytes are arranged in cords with radiating nature. The central veins made up of blood vessels. Pancreas is extended into liver forming a hepatopancreas. It is made up of exocrine cells, which are large in nature and columnar or cuboidal shape with a large nucleus. Each exocrine cell has a basal portion containing homogeneous cytoplasm and an apical part containing a large number of zymogen granules. Due to diesel toxicosis in the liver of L. bata, distortion of the hepatic cords, dilation of the sinusoids, degeneration of endothelial lining cells, shrinkage of acinar cells, bile stagnation and early signs of necrosis were the prominent observations. In L. rohita, the hepatic cords were also disrupted. Apart from that distorted nucleus, nuclear megalocity, dilated sinusoids were also noticed. In C. mrigala, vacuolation of the sinusoids, degeneration of the hepatic cords, bile stagnation, formation of small vacuoles, and mild necrosis in some places were the major symptoms of toxicity (Figure 3).
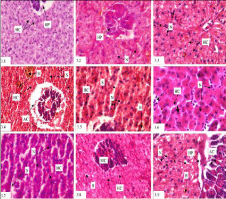
Figure 3: Histopathological photomicrographs of liver (Haematoxylin-Eosine
Staining) of L. bata (3.1) 1000 X, L. rohita (3.2) 1000 X and C. mrigala (3.3)
1000 X in control conditions under light microscopy, field condition of different
fishes L. bata (3.4 X 400 and 3.5 X 1000), L. rohita (3.6 X 1000 and 3.7
X 1000) and C. mrigala (3.8 X 400 and 3.9 X 1000). The hepatocytes are
arranged in cords (HC) with radiating manner showing hepatopancreas (HP)
(3.1). Extended pancreas into liver forming a HP have nucleus (N) (3.2 and
3.3). Distortion (broken arrow) of the hepatic cords (HC), dilation (broken
arrow) of the sinusoids (S), shrinkage of acinar cells (AC), bile stagnation
(B) and early signs of necrosis in L. bata (Treated) (3.4 and 3.5). Irregular
shaped hypertrophied and pyknotic nuclei nucleus (N), nuclear megalocity
(arrow head), dilated sinusoids (S) and distortion (broken arrow) of hepatic
cords (HC) in L. rohita (3.6 and 3.7). Vacuolation of the sinusoids (V) formed
(broken arrow) in the hepatic cords (HC), bile stagnation (B) in C. mrigala
(3.9 and 3.8).
A number of investigations have been reported to show the observations in liver such as, vacuolar degeneration (focal and diffuse) of hepatocytes, regeneretion and inflammation, foci of necrosis, piknotic nuclei in the hepatocytes etc., which may be caused due to the exposure of fishes to a number of different chemical compounds, like crude oil, high ammonia concentrations, heavy metals released from paper and pulp mill effluents, and complex environmental pollution [27-32]. In the study of Belicheva and Sharova (2011), it has been revealed that fish contains signs of neoplastic changes in liver. The affected liver cells possess the characteristics of variable degrees of cellular pleomorphism, high mitotic activity, nuclear atypia, along with abnormal architecture. Non-neoplastic proliferative changes related to diesel oil pollution, which are characterized with small islands of regenerating hepatocytes, enclose noticeably basophilic and rear cytoplasmic vacuoles. Due to the effect of water soluble fractions of diesel, the most relevant changes has been studied in the liver of the exposed fish are like nuclear hypertrophy, irregular shaped nucleus situated in lateral position, cellular hypertrophy and irregular, cytoplasm with eosinophilic granules, cellular atrophy, melanomacrophage aggregates, nuclear atrophy, peripheral nuclei, cytoplasmic vacuolation along with biliary stagnation [33]. The study conducted by Myers et al., in 1998 revealed the presence of several hepatic lesions in the fish flounder Pleuronectesvetulus, which has been exposed to sediment PAH. In another study, various histological alterations in liver has been found in the fish Pleuronectesamericanus (Flounder),collected near an oil refinery considered as an indicator to show the ill effects of oil on fish health [34,35]. Necrosis areas, cellular vacuolization, inflammatory response, pre-carcinogenic or carcinogenic lesions can be frequently observed in liver of fish, which has been environmentally exposed to PAH´s, PCB´s, heavy metals, sewage, harbour or industrial effluents [36- 41]. According to the study of (42), the liver of the milk fish, which has been exposed to petroleum hydrocarbons revealed the histological alterations, like sinusoid dilation, lipidosis, necrosis, vacuolations, nucleus pleomorphism and bile stagnation. The blood sinusoids are dilated between the cords of hepatocytes, which is almost similar to the present observations.
Enzymatic assay
Presence of high concentration specific enzymes like alanine aminotransferase (ALT or SGPT) and aspartate aminotransferase (AST or SGOT), through simple blood test can reveal the liver injury because enzymes present in liver cell spilled out into the blood stream during injury and thus raised the enzyme concentration in blood, which plays as an indicator of liver injury [43].
Several hazardous compounds produced from diesel act as an effective carcinogenic and immunotoxicant for living cells. It can promote several degenerative alteration in structure and enzymes in hepatic cells. Diesel can induce variable degenerative alterations in the structural integrity of liver cells along with its enzymes [44].
In the present observation, the herbivorous fishes like C. mrigala, L. bata and L. rohita, which were collected from the diesel contaminated pond, the mean plasma SGOT (AST) values were 276.3±31.086 u/l, 199.0±40.037 u/l and 232.0±39.661 u/l respectively, which were higher than the values in the fish of some species of the control pond (172.3±57.873 u/l, 159.0±43.486 u/l and 182.7±35.921 u/l respectively) (Figure 4.1). The mean plasma SGPT (ALT) values of the affected fishes were 17.00±2.00u/l in C. mrigala, 31.167±6.825 u/l in L. bata, and 34.667±6.506 u/l in L. rohita. They also showed higher mean values than the control fishes i.e, 12.333± 2.517 u/l, 21.933±1.069 u/l and 22.333±5.508 u/l, respectively (Figure 4.2). The plasma alkaline phosphatase activity (Figure 4.3) of the diesel affected fishes became higher (160.667±9.074 IU/l, 117.067±20.368 IU/l and 412.0±128.550 IU/l in C. mrigala, L. bata and L. rohita, respectively) than the control fishes (Table 3).The mean plasma ALPase and SGOT (AST) activity of all the affected set of fish, which have been exposed to sublethal concentrations of diesel oil were significantly higher (p<0.05) in respect to the set of control fish. The mean plasma of SGPT activity of all the set of affected fish groups were significantly higher (p<0.05) than that of the set of control fish. Azad [45] observed same pattern of diesel toxicity on different tissues and organs of fish. Due to the effect of diesel contamination, the fishes (L. bata, L. rohita, and C. mrigala) showed elevated level of plasma enzymes than the control fishes. These increments may be due to the malfunctioning of liver under stress condition. The fluctuations in the liver enzyme (SGOT, SGPT, alkaline phosphatase) level may be caused by the changes in metabolic pathways due to petroleum exposure. The increase in SGOT and SGPT activities is the indicator of liver damage occurred due to petroleum exposure leads to the seepage of these enzymes to blood. Elicited level of different hepatic biotransformation of enzymes with different sensitivity level after the severe exposure of the fish Scophthalmus maximus (juvenile turbot) to Prestige fuel oil has been demonstrated by [46]. It has been reported by [47] that temporary exposure of petroleum oil spill on Rainbow fish can potentially affect the level of metabolic and detoxification enzymes. According to [44], the average serum plasma ALP activity of all the treated fish sets, which have been treated with sub lethal concentrations of graded diesel oil are significantly higher (p<0.05) as compared to the control fish group after 14, 21 and 28 days of treatment, respectively. After 14, 21 and 28 days of treatment with diesel, the measured mean plasma SGPT activity of all the diesel exposed fish groups were significantly higher (p<0.05) than the untreated fish group (control). Sometimes the levels fluctuated due to the degree of death of the hepatic cells of fish liver, which has been exposed to different concentrations of diesel oil. From the obtained results, it can be inferred that exposure of diesel increased the mean serum SGPT level, which is a strong indication of cirrhosis and stress, myocardial infarction; similar type of increments were found in serum mean ALP, SGOT and SGPT in plasma. This is similar to the finding of [48], where alterations in the level of plasma enzymes, which have been exposed to the lowest concentration of stressors, showed the highest effect. A related work has been recorded by [49], where freshwater fish was exposed to diethylphthalate. For determination of the physiological status of cells or tissues, measurement of liver and plasma enzyme activities can be considered as a promising diagnostic tool [44]. There are several reports on changes in immune system, cells, tissues and organs of fish for alteration of plasma enzyme activities [50,51]. According to [52], significant increase in ALP and SGPT is the indication of random pathological changes and damage to specific organs of the fish C. gariepinus. Another study of [53] shows the increase of plasma ALP, SGPT and SGOT in C. gariepinus by exposing at sublethal concentration of Diazinons. No tissue damage takes place. Decrease in transaminases, liver enzyme cannot show any tissue injury [54,55]. Low metabolism and reduction in the metabolic transport lessen the synthesis rate of glycogen, which may decrease the SGPT level [56]. Enzymological study reveals that activity of SGOT (AST) in C. mrigala is higher than other target species, but SGPT (ALT) and ALPase activities are higher in L. rohita, which indicates that whole water body is contaminated by PAHs and especially the column and bottom strata.
SGOT AST (u/l)
SGPT ALT (u/l)
ALPase (IU/l)
L. bata
L. rohita
C. mrigala
L. bata
L. rohita
C. mrigala
L. bata
L. rohita
C. mrigala
Control
159.0
±
43.486
182.7
±
35.921
172.3
±
57.873
21.9
±
1.069
22.3
±
5.508
12.3
±
2.517
71.8
±
19.937
204.0
±
66.903
149.3
±
3.512
Treatment
199.0
±
40.037
232.0
±
39.661
276.3
±
31.086
31.1
±
6.825
34.6
±
6.506
17.0
±
2.000
117.0
±
20.368
412.0
±
128.550
160.6
±
9.074
Table 3: Comparative analysis of plasma enzymes between the control and diesel-fed pond fishes.
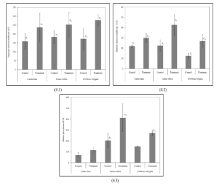
Figure 4: Liver enzyme activity of different three test fish species. Data
of aspartate aminotransferase (4.1), alanine aminotransferase (4.2) and
alkaline phosphatase (4.3) are expressed as mean±SEM (n = 12). Values
with different lowercase superscripts (alphabet) [a (L. bata), b (L. rohita) and c
(C. mrigla)] differ significantly between control and treated fishes and different
numeric superscripts indicate differ significantly at p<0.05(1) and p<0.05(2).
Conclusion
PAHs are the mixture of hundred separate chemicals with wide and varied group of compounds. This study attempted to examine the impact of PAHs on fresh water fish to reveal the toxicity level in the organism because it may be further affect on human, ultimate consumer of the food chain. The major outcome from this work was identification of different PAHs molecules from Loco tank, according to USEPA, among them 16 PAHs molecules are considered to be carcinogenic. All are found to be present in the S1 and S2 sites, the discharge points, of the diesel-fed pond in a considerable amount. High molecular weight of PAHs are less soluble in water, more stable/ persist in environment and toxic for ecosystem. Due to their effect, several histo-pathological lesions have been found on the liver of the three teleostean fishes like L. bata, L. rohita and C. mrigala. The plasma enzymes, which have been taken from the plasma, showed significant increase in activities than the control one at p<0.05 level [57-69].
References
- Rangnekar S, Malik A, Jadhav A, Parulekar T. Determination of water quality parameters after artificial idol immersion on a lake in Mumbai, India. Int J Pl. An and Env Sci. 2016; 6: 77-83.
- Haque S, Mondal S, Ghosh AR. Isolation and identification of diesel degrading microorganism from Barddhaman loco shed pond, Burdwan, West Bengal, India. The Bioscan. 2012; 7: 719-722.
- Rodrigues RV, Miranda-Filho KC, Gusmao EP, Moreira CB, Romano LA, Sampaio LA. Deleterious effects of water-soluble fraction of petroleum, diesel and gasoline on marine pejerrey Odontesthes argentinensis larvae. Sci Total Environ. 2010; 408: 2054-2059.
- Das N, Chandran P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol Res Int. 2011; 1-13.
- Almeda R, Wambaugh Z, Chai C, Wang Z, Liu Z, Buskey EJ. Effects of crude oil exposure on bioaccumulation of polycyclic aromatic hydrocarbons and survival of adult and larval stages of gelatinous zooplankton. PLoS ONE. 2013; 8: e74476.
- Kundu D, Mondal S, Dutta D, Haque S, Ghosh AR. Accumulation and contamination of lead in different trophic levels of food chain in sewage-fed East Kolkata Wetland, West Bengal, India. International Journal of Environmental and Technological Sciences. 2016; 2: 61-68.
- Carvalho CS, Bernusso VA, Araujo HSS, Espíndola ELG, Fernandes MN. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere. 2012; 89: 60-69.
- Authman MMN, Ibrahim SA, El-Kasheif MA, and Gaber HS. Heavy Metals Pollution and Their Effects on Gills and Liver of the Nile Catfish Inhabiting El-Rahawy Drain, Egypt. Glob Vet. 2013; 10: 103-115.
- Zikic RV, Stajn S, Pavlovic Z, Ognjanovic BI, Saicic ZS. Activities of superoxide dismutase and catalase in erythrocyte and plasma transaminases of gold fish (Carassius auratus gibelio Bloch.) exposed to cadmium. Physiological Research. 2001; 50: 105-111.
- Singh A, Bhat TK, Sharma OP. Clinical biochemistry of hepatotoxicity. J Clin Toxicol. 2011; S4: 1-19.
- Al-Ghanim KA. Effect of cypermethrin toxicity on enzyme activities in the freshwater fish Cyprinus carpio. Afr J Biotechnol. 2014; 13: 1169-1173.
- Baghshani H, Ghodsi V. Evaluation of some enzymatic changes in the liver and kidney of rats following exposure to sublethal concentration of potassium cyanide. Iranian Journal of Toxicology. 2016; 10: 9-12.
- Gabriel UU, Akinrotimi OA, Ariweriokuma VS. Changes in metabolic enzymes activities in selected organs and tissue of Clarias gariepinus exposed to cypermethrin. Journal of Chemical Engineering. 2012; 1: 25-30.
- Reitman S, Frankel S. Standard methods in Clinical Chemistry. Am J Clin Chem. 1957; 28: 56-59.
- Adhikari S, Chaurasia VS, Naqvi AA, Pillai B.R. Survival and growth of Macrobrachium rosenbergii (de Man) juvenile in relation to calcium hardness and bicarbonate alkalinity. Turkish J Fish Aquat Sci. 2007; 7: 23-26.
- Ara H, Rahamana MS, Islam MM, Mallick A, Hossain MS. A laboratory approach for determining effects of potential oil spillage on water quality of Sundarbans mangrove forest, Bangladesh. J Life Earth Sci. 2009; 3-4: 23-28.
- Khaiwal R, Ameena, Meenakshi, Monika, Rani, Kaushik A. Seasonal variations in physico-chemical characteristics of river Yamuna in Haryana and its ecological best designated use. J Environ Monitor. 2003; 5: 419-426.
- Guillen MD, Sopelana P, Partearroyo MA. Determination of polycyclic aromatic hydrocarbons in commercial liquid flavouring of different compositions by gas chromatography–mass spectrometry. J Agric Food Chem. 2000; 48: 126-131.
- Gehle K. Agency for Toxic Substances and Disease Registry (ATSDR) case studies in environmental medicine toxicity of polycyclic aromatic hydrocarbons (PAHs). 2009.
- Banudevi S, Krishnamoorthy G, Venkatataman P, Vignesh C, Aruldhas MM, Arunakaran J. Role of a-tocopherol on antioxidant status in liver, lung and kidney of PCB exposed male albino rats. Food ChemToxicol. 2006; 44: 2040-2046.
- Glusczak L, Miron DS, Crestani M, Fonseca MB, Pedron FA, Duarte MF, et al. Effect of glyphosate herbicide on acetylcholinesterase activity and metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol Environ Saf. 2006; 65: 237-241.
- Monteiro A, Glaser G, Stockslagger S, Glansdorp N, Ramos DM. Comparative insights into questions of lepidopteran wing pattern homology. BMC Dev Biol. 2006; 6: 52.
- Modesto KA, Martinez CBR. Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere. 2010; 78: 294-299.
- Solangi MA, Overstreet RM. Histopathological changes in two estuarine fishes, Menidia berylina (Cope) and Trinectes maculatus (Bloch and Schneider) exposed to crude oil and its water-soluble fractions. J Fish Dis. 1982; 5: 13-35.
- Kiceniuk JW, Khan RA. Toxicology of chronic crude oil exposure: Sublethal effects on aquatic organisms. Nriagu JO, editors. In: Aquatic Toxicology. John Wiley and Sons. 1983; 425-436.
- Sindermann CJ. Implication of oil production of disease in marine organisms. Phil Trans R Soc B. 1982; 297: 385-399.
- Schwaiger J, Bucher F, Ferling H, Kalbfus W, Negele RD. A prolonged toxicity study on the effects of sublethal concentrations of bis(tri-n-butyltin)oxide (TBTO): Histopathological and histochemical findings in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol. 1992; 23: 31-48.
- Giari L, Dezfuli BS, Lanzoni M, Castaldelli G. The impact of an oil spill on organs of bream Abramis brama in the Po River. Ecotoxicol Environ Saf. 2012; 77: 18-27.
- Khan RA, Barker DE, Hooper R, Lee EM, Ryan K, Nag K. Histopathology in winter flounder (Pleuronectes americanus) living adjacent to a pulp and paper mill. Arch Environ Contam Toxicol. 1994; 26: 95-102.
- Brand DG, Fink R., Bengeyfield W, Birtwell IK, McAllister CD. Salt water acclimated pink Salmon Fry (Oncorhynchus gorbuscha) develop stress-related visceral lesions after 10-day exposure to sublethal concentrations of the water soluble fraction of north slope crude oil. Toxicol Pathol. 2001; 29: 574-584.
- Sepulveda MS, Gallagher EP, Gross, TS. Physiological changes in largemouth bass exposed to paper mill effluents under laboratory and field conditions. Ecotoxicol. 2004; 13: 291-301.
- Benli ACK, Köksal G, Özkul A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere. 2008; 72: 1355-1358.
- Simonato JD, Guedes CLB, Martinez CBR. Bio-chemical, physiological, and histological changes in the neo-tropical ?sh Prochilodus lineatus exposed to diesel oil. Ecotoxicol Environ Saf. 2008; 69: 112-120.
- Khan RA, Kiceniuk JW. Effects of petroleum aromatic hydrocarbons on monogeneids parasitizing Atlantic cod, Gadusmorhua (L.). B Environ Contam Tox. 1988; 41: 94-100.
- Khan RA. Health of ?at?sh from localities in Placentia Bay, Newfoundland, contaminated with petroleum and PCBs. Arch Environ Contam Toxicol. 2003; 44: 485-492.
- Schmalz WF, Hernandez AD, Weis P. Hepatic histopathology in two populations of the mummichog Fundulus heteroclitus. Mar Environ Res. 2002; 54: 539-542.
- Norena-Barroso E, Sima-Alvarez R, Gold-Bouchot G, Zapata-Perez O. Persistent organic pollutants and histological lesions in Mayan catfish Ariopsis assimilis from the estuary of Chetumal, Mexico. Marine Poll Bull. 2004; 48: 263-269.
- Oliveira Ribeiro CA, Vollaire Y, Sanchez-Chardi A, Roche H. Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the Eel (Anguilla anguilla) at the Camargue Nature Reserve, France. AquatToxicol. 2005; 74: 53-69.
- Schlacher TA, Mondon JA, Connolly RM. Estuarine fish health assessment: Evidence of wastewater impacts based on nitrogen isotopes and histopathology. Marine Poll Bull. 2007; 54: 1762-1776.
- Katsumiti A, Valdez-Domingos FX, Azevedo M, Silva MD, Damian RC, Almeida MIM, et al. An assessment of acute biomarker responses in the demersal catfish Cathoropsspixii after the Vicuña Oil Spill in a harbour estuarine area in Southern Brazil. Environ Monit Assess. 2008; 152: 209-222.
- Silva DN, Zagatto PJP, Guardani R, Nascimento CAO. Remediação de Solos Contam-inados com Linear Alquil Benzenos Usando Reagentes de Fenton. COBEQ. 2004.
- Biuki NA, Savari A, Mortazavi MS, Hossein Z, Salamat N. Liver histopathological changes in milkfish (Chanos chanos) exposed to petroleum hydrocarbon exposure. World Appl Sci J. 2012; 18: 1315-1320.
- D’Souza R, Sinnott P, Glynn MJ, Sabin CA, Foster GR. An unusual form of autoimmune hepatitis in young Somalian men. Liver Int. 2005; 25: 325-330.
- Ezenwaji-Evelyn N, Bebe Y, Chukwuemeka N. Changes in liver and plasma enzymes of Clarias gariepinus exposed to sublethal concentration of diesel. Afr J Biotechnol. 2013; 12: 414-418.
- Azad M. Toxicity of water soluble fractions of crude oil on Meta mysidopsis insularis, an indigenous tropical mysid species. Environ Monit Assess. 2005; 104: 37-40.
- Martin-Skiltona, R, Saborido-Rey F, Portea C. Endocrine alteration and other biochemical responses in juvenile turbot exposed to the Prestige fuel oil. Sci Total Environ. 2008; 404: 68-76.
- Pollino CA, Holdway DA. Hydrocarbon induced changes to metabolic and detoxification enzymes of the Australian crimson-spotted rainbow fish (Melanotaenia fluviatilis). ?Environ Toxicol. 2003; 18: 21-28.
- Wedemeyer GA, Yasutake WT. Clinical methods of the effect of environmental stress on fish health. Aquaculture Science. 1977; 15: 47-49.
- Nivedita G, Vatsal M, Madhuri K, Pushph S, Simta K, Vaman CR. Toxicity study of diethyl phthalate on freshwater. Fish Girrihinamri gala. Ecotoxicol Environ Saf. 2002; 53: 255-258.
- Begum G. Carbofuran insecticide induced biochemical alternations in linear and muscle tissue of Clariasbotrachusand recovery respond. Aquat Toxicol. 2004; 66: 83-91.
- Gabriel UU, George A. Plasma enzymes in Clarias gariepinus exposed to chronic level of round up (glyphosate). Journal of Environment and Ecology. 2005; 23: 271-276.
- Fatma AS, Nahed SG. Environmental pollution induced biochemical changes in tissues of Tilapia zilli; Solea vulgaris and Mugil capito from lake qarum, Egypt. Glob Vet. 2000; 2: 327-336.
- Inyang IR, Daka ER, Ogemba EN. Changes in electrolyte activities Clariasgariepinus exposed to diazinon. J Trop Biol Environ Sci. 2010; 7: 198-200.
- Ayalogu OE, Igbo NM, Dede EB. Biochemical changes in the serum and liver of albino rats exposed to petroleum samples (gasoline, kerosene and crude petroleum). J ApplSci Environ Manag. 2001; 20: 97-100.
- Luskova, V, Svoboda M, Kolarava J. The effects of diazinon on blood plasma biochemistry in carp (Cyprinus carpio L.). J Equine Vet Sci. 2002; 71: 117-125.
- Kulkarni RS, Barad VS. Effect of starvation on haematological and serum biochemical changes in the fresh water fish, Notopterus Notopterus (Pallas). International Journal of Innovative Studies in Aquatic Biology and Fisheries. 2015; 1: 24-29.
- Abbas H, Ali F. Study the effect of hexavalent chromium on some biochemical, cytotoxicological and histopathological aspects of the Oreochromis spp. Fish Pak J Biol Sci. 2007; 10: 3973-3982.
- Adeyemi OT, Osilesi O, Adebawo OO, Onajobi FD, Oyedemi SO, Afolayan AJ. Alkaline Phosphatase (ALP), Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) activities in selected tissues of rats fed on processed atlantic horse mackerel (Trachurus trachurus). Adv Biosci Biotechnol. 2015; 6: 139-152.
- Akaishi FM, Silva de Assis HC, Jakobi SCG, Eiras-Stofella DR, St-Jean SD, Courtenay SC, et al. Morphological and neurotoxicological findings in tropical freshwater fish (Astyanax sp.) after waterborne and acute exposure to Water Soluble Fraction (WSF) of crude oil. Arch Environ Contam Toxicol. 2004; 46: 244-253.
- Standard methods for the examination of water and wastewater. American water works association and water pollution control federation, 21st ed. Washington, DC. APHA (American Public Health Association). 2005.
- Das B, Mukherjee S. A histopathological study of carp (Labeo rohita) exposed to hexachlorocyclohexane. Vet Arhiv. 2000; 70: 169-180.
- Elnemaki F, Abuzinadah O. Effect of contra/insect 500/50 E.C. on the histopathology of Oreochromis spilurus fish. Egyptian Journal of Aquatic Research. 2003; 29: 221-253.
- Myers IB, McCaulley MH, Quenk NL, Hammer AL. MBTI manual: A guide to the development and use of the myers-briggs type indicator. 3rd edition. Consulting Psychologists Press. 1998.
- Nour A, Amer A. Impairment of muscle performance in the Nile catfish Clarias lazera in response to hostathion insecticide contamination and/or gamma irradiation. Journal of Egyptian German Society of Zoology. 1995; 18: 153-175.
- Plohl K, Leskovsek H, Bricelj M. Biological degradation of motor oil in water. Acta Chim Slov. 2002; 49: 279-289.
- Scullion J. Remediating polluted soils. Naturwissenschaften. 2006; 93: 51-65.
- Thophon S, Kruatrachue M, Upatham ES, Pokethitiyook P, Sahaphong S, Jaritkhuan S. Histopathological alterations of white sea bass, Lates calcalifer, in acute and subchronic cadmium exposure. Environ. Pollut. 2003; 121: 307-320.
- Water: Basic information about regulated drinking water contaminants. List of all regulated contaminants. USEPA (United States Environmental Protection Agency). 2009.
- Ziolli RL, Jardim WF. Operational problems related to the preparation of the seawater soluble fraction of crude oil. Journal of Environmental Monitoring. 2002; 4: 138-141.
