Abstract
Phosphate solubilising microbial inoculants are one of the biological tools that can reduce the input of chemical phosphorus (P) fertilizers, improve P availability in soil and affect the soil quality positively. The present investigation evaluates the impact of three rates of seed based wettable formulation (2.650, 5.302 and 10.604 g kg-1 seed) of phosphate dissolving Penicillium bilaii (Jump Start) and two rates of single super phosphate (60 and 30 kg P2O5 ha-1) on biological function of field grown mustard soil. Dehydrogenase, fluorescein diacetate, cellulase, protease, acid and alkaline phosphatase enzymes involved in major nutrient transformation were measured during crop growth. Biochemical assay of the soil revealed that integrated application of 30 kg P2O5 ha-1 as chemical P and fungal formulation @2.650 g kg-1 seed, improved the dehydrogenase, protease and alkaline phosphatase activity by 22.0, 18.0 and 27.14% respectively over un-inoculated control at harvest. However, fluorescein diacetate and cellulase registered an increase of 18.91 and 29.31 % respectively with 30 kg P2O5 ha-1 as chemical P+ fungi @5.302 g kg-1 seed, compared to uninoculated counterpart, at crop maturity. Acid phosphatase activity titer in soil fertilized with 60 kg P2O5 ha-1 was at par with 30 kg P2O5 ha-1+ fungal inoculant @2.650 g kg-1 seed at 120 d sampling. Soil ergosterol recorded its peak value with 50 % chemical P+ P.bilaii @5.302 g kg-1 seed at maturity. Integrated application of seed based fungal formulation @2.650-5.302g kg-1 seed+½ the recommended chemical P can be economical and beneficial for soil biological health.
Keywords: Enzymes; Ergosterol; Microbial biomass carbon; Mustard; Penicillium bilaii
Introduction
Soil is one of the most precious natural resources that performs different ecological functions for the survival and nourishment of life on earth. To maintain its health is the moral responsibility of mankind. However, the urge for producing more food, feed and fuel is causing an irreparable damage to its environment. The excessive mineral fertilization and modern cultivation practices are adding to the deterioration of soil quality with negative environmental outcomes. Environmental and soil concern firmly emphasize the need to adopt eco-friendly agricultural practices for sustainable food production. The manipulation of soil microorganisms in the form of microbial inoculants hold good promise for reducing the input of chemical fertilizers and optimize crop productivity. A successful microbial inoculant has to invade, persist in the context of indigenous microbes and establish a compatible interaction with the host [1]. Regardless of the purpose for which beneficial microorganisms are introduced in soil, for optimum functionality of the inoculants, they need to be applied in large amount [2]. Microbial inoculants are applied (mainly in research) in the forms of liquids (as sprays, root dips, drenches) or as dry formulations. Seed based application delivers microorganisms directly to the plant rhizosphere (the narrow zone of soil that surrounds the roots) where plants interact directly with microorganisms [3]. Seed bacterization can influence, at least temporarily, the resident microbial communities. The composition of the microbial groups in soil determines the potential for enzyme synthesis and root exudates modify the actual rate of enzyme production and their activity. The type of inoculated microorganisms may result in increase, decrease or no effect on enzyme activity levels. The impact of microbial inoculation on functional capabilities of the soil microbial communities needs to be examined and the present investigation was an attempt in this direction.
Novozymes, the largest suppliers of industrial enzymes and microorganisms has developed a fungus-based P fertilizer (Jumpstart) that contains Phosphate Solubilizing Fungus (PSF) Penicillium bilaii. Actively sporulating fungi is not only a good P mobilizer but also has a broad ecological range, indicating its potential to be used as an inoculant for a range of plant production systems [4]. Application of P bilaii for improving P availability, root hair and root growth has been explored in soil under wheat, canola, and corn [5]. Seed inoculation with Penicillium sp. +100 % recommended dose of chemical P improved the P uptake by maize plants at harvest [6], besides plant biometric parameters. JumpStart® has also been evaluated for improving P availability and distribution of inorganic P in different fractions under mustard and wheat soil [7]. However, effect of P. bilaii inoculation on microbial functional diversity is lacking. Enzymes (responsible for specific nutrients transformation) reflect the changes in soil microbial diversity much faster, compared to chemical parameters and can be used as an indicator of soil biological health. Hence, the present investigation was conducted to compare the impact of mineral P fertilizer applied at 30 and 60 kg P2O5 ha-1 with integrated use of 30 kg P2O5 ha-1 + three rates of seed based fungal formulation, to select the best treatment for improving soil biological properties under mustard crop. The soil enzymatic activities, microbial biomass carbon and ergosterol content were estimated during different stages of mustard crop growth to generate variation among different P fertilization treatments.
Fungal Inoculants
Penicillium bilaii (commercial product, Jumpstart) was obtained from Novozymes Asia Pvt. Ltd. Bangalore. It had fungal spores’ population of 7.2 × 108 colony-forming unit’s g-1 (CFU g-1).
Field Experiment
Experimental details
The experiment was conducted during winter season of 2014-15 at the research farm of ICAR-Indian Agricultural Research Institute (IARI), New Delhi, India, situated at an latitude of 28° 40’ N and longitude of 77° 12’ E, altitude of 228.6 meters above mean sea level. The field soil belonged to order Inceptisol, Mahauli series with sandy loam texture, well leveled, deep percolating and well drained, hypothermic family of the Typic Ustochrept (old alluvium). The experimental field soil had a bulk density-1.49 Mg m-3, field capacity-13.9 % (w/w), wilting point- 5.7 % (w/w), water holding capacity- 39.2 %, an initial pH- 8.3, EC- 0.26 dS m-1, organic carbon- 0.38 %, alkaline KMnO4 oxidizable N- 66.7 mg kg-1 soil, 0.5 M NaHCO3 extractable P- 26.2 mg kg-1 soil and exchangeable K- 62.6 mg kg-1 soil. Zinc, copper, iron, and manganese content of soil was 30.0, 5.2, 620 and 17 ppm respectively. The mustard (Brassica campestris cv. Pusa M-30) seeds were treated with the wettable powder formulation of Penicillium bilaii at three different rates of 2.651, 5.302 and 10.604 g kg-1 seed (as per directions from Novozyme Asia Pvt. Ltd. Banglore) on 14th Nov, 2014. The fungus treated seeds (seed rate 4 kg ha-1) were spread on a sheet (in a flat layer) under shade, and allowed to dry for 2-3 hrs, prior to sowing. Experiment was carried out on 10 sub-plots of 12 m2 each, with three replicates, following randomized block design. A row width of 30 cm was observed. Different treatments included T1- recommended dose of chemical P- 60 kg P2O5 ha-1, T2- ½ the recommended dose of chemical P (30 kg P2O5 ha-1), T3- 30 kg P2O5 ha-1 + 2.651g kg-1 seed based fungal inoculant, T4- 30 kg P2O5 ha-1 +5.302g kg-1 seed based fungal inoculant, T5- 30 kg P2O5 ha-1 +10.604 g kg-1 seed based fungal inoculant. Urea and muriate of postah were used for N and K fertilization at N80 K40. However, single super phosphate was used as chemical P. The chemical fertilizers were evenly spread on the soil surface of each plot on the date of sowing. For proper seed distribution along the rows, strip paper containing inoculated and un-inoculated seeds were used. The field was irrigated as and when required with manual weeding done at regular intervals. The aim was to expose the crop to three different rates of seed inoculation with fungal based product and two rates of chemical P fertilization to generate variability in enzymatic activities of soil. The crop was harvested in the last week of March 2015.
Soil sampling
Three sub-samples of soil were taken at 60 d and 120 d of crop growth from different locations of each treatment plot, at a depth of 0-15 cm and mixed together to make a composite sample that was pooled in a sterile plastic bag and transported to the laboratory. Field moist samples were passed through a 2 mm sieve and stored in cold room for enzyme assay, microbial biomass carbon and ergosterol content.
Enzymes assay
The levels of tested enzyme activities were measured at 60 and 120 d interval. Dehydrogenase Activity (DHA) was assayed by mixing 6 g soil with tri-phenyl tetrazolium chloride, followed by incubation at 30° C for 24 h. Methanol was used as an extractant. Tri-Phenyl Formazon (TPF) liberated was assayed at 485 nm and DHA activity was expressed as μg TPF g-1d-1 [8]. The fluorescein diacetate activity (FDA) was determined by exposing 1 g fresh soil to 200 μl of FDA solution (1 mg ml-1) and phosphate potassium buffer, (60 mM at pH 7.6) for 1 h at 30° C. Enzyme assay was carried out at 490 nm and the result for FDA activity was expressed as μg fluorescein released g-1 soil h-1 [9]. The protease and cellulase (CMCase) activity in soil was assayed according to the method described by Tsuchida et al [10] and Wood and Bhat [11] respectively. Modified universal buffer of pH 6.5 and 11.0 was added to soil to determine the acid and alkaline phosphatase activity respectively [12]. The quantified activity was expressed as μg p-nitrophenol g-1soil h-1.
Microbial biomass carbon
The Microbial Biomass Carbon (MBC) of soil was determined using fumigation-extraction method [13]. Seven g moist soil (on an oven-dry basis) was split into two portions of 3.5 g each. 3.5 g was fumigated with chloroform and 3.5 g kept as non-fumigated treatment. Both fumigated and non-fumigated samples were extracted with 14 ml of 0.5 M K2SO4. Organic C in the extracts was measured using dichromate oxidation method [14].
Soil microbial biomass C = Ec/KEC
where Ec is [(organic C extracted from fumigated soil) - (organic C extracted from non-fumigated soil)] and KEC = 0.45 [15]
Estimation of ergosterol
Ergosterol content in soil was estimated by Microwave Assisted Extraction (MAE) method [16]. 250 mg of soil was placed into a 25 ml screw capped culture tube, treated with 2 ml of methanol and 0.5 ml of 2 M NaOH. Each culture tube was then placed within screw capped plastic bottle and tightly sealed. This combination was then placed at the centre of a microwave oven operating at 260° C for 18 s. After cooling for 15 min, the culture tubes were irradiated for an additional 17 s at medium power. After cooling, the tubes were removed from the plastic outer bottle. The contents were neutralized with 1 M HCl, treated with 2 ml of methanol, vortexed and then extracted with pentane (3 ca. 2 ml), all within the culture tube. The combined pentane extracts were passed through a 0.45 mm disc filter and evaporated to dryness. The residues were then made up to 200 ml in methanol. Ergosterol concentration was determined by HPLC [16].
Statistical analysis
Data were statistically analyzed with the aid of online software WASP (Web Agriculture soft package) 1.0 and Microsoft Excel (Windows 2008) using Randomized Block Design.
Results and Discussion
Soil enzyme activities have been proposed as an important index of total microbial activity and soil quality [17]. They indicate the nature of perturbations caused to ecosystem function. They reflect the changes in soil ecology, resulting from the interactions between inoculants and indigenous microbial population. Mustard grown soil receiving chemical fertilization and conjoint application of 30 kg P2O5 ha-1 as chemical P + seed based fungal formulation at three different rates registered variations in the estimated soil enzymes activities. DHA, FDA and CM Case showed an upward trend in their activity titer with crop growth, whereas protease activity declined as the crop matured.
Under field conditions, DHA activity of mustard grown soil was affected by both the fertilization and the environmental conditions. Application of P.bilaii improved the DHA activity that recorded high values at 120 d compared to 60 d crop growth. At crop maturity, DHA activity in T3 increased by 22 %, compared to T2 (un-inoculated control). However, both T3 and T5 registered an increase of 5 and 4 fold respectively at 120 d soil sampling, compared to their 60 d values (Figure 1). Microbial inoculation with Bacillus megaterium has also been reported to improve the DHA activity of soil under L.dantata [18]. Higher activity of DHA in soil can be interpreted as a greater functional diversity of the microbial community. Moreover, the improved DHA with crop growth may be explained by the favorable temperature and improved P availability that might have exerted positive effect on viable microbial population of soil. Inorganic fertilizers have been reported to either preserve or decrease several enzyme activities in the rhizosphere, compared to organic treatments that enhance most enzyme activities in both rhizosphere and bulk soil [19].
FDA hydrolysis measures the total microbial activity in soils as it is mediated simultaneously by protease, esterase and lipase and reflects the activities of these enzymes in soil. FDAse activity followed a trend similar to DHA activity (Figure 2). The activity titer recorded an upward trend towards crop maturity. The maximum FDAse activity was observed in T4. Exudation of carbonaceous and nitrogenous compounds by plant roots stimulates microbial activity and production of extracellular enzymes in the soil adjacent to rhizosphere [20]. The increased FDA hydrolysable activity could be related to the metabolic versatility of the microbial groups in agreement with the reports of Aseri and Tarafdar [21].
Protease showed a trend opposite to DHA, FDAse and cellulase activity, as the former was higher at 60 d, compared to latter that registered high activity values at 120 d sampling (Figure 1-4). Protease activity titers were statistically at par in T1, T2 and T3 treatments at 60 d sampling. As the rate of fungal based formulation increased, the protease activity decreased in the following days of crop growth. This may be caused by the fungal degradation of soluble protein to free amino acids. Microbial inoculants could cause a change in microbial community and that may result in a decrease or an increase in functional diversity [22]. The cellulase activity improved towards crop maturity and recorded its peak value in T4 registering an increase of 29.3 % (Figure 4), compared to its un-inoculated counterpart (T2). The activity can be triggered when there is C and N starvation toward crop maturity [23].
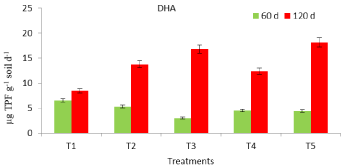
Figure 1: Dehydrogenase activity of mustard grown soil in response to
P.bilaii inoculation.
T1- 60 kg P2O5 ha-1, T2-30 kg P2O5 ha-1, T3-30 kg P2O5 ha-1+ PSF seed
inoculation @ 2.0651g kg-1 seed, T4-30 kg P2O5 ha-1+PSF seed inoculation
@5.302g kg-1, T5-30 kg P2O5 ha-1 +PSF seed inoculation @10.604g kg-1
seed.
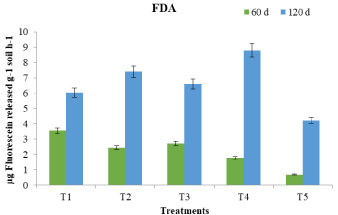
Figure 2: Effect of P.bilaii inoculation on fluorescein diacetate activity of soil
under mustard crop.
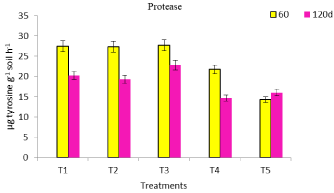
Figure 3: Protease activity of mustard grown soil in response to fungal
inoculation.
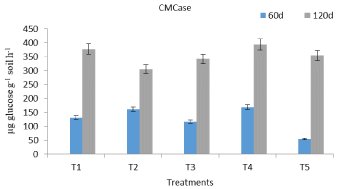
Figure 4: Effect of chemical fertilization and P.bilaii inoculation on cellulase
activity of mustard grown soil.
Acid phosphatase activity is produced by both roots and microorganisms whereas alkaline phosphatase activity is produced only by microorganisms. Acid and alkaline phosphatase activity in chemical P treated soil (T1 and T2) followed a trend different from soil that was fertilized with 50% chemical P and fungal based formulation (T3, T4, T5). The former showed a steady decline towards crop maturity, whereas the latter recorded an enhanced phosphatase activity titer with crop growth (Figure 5,6). The increased phosphatase activities in fungal formulation treated soil may either be due to stimulation of microbial activity or depletion of Pi [24]. The increased soil acid phosphatase activity due to applied soil phosphorus activator has also been reported in paddy crop at maturity stage [25]. At 120 d soil sampling, acid and alkaline phosphatase activity values did not differ greatly. Penicillium sp. has been reported to release more extracellular phytase than phosphatase but at high percentage of phytin and glycerophossphate [26]. Phosphate-solubilizing fungi are the potential tools that can support plant growth due to production of organic acids that dissolve inorganic P and mineralize organic P due to extracellular production of phytases and phosphatases in agreement with the reports of Menezes-Blackburn et al, [27]. These enzymes may also preserve their activity even if complexed with soil colloids [27].
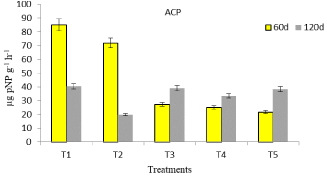
Figure 5: Acid phosphatase activity of mustard grown soil in response to
fungal inoculation.
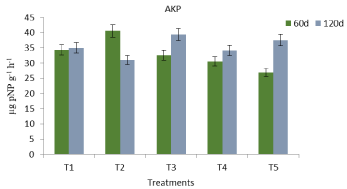
Figure 6: Alkaline phosphatase activity of mustard grown soil in response to
fungal inoculation.
Soil Microbial Biomass Carbon (MBC) is a sensitive indicator of changing soil conditions and reflects the state and change of total soil organic matter. Microbial biomass C may lead to greater nutrient mineralization and results in an increased availability of N and P. A significant increase in MBC with inorganic P fertilizer was observed, compared to fungal inoculated treatments (Figure 7). The high MBC in T1 and T2 may be attributed to easy availability of mineralizable C, N and P. Effects of P. bilaii on MBC was less pronounced in T3, T4 and T5. Fungi with thick cell walls might be less affected by CHCl3 fumigation [28], leading to under estimation of MBC. The results were in agreement to the findings of Cavagnaro et al. [29], who observed no significant change in MBC under tomato crop in relation to plant genotype and fertilization practices under on-farm organic management. The shift in soil pH induced by the application of N and P fertilizer must be a decisive factor in determining microbial abundance and community structure. In addition to soil pH, soil mineral N and P availability also changes microbial community structure [30]. Fungi introduced in soil may affect the soil microorganisms associated with their mycelium, leading to the formation of a specific zone of soil called the myco-rhizosphere, where fungi may exert negative [31], positive, or no effect on MBC [32].
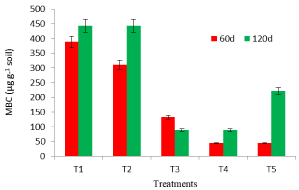
Figure 7: Microbial biomass carbon of mustard grown soil in response to
P.bilaii inoculation.
Ergosterol is a sterol common to many fungi and is used as an indicator of fungal biomass. A direct relation between MBC and ergosterol content was observed. In the present study, soil ergosterol content also recorded much higher values under chemical P fertilization, compared to integrated application of fungal inoculants and chemical P at 60 d interval. However, the trend was vice versa at 120 d soil sampling (Table 1). All living fungal species (both native and introduced) or strains do not grow in a synchronized manner in soils. Thus, their lag, exponential and stationary growth phases occur at different rates, and periods that cannot be differentiated with the methods used to draw and handle the soil samples. Therefore, ergosterol concentration varied among different fertilized treatments.
Treatments
Ergosterol (μg g-1)
Fungal biomass (μg g-1)
60 d
120 d
60 d
120 d
T1
10.9
3.94
4387
1585
T2
12.84
3.98
5168
1601
T3
3.71
4.68
1493
1883
T4
5.25
5.38
2113
2165
T5
7.46
4.38
3002
1762
Fungal biomass (μg g-1) = Ergosterol × f × Rf
where f = 250 (1/4 1000, mg biomass μg g-1 ergosterol), and Rf = 1.61 (correction factor for average percent recovery, 1/0.62).
T1- 60 kg P2O5 ha-1, T2- 30 kg P2O5 ha-1, T3- 30 kg P2O5 ha-1+ PSF seed inoculation @ 2.0651g kg-1 seed, T4- 30 kg P2O5 ha-1+PSF seed inoculation @5.302g kg-1, T5- 30 kg P2O5 ha-1 + PSF seed inoculation @10.604g kg-1 seed
Table 1: Ergosterol and fungal biomass content of mustard soil in response to chemical fertilization and P.bilaii inoculation.
Conclusion
The response of introduced microorganisms on ecosystem processes (regulated by soil biota) is important to understand the inoculation effect on microbial functional diversity. Specific enzyme activity is a more accurate measure for comparing the extent to which different fertilization amendments affect the functioning of the soil microbial community [33]. Integrated application of ½ the recommended mineral P + P. bilaii inoculation affected the DHA, FDAse, protease and cellulase activities positively in mustard grown soil. These activities increased to a level better than mineral P fertilizer treatments. The application of phosphate dissolving fungi increased the soil acid phosphatase activity in soil over the growing season, but only at the maturing stage. The application rate of wettable fungal formulation was found to be variable for specific enzyme activity. Highest dose of 10.604 g kg-1 seed did not result in proportionate increase in tested enzyme activities. MBC and ergosterol content of soil under different treatments were highest in soil fertilized with mineral P and showed a good relation between themselves. However, with crop growth, the biomass carbon values declined in mineral P treated soil, compared to fungal inoculated treatments. Thus, seed based inoculation of P. bilaii @ 2.604 -5.320 g kg-1 seed was good enough to improve the soil biological health. Being economical and ecologically safe, this fungal inoculant can protect the soil from ill effects of overuse of chemical P fertilizer.
References
- Finkel OM, Castrillo G, Herrera Paredes S, Salas González I, Dangl JL. Understanding and exploiting plant beneficial microbes. Curr. Opinion Pl. Biol. 2017; 38: 155-163.
- Callaghen OM. Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl Microbiol Biotechnol. 2016; 100: 5729-5746.
- Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013; 11: 789-799.
- Harvey PR, Warren RA, Wakelin S. Potential to improve root access to phosphorus: the role of non-symbiotic microbial inoculants in the rhizosphere. Crop Pasture Sci. 2009; 60: 144-151.
- Gulden Robert, Vessey H, Kevin J. Penicillium bilaii inoculation increases root-hair production in pea. Canadian J Pl. Sci. 2000; 80: 801-804.
- Patil MP, Kuligod VB, Hebsur NS, Patil CR, Kulkarni G N. Effect of phosphate solubilizing fungi and phosphorus levels on growth, yield and nutrient content in maize (Zea mays). Ag. Sci. 2012; 25: 58-62.
- Singh YV, Gaind Sunita. Inorganic phosphorus fractions and crop productivity in response to different rates of phosphate-solubilising fungi Penicillium bilaii. Indian J Ag Sci. 2019; 89: 238-245.
- Casida, LE, Klein DA. Jr, Santaro T. Soil dehydrogenase activity. Soil Sci. 1964; 98: 372-376.
- Schruner J, Rosewall T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol. 1982; 43: 1256-1261.
- Tsuchida O, Yamagata Y, Ishizuka T, Arai T, Yamada J, Takeuchi M, et al. An alkaline protease of an alkalophilic Bacillus sp. Curr Microbiol. 1986; 14: 7-12.
- Wood TM, Bhat KM. Methods for measuring cellulase activities. Methods Enzymol. 1988; 160: 87-112.
- Tabatabai MA, Bremner JM. Use of p-nitrophenylphosphate in assay of soil phosphatase activity. Soil Biol Biochem. 1969; 1: 301-307.
- Vance ED, Brookes PC, Jenkinson D. An extraction method for measuring microbial biomass carbon. Soil Biol. Biochem.1987; 19: 703-707.
- Walkley A, Black IA. An examination of Degtareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934; 27: 29-38.
- Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC. Measurement of soil microbial biomass C by fumigation-extraction: an automated procedure. Soil Biol Biochem. 1990; 22: 1167-1169.
- Montgomery HJ, Monreal CM, Young JC, Seifert KA. Determination of soil fungal biomass from soil ergosterol analyses. Soil Biol. Biochem. 2000; 32:1207-1217.
- Trasar-Cepeda C, Leiros C, Gi-Sotres F, Seoane S. Towards a biochemical quality index for: An expression relating several biological and biochemical properties. Biol.Fertil. Soils. 1998; 26: 100-106.
- Carmen Mengual,*, Mauricio Schoebitz, Rosario Azcón, Antonio Roldán aMicrobial inoculants and organic amendment improves plant establishment and soil rehabilitation under semiarid conditions J. Environ Management. 2014; 134:1-7.
- Ai C, Liang G, Sun J, Wang X, Zhou W. Response of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma. 2012; 173– 174: 330-333.
- Brzostek ER, Greco A, Drake JE, Finzi AC. Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry. 2013; 115: 65–76.
- Aseri GK, Tarafdar JC. Fluorescein Diacetate: A Potential Biological Indicator for Arid Soils. Arid Land Res.Management. 2006; 20:1–13.
- Trabelsi Darine, Mhamdi Ridha Microbial inoculants and their impact on soil microbial communities: a review. Biomed Res Int. 2013; doi: 10.1155/2013/863240
- Gaind Sunita, Pandey AK, Lata. Microbial biomass, P nutrition and enzyme activities of wheat soil in response to phosphorus enriched organic and inorganic manures. J Environmental Science and Health Part B Pesticides, Food Contaminants and Agricultural Wastes. 2006; 41B (2), 177-187.
- George TS, Gregory PJ, Wood M, Read D, Buresh RJ. Phosphatase activity and organic acids in the rhizosphere of potential agro-forestry species and maize. Soil Biol. Biochem. 2002; 34: 1487–1494.
- LiuXiao-jie, DuanXue-jiao, MaNa, SunTao, XuJing-gang. Impact of microbial Inoculants on microbial quantity, enzyme activity and available nutrient content in paddy soil. J. Northeast Ag. Univ. 2015; 22: 7-14.
- Yadav BK, Tarafdar JC. Phytase and phosphatase producing fungi in arid and semi-arid soils and their efficiency in hydrolyzing different organic P compounds. Soil Biol. Biochem. 2003; 35: 745-751.
- Menezes-Blackburn D, Jorquera MA, Greiner R, Gianfreda L, Mora ML. Phytases and phytase-labile organic phosphorus in manures and soils. Crit. Rev. Environ. Sci. Technol. 2013 ; 43: 916-954.
- Ingham ER, Griffiths RP, Cromack K, Entry JA. Comparison of direct vs. fumigation incubation microbial biomass estimates from ecto-mycorrhizal mat and non-mat soils. Soil Biol. Biochem. 1991; 23: 465-472.
- Cavagnaro TR, Jackson LE, Six J, Ferris H, Goyal S, et al. Arbuscular mycorrhizas, microbial communities, nutrient availability, and soil aggregates in organic tomato production. Plant Soil. 2006; 282: 209–225.
- Qing Wang, Cong Wang, Wei Wei Yu, Ali Turak Diwen Chen, Ying Huang, Junhua Ao,* Yong Jiang, Zhengrui Huang. Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity, and community composition in Chinese fir plantations. Front. Microbiol. 2018; 9:1543
- Linderman RG. Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizosphere effect. Phytopathology. 1988; 78: 366–371.
- Albertsen A, Ravnskov S, Green H, Jensen DF, Larsen J. Interactions between the external mycelium of the mycorrhizal fungus Glomus intraradices and other soil microorganisms as affected by organic matter. Soil Biol. Biochem. 2006; 38(5): 1008–1014.
- Olsson PA, Bååth E, Jakobsen I, Söderström B. Soil bacteria respond to presence of roots but not to mycelium of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 1996; 28 (4-5):463–470.
