Abstract
Particulate matter and ozone, which affect human and environmental health, are the atmospheric pollutants most frequently exceeding the national standards in Chilean cities. The objective of this work was to assess the effect of both pollutants on the native urban tree Quillaja saponaria Molina leaves. Batches of ten individuals of Q. saponaria were located close to nine official pollution monitoring stations, belonging to the Air Quality Monitoring Network of the Metropolitan Region of Santiago, Chile. Morpho-anatomical traits of Q. saponaria leaves were analyzed after twelve months of exposure to the environmental condition. There is a significant positive relationship between leaf area and particulate matter, while stomata width and palisade thickness decreased. Leaf traits were independent of ozone concentration. Therefore, particulate matter changed some morpho-anatomical traits, and affects the physiological functioning of the tree. These findings are important to take better decisions for urban planning and to improve the air quality of cities.
Keywords: Particulate matter; Ozone; Morpho-anatomical leaf traits; Quillaja saponaria; Santiago chile
Introduction
One of the main problems affecting quality of life in urban cities is anthropogenic atmospheric pollution produced by atmospheric aerosols and particulate matter (i.e. mainly particulate matter of ≤2.5 and ≤10 μm in aerodynamic diameter, namely PM2.5 and PM10, respectively), inorganic and organic gaseous compounds. There is increasing awareness and concern about the negative effects of pollution on human health, ecosystems, visibility, infrastructure, as well as economic and social welfare. A direct relationship has been established by WHO between exposure to high concentrations of coarse and fine particles and the levels of mortality and morbidity of the population [1].
In several cities worldwide, the use of urban vegetation (trees, shrubs and grasses) has been integrated as part of programs, policies and measures for the reduction of pollutant concentration and for human health protection [2,3,4]. This reduction can occur directly by deposition on the tree surface and/or by stomatal uptake of atmospheric pollutants [5]. Plants exhibit a large foliage area per unit volume, increasing the probability of interception and deposition that varies substantially depending on particle size and leaf roughness [6]. Particle deposition can also be increased by the presence of epicuticular waxes in which become stuck or immersed [7,8].
Trees can grow in places with high levels of pollutant concentration and accumulate organic and inorganic chemical species in their cells. The response of each plant species to a particular type of pollutant is different depending on a set of environmental conditions [9,10]. Leaf functional traits are reliable markers that show significant variations between plants, as well as among different biotic and abiotic stressors [11].
Particulate matter contains solid and liquid particles of different origins, sizes, shapes and chemical composition that can cause diverse effects on plants and ecosystems [12]. The presence of PM on leaves has an adverse effect on plants, mainly by limiting the amount of light reaching the mesophyll, which is reflected, reducing the amount of absorbed photosynthetic active radiation [13,14]. The biological effects of PM deposited on the leaves may increase acidity, salinity, nutrient content, trace metal content and change surfactant properties of leaves [12].
Tropospheric ozone can also cause adverse effects on vegetation. The reactions of ozone and the internal components of leaves can lead to the formation of reactive oxygen species leading to oxidative stress and damage to plants [15]. As a strong oxidant, ozone causes several types of visible lesions including chlorosis and necrosis [16,17], affects the metabolic processes of the plant that leads to reduction of carbon assimilation [18], growth [19], foliar area [20] and stomatal control [21,22]. In addition, ozone pollution can induce programmed cell death, accelerate senescence and weaken the defense against pests and diseases [23-25].
Santiago (33 ° 26’16 “S 70 ° 39’01” W) is the capital city of Chile located in the Maipo river basin, in the central valley (400 to 1200 m asl), between the Andes to the east, and the Coastal mountains to the west. The population of Santiago is about 40.5% (7,112,808 inhabitants) of the country’s population [26]. Santiago shows high concentrations of PM and O3 which generates effects on the socioeconomic and environmental ecosystems. High PM concentration levels usually occur in the autumn-winter months mediated by poor ventilation conditions, sometimes producing critical episodes (concentrations 2-3 times over national quality standard). Anthropogenic emissions (volatile organic compounds and nitrogen oxides in the basin) generate ozone in the summer months [27]. In addition, population growth and urban expansion have replaced agricultural lands and natural habitats [28] negatively affecting the air quality, decreasing vegetation cover and increasing temperatures [29].
Models predict that urban vegetation, mainly trees, can reduce PM and some gases [30-32]. Experimental research shows that PM retention is species specific [33-35], having a beneficial effect on the population. However, the effect on morpho-anatomical traits of air pollution on plants has been less studied.
The aim of this work is to assess the effect of PM and ozone on morpho-anatomical traits of the native species Quillaja saponaria Molina. Hernández and Villaseñor [36] report that this a native evergreen tree with an abundance of 3.48% in the native urban green infrastructure of Santiago city (13.52%).
Materials and Method
Plant material
Pots of ten two-years-old plants (around 1.5 m tall) of Q. saponaria (QS) from the nursery of the Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Universidad de Chile, were located in November-December 2014 near (2-700 m) nine official Monitoring Stations (MS) within network for the Metropolitan Region of Santiago, Chile. Plants for this experiment originate from seeds of a single population and genetic background. For each station, random plants were selected that had the same initial size. The MS included in this study were (Table 1): Cerrillos (MS-C), Cerro Navia (MS-CN), El Bosque (MS-EB), Independencia (MS-I), Las Condes (MS-LC), La Florida (MS-LF), Puente Alto (MS-PA), Parque O’Higgins (MS-PO) and Quilicura (MS-Q), located between 481 masl (west) and 785 masl (east) [37]. From each individual plant, ten fully expanded leaves from the upper third of the upper canopy were harvested between November 2014 and January 2016. All plants were exposed to the environmental conditions of the corresponding monitoring station (temperature, humidity, radiation) and regular irrigation.
Monitoring station
MS-Q
MS-C
MS-CN
MS-EB
MS-PO
MS-I
MS-LC
MS-PA
MS-LF
Latitude (º)
-33.365804
-33.49289
-33.433109
-33.547125
-33.464154
-33.422224
-33.376764
-33.591352
-33.516618
Longitude (º)
-70.748223
-70.719399
-70.732085
-70.666158
-70.660778
-70.651177
-70.523249
-70.594763
-70.588072
Pots distance to MS (m)
93
55
10
66
702
48
4
2
6
N° of exposition days
416
386
415
384
415
415
415
389
393
Mean spring-summer RH
52.3±18.3
59.9±20.9
53.1±19
53.6±18.7
53.7±19.3
54.2±20.1
54.3±20.3
55.2±17.9
51.7±18.3
Mean autumn-winter RH
61.6±19.3
65.7±21
61.4±19.3
58.9±19.5
61±19.8
58.3±21
54.4±22.2
57.5±19.6
58.3±19.4
Mean spring-summer T(°C)
19.8±6.56
20.2±5.9
19.1±6.6
18.8±6.72
19.1±6.72
21±6.14
19.5±5.93
18±6.31
21.9±6.4
Mean autumn-winter T(°C)
13±6.4
15±4.98
12.6±6.19
12.5±6.26
12.6±6.26
14.9±5.4
15±5.71
12.3±5.81
11.7±6.42
MS-C: Cerrillos; MS-CN: Cerro Navia; MS-EB: El Bosque; MS-I Independencia; MS-LC: Las Condes; MS-LF: La Florida; MS-PA: Puente Alto; MS-PO: Parque O’Higgins and MS-Q: Quilicura. Source: SINCA 2018.
Table 1: Location of the air quality monitoring station where batches of 10 plants of Quillaja saponaria were grown from September 2014 to July 2016. Period of exposition at each monitoring station. Mean values and standard deviation for air relative humidity RH (%) and air temperature T (°C) from the official monitoring stations for the period between November 2014 and January 2016.
Morpho-anatomical traits
The Leaf Area (LA) and the Leaf Perimeter (LP) from each foliage sample were measured using scanned images (HP Scanjet 3670) and processed using IMAGEJ (National Institute of Health, USA). For epidermal traits, three leaves from each individual were selected at each MS. To observe the adaxial and abaxial epidermis, the central third of each selected leaf was diaphanized using a modification of the protocol proposed by Dizeo de Strittmatter [38], replacing the use of alcohol with sodium hypochlorite for greater separation of the epidermis. The morpho-anatomical variables studied were: Adaxial Stomata Length (L-ADA), Abaxial Stomata Length (L-ABA), Adaxial Stomata Width (W-ADA), Abaxial Stomata Width (W-ABA), Adaxial Stomatal Density (D-ADA) and Abaxial Stomatal Density (D-ABA). Twenty epidermis samples for each MS on the diaphanized preparations were photographed with a Carl Zeiss Axiostar light microscope with an attached Canon digital camera. The length and width of the stomata were observed with magnification 40X and measured using the IMAGEJ program. The stomatal density was measured as the number of stomata within 1 mm2 using IMAGEJ on images with magnification 10X from each epidermis sample according to the protocol proposed by Dunlap and Stettler [39].
The thickness of the epidermal tissue and the mesophyll components were determined on one leaf from each of four individual QS from each station. The central third of each leaf was analyzed and stored in 70% ethanol to obtain permanent histological sections of 15 μm thickness with a rotating Wetzlar microtome using the method proposed by Johansen [40]. The morpho-anatomical variables studied were: Leaf Thickness (LT), Epidermis Thickness (ET), Mesophyll Thickness (MT), Parenchymal Palisade Thickness (PPT) and Spongy Parenchymal Thickness (SPT). Cross sections obtained from five samples of each MS were observed and photographed in a Carl Zeiss Axiostar light microscope coupled with a Canon digital camera using 10X lens. To measure tissue thickness, the IMAGEJ was used.
Concentrations of pollutants and environmental variables values
The official validated records of concentrations (PM2.5, PM10 and Ozone), Relative Humidity and Temperature reported by Sistema de Information Nacional de Calidad de Aire, SINCA [41] were used. The values of all variables correspond to the period between November 2014 and January 2016 and were downloaded from each of the nine monitoring stations included in the study. On the other hand, two seasons (spring-summer and autumn-winter) were considered to calculate the number of days that exceeded the daily national quality standard of PM2.5 (50 μg/m3, 24h) and PM10 (150 μg/m3, 24h).
Statistical analysis
Variables were tested for normality and homogeneity of variance, and transformations were made as necessary to meet the underlying statistical assumptions of the models used. A one-way ANOVA was applied to analyze the differences between the monitoring stations, which, when significant, was followed by a multiple comparisons Tukey Test in order to identify specific differences among pairs of stations. The Pearson correlation coefficient was used to assess the strength and direction of relationships/ linear regression between the morpho-anatomical variables and pollutant concentrations for spring-summer and autumn-winter periods. The analyses were performed using R [42].
Results
Particulate material and ozone concentrations across monitoring stations
Table 1 shows the mean values and standard deviation for relative humidity (%) and temperature (°C) data registered by the official monitoring stations belonging to SINCA for the spring-summer and the autumn-winter seasons. Figure 1 shows total concentrations of PM2.5, PM10 and O3 and seasonal concentration values (summerspring and autumn-winter) obtained from the official monitoring stations. Also the number of days exceeding the daily standards of PM2.5 and PM10 for the studied period are indicated. Note that the highest number of critical episodes occur during the studied period. The Ministry of Environment reported 48, 51 and 42 critical episodes for PM2.5, and 12, 27 and 2 for PM10, for the years 2014, 2015 and 2016 [43]. MS-Q and MS-CN, located in the west and north-west sector of the Santiago valley, reported highest values PM2.5 and PM10 concentration in autumn-winter months, respectively. On the other hand, MS-PA even MS-LC, located in the east sector of the valley, exhibited the highest concentrations of ozone in the springsummer months.
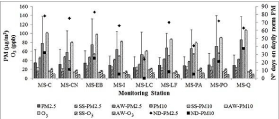
Figure 1: Mean values and standard deviation for total concentrations of
PM2.5, PM10 and O3 and for the Summer-Spring (SS) and Autumn-Winter
(AW) season obtained from the official monitoring stations for the period
between November 2014 and January 2016. The number of days over the
daily standard of PM2.5 (♦) and PM10 (■) are also indicated. Monitoring
stations: MS-C: Cerrillos; MS-CN: Cerro Navia; MS-EB: El Bosque; MS-I
Independencia; MS-LC: Las Condes; MS-LF: La Florida; MS-PA: Puente
Alto; MS-PO: Parque O’Higgins and MS-Q: Quilicura.
Observed differences in morpho-anatomical variables across monitoring stations
Morpho-anatomical traits ranged from 317.7 to 819.1 mm2 for leaf area; 5.58 to 12.35 μm for adaxial and abaxial stomata widths and 8.84 and 28.03 μm for epidermis thickness. Significant differences (p‹0.05) were observed in the morpho-anatomical variables of leaves of QS among different MS. Foliar perimeter significantly (F = 2.446, p‹0.05) differed between MS-EB (largest) compared to MS-I (smallest), with all other MS data falling between these two extremes (Figure 2). For stomatal characteristics, the L-ADA was largest in MSPA and smallest in MS-EB (Figure 3a), while L-ABA was largest in MS-I and smallest in MS-EB and MS-CN (F = 2.502, p‹0.05) (Figure 3b). D-ADA was largest in MS-LF and smallest in MS-C, MS-EB and MS-PO (F = 10.64, p‹0.001) (Figure 3c). D-ABA was largest in MS-LF and smallest in MS-CN, MS-I, and MS-Q (F = 2.567, p‹0.05) (Figure 3d).
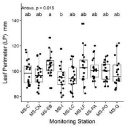
Figure 2: Box plots of Leaf Perimeter (LP) values for Q. saponaria. Different
letters (a, b) indicate significant differences (p‹0.05) between monitoring
stations (MS). MS-C: Cerrillos; MS-CN: Cerro Navia; MS-EB: El Bosque;
MS-I Independencia; MS-LC: Las Condes; MS-LF: La Florida; MS-PA:
Puente Alto; MS-PO: Parque O’Higgins and MS-Q: Quilicura.
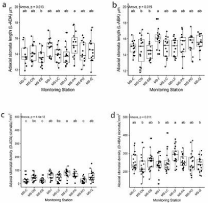
Figure 3: Box plots of stomata traits values for Q. saponaria. a) adaxial
(above) stomata length (L-ADA). b) abaxial (below) stomata length (L-ABA).
c) adaxial stomatal density (D-ADA), and d) abaxial stomatal density (D-ABA).
Different letters (a, b, c) indicate significant differences (p‹0.05) between
Monitoring Stations (MS). MS-C: Cerrillos; MS-CN: Cerro Navia; MS-EB: El
Bosque; MS-I Independencia; MS-LC: Las Condes; MS-LF: La Florida; MSPA:
Puente Alto; MS-PO: Parque O’Higgins and MS-Q: Quilicura.
Figure 4 shows thickness of leaves, mesophyll, palisade and spongy parenchyma from samples across MS. Significant differences were observed in the leaf thickness (F = 4.134, p‹0.01) (Figure 4a), in the thickness of the mesophyll (F = 4.2005, p‹0.01) (Figure 4b), and in the thickness of the palisade parenchyma (F = 3.361, p‹0.01) (Figure 4c). MS-PA and MS-LF show the highest thickness of the palisade parenchyma and MS-C and MS-PO as the smallest ones. The spongy parenchyma thicknesses significantly differ across the MS (F = 3.518, p‹0.01) being greatest in MS-PA and smallest in MS-C and MS-PO with all other MS situated between these two extremes (Figure 4d). Other leaf traits did not show significant differences among MS.
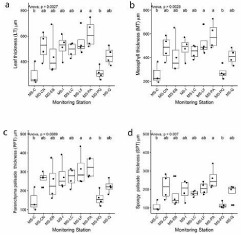
Figure 4: Box plots of thickness traits values for Q. saponaria. a) Leaf
Thickness (LT), b) Mesophyll Thickness (MT), c) Parenchyma Palisade
Thickness (PPT), and d) Spongy Parenchymal Thickness (SPT). Different
letter (a, b) indicate significant differences (p‹0.05) between Monitoring
Stations (MS). MS-C: Cerrillos; MS-CN: Cerro Navia; MS-EB: El Bosque;
MS-I Independencia; MS-LC: Las Condes; MS-LF: La Florida; MS-PA:
Puente Alto; MS-PO: Parque O’Higgins and MS-Q: Quilicura.
Association between morpho-anatomical variables and PM and ozone pollution
Statistically significant linear associations were found between morpho-anatomical variables: leaf area, stomatal width and palisade parenchyma thickness of QS leaves, and PM10 and PM2.5 (Figure 5). Ozone concentration did not correlate to any morpho-anatomical variable. Figure 5a shows a positive relationship between leaf area and the number of days exceeding the daily standard of PM10 (r = 0.758, p‹0.05); on the other hand, Figure 5b shows a negative relationship between stomata width and PM2.5 values in the autumn-winter months (r = -0.706, p‹0.05). Similarly, Figure 5c shows a negative relationship between the palisade parenchyma thickness and the number of days exceeding the national daily standard for PM10 (r = -0.730, p‹0.05). The Pearson correlation analysis indicates that palisade parenchyma thickness also correlates negatively with mean value of relative humidity during autumn-winter (r = -0.732, p‹0.05), while other variables did not present significant correlations. The number of days exceeding the daily standard of PM10, Yáñez et al. [44] showed that variables derived from relative humidity contributed differently to the models (Generalized Additive Models, GAMs), having a higher effect on PM coarse (PM10-2.5) than on PM2.5, and exhibiting both negative and positive relationships.
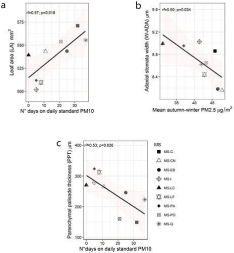
Figure 5: Linear relationships between morpho-anatomical traits of Q.
saponaria and particulate matter across monitoring stations, a) Relationship
between leaf area and the number of days exceeding the national standard
for PM10; b) Relationship between adaxial stomata width and mean autumnwinter
PM2.5; c) Relationship between parenchymal palisade thickness and
the number of days exceeding the national standard for PM10.
Monitoring stations (MS): MS-C: Cerrillos; MS-CN: Cerro Navia; MS-EB: El
Bosque; MS-I Independencia; MS-LC: Las Condes; MS-LF: La Florida; MSPA:
Puente Alto; MS-PO: Parque O’Higgins and MS-Q: Quilicura.
Discussion
The significant relationship between PM and morpho-anatomical traits of QS leaves found in this study is consistent with reports for several tree species [45-54, 10]. There is scarce information for this native species. No relationship was observed between ozone concentrations and the morpho-anatomical traits in this tree, as shown for other species at physiological and biochemical levels [55- 57].
The observed pattern shows negative relationship between the higher concentrations of PM2.5 and the stomata width; the reason for this may that smaller stomata sizes contribute to better control of absorption of contaminants, however this can also increase obstruction and reduce photosynthesis, as reported by Pourkhabbaz et al. [46] and eventually growth [58], as PM can obstruct leaf stomata, affecting gas exchange and altering the physiological activity of the plant. The PM deposited on the leaf surface [59] could lead to stomata closure or affect interception of PAR radiation by the leaf, therefore reducing photosynthetic rates. Particles can also be located in the openings of the stomata, as reported for Q. saponaria, Schinus molle L., Olea euroapaea L. and Melia azedarach L., urban trees in Santiago [59], where they reduce the intensity of transpiration, negatively affecting the heat tolerance of the plant [60, 61].
The important concentrations of PM associated to the MS-C respond to the lowest values of palisade parenchyma thickness and the largest leaf area. Thicker leaves have a higher cellular chlorophyll density and higher photosynthesis capacity per area unit than thinner leaves. Thin, but larger leaves (as seen in other species), would allow a higher interception of light [62]. The individual plants of MS-C and others growing in places with similar characteristics may present similar changes to those observed in the species studied, that is, a greater capture of light. Singh et al. [54] report the influence of polluted environments over leaf thickness as an important factor controlling stomatal function, reflecting the adaptive plasticity of plants to cope with severe environmental conditions.
According to indications by Singh et al. [54], changes at physiological, morphological and biochemical levels induce structural and functional changes in the plant. In this study, changes in the morpho-anatomical traits observed in QS may result in relevant changes for tree growth and survival. Leaf anatomical traits may mediate the photosynthetic performance of Q. saponaria (with greater photosynthetic capacity than Cryptocarya alba (Mol.) Looser and Lithraea caustica (Mol.) Hool. et Arn, both native and evergreen species), which is related to mesophyll conductance limitations, and therefore PM could also affect this photosynthetic variable [62]. Among native trees, other species with similar requirements could be subjects of study to promote an increase of their use in the public green infrastructure of the cities of Chile with Mediterranean climate [64].
The response of leaf characteristics to environmental factors is species-specific and related to the protective or adaptive mechanisms of plants [65]. Analysis of plant traits can help in the selection of tree species to enhance the resilience of urban forests [66]. In addition, more complex relationships may become apparent when the pollution variables are mixed, showing an interaction of synergistic or antagonistic effects between them. For better air quality, the health of trees and the species used in urban trees should be considered.
Conclusion
A positive relationship between leaf area and number of days exceeding the national daily standard for PM10 was found; also a negative relationship was observed between stomata width and the mean PM2.5 in the autumn-winter season and the palisade thickness and the number of days exceeding the national daily standard for PM10. In this study period many days above national dayly standards and several critical episodes are included. Therefore, particulate matter induced specific morpho-anatomical trait changes of Q. saponaria that affect structural leaf properties and could generate modifications in the normal biological functions and the ecosystemic services of the tree. The foliar perimeter, stomata length and stomata density of adaxial and abaxial epidermis, thickness of leaves, mesophyll, palisade parenchyma and spongy parenchyma from individuals of Q. saponaria presented differences, under the same irrigation conditions, between the monitoring stations. This finding indicates an effect of the local urban environmental growing condition on these morpho-anatomical traits. This information should help taking better decisions for urban planning and to improve air quality of cities.
Acknowledgements
Authors acknowledge project REDES-Conicyt 170074 for financial support. MP acknowledges D. Seelenfreund for critical review of the manuscript.
References
- WHO. World Health Organization. Ambient (outdoor) air quality and health. 2019.
- Escobedo FJ, Kroeger T, Wagner JE. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environmental pollution. 2011; 159: 2078-2087.
- Nowak DJ, Poudyal NC, McNulty SG. Forest Ecosystem Services: Carbon and Air Quality. 2017.
- Sills EO, Moore SE, Cubbage FW, McCarter KD, Holmes TP, Mercer DE. Trees at work: economic accounting for forest ecosystem services in the U.S. South. Asheville, USA: US Department of Agriculture Forest Service, Southern Research Station. 2017; 49-63.
- Niinemets Ü, Fares S, Harley P, Jardine KJ. Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition. Plant, Cell & Environment. 2014; 37: 1790-1809.
- Janhäll S. Review on urban vegetation and particle air pollution – Deposition and dispersion. Atmospheric Environment. 2015; 105: 130-137.
- Dzierzanowski K, Popek R, Gawronska H, Sæbø A, Gawronski SW. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. International Journal of Phytoremediation. 2011; 13: 1037–1046.
- Leonard RJ, McArthur C, Hochuli DF. Particulate matter deposition on roadside plants and the importance of leaf trait combinations. Urban Forestry & Urban Greening. 2016; 20: 249–253.
- Calfapietra C, Peñuelas J, Niinemets Ü. Urban plant physiology: adaptationmitigation strategies under permanent stress. Trends in Plant Science. 2015: 20: 72-75.
- Khalid N, Noman A, Sanaullah T, Akram MA, Aqeel M. Vehicle pollution toxicity induced changes in physiology, defence system and biochemical characteristics of Calotropis procera L. Chemistry and Ecology. 2018; 34: 565-581.
- Mukherjee A, Agrawal M. Use of GLM approach to assess the responses of tropical trees to urban air pollution in relation to leaf functional traits and tree characteristics. Ecotoxicology and environmental safety. 2018; 152: 42-54.
- Grantz DA, Garner JHB, Johnson DW. Ecological effects of particulate matter. Environment International. 2003; 29: 213-239.
- Przybysz A, Popek R, Gawronska H, Grab K, Loskot K, Wrochna M, et al. Efficiency of photosynthetic apparatus of plants grown in sites differing in level of particulate matter. Acta Scientiarum Polonorum Hortorum Cultus. 2014; 13: 17-30.
- Popek R, Lukowski A, Karolewski P. Particulate matter accumulation—further differences between native Prunus padus and nonnative Prunus serotina. Dendrobiology. 2017; 78: 85-95.
- Dizengremel P, Vaultier M.N, Le Thiec D, Cabané M, Bagard M, Gérant D, et al. Phosphoenolpyruvate is at the crossroads of leaf metabolic responses to ozone stress. New Phytologist. 2012; 195: 512-517.
- Mills G, Pleijel H, Braun S, Büker P, Bermejo V, Calvo E, et al. New stomatal flux-based critical levels for ozone effects on vegetation. Atmospheric Environment. 2011; 45: 5064-5068.
- Feng Z, Sun J, Wan W, Hu E, Calatayud V. Evidence of wide-spread ozoneinduced visible injury on plants in Beijing, China. Environmental Pollution. 2014; 193: 296-301.
- Fares S, Vargas R, Detto M, Goldstein AH, Karlik J, Paoletti E, et al. Tropospheric ozone reduces carbon assimilation in trees: estimates from analysis of continuous flux measurements. Global Change Biology. 2013; 19: 2427-2443.
- Hoshika Y, Watanabe M, Inada N, Mao Q, Koike T. Photosynthetic response of early and late leaves of white birch (Betula platyphylla var. japonica) grown under free-air ozone exposure. Environmental Pollution. 2013; 182: 242-247.
- Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative metaanalysis. Global Change Biology. 2009; 15: 396-424.
- Hoshika Y, Carriero G, Feng Z, Zhang Y, Paoletti E. Determinants of stomatal sluggishness in ozone exposed deciduous tree species. Science of the Total Environment. 2014; 481, 453-458.
- Hoshika Y, Watanabe M, Kitao M, Häberle KH, Grams TEE, Koike T, et al. Ozone induces stomatal narrowing in European and Siebold’s beeches: a comparison between two experiments of free-air ozone exposure. Environmental Pollution. 2015; 196: 527-533.
- Ribas A, Peñuelas J, Elvira S, Gimeno BS. Ozone exposure induces the activation of leaf senescence-related processes and morphological and growth changes in seedlings of Mediterranean tree species. Environmental Pollution. 2005; 134: 291-300.
- Gielen B, Löw M, Deckmyn G, Metzger U, Franck F, Heerdt C, et al. Chronic ozone exposure affects leaf senescence of adult beech trees: a chlorophyll fluorescence approach. Journal of Experimental Botany. 2007; 58: 785-795.
- Matyssek R, Karnosky DF, Wieser G, Percy K, Oksanen E, Grams TEE, et al. Advances in understanding ozone impact on forest trees: messages from novel phytotron and free-air fumigation studies. Environmental Pollution. 2010; 158: 1990-2006.
- INE. Instituto Nacional de Estadísticas. Resultados CENSO 2017.
- Préndez M, Corada K, Morales J. Natural organic compounds from the urban forest of the Metropolitan Region, Chile: impact on air quality. In: Khaled Chetehouna (Ed.) Volatile Organic Compounds: Emission, Pollution and Control. New York, USA: Nova Sciences Publishers Inc. 2014; 103-142.
- Romero H, Vásquez A, Fuentes C, Salgado M, Schmidt A, Banzhaf E. Assessing urban environmental segregation (UES). The case of Santiago de Chile. Ecological Indicators. 2012; 23: 76-87.
- Krellenberg K, Müller A, Schwarz A, Hofer R, Welz J. Flood and heat hazards in the Metropolitan Region of Santiago de Chile and the socio- economics of exposure. Applied Geography. 2013; 38: 86-95.
- De la Maza CL, Rodríguez M, Hernández J, Serra MT, Gutiérrez P, Escobedo F, et al. Silvicultura urbana: Vegetación urbana como factor de descontaminación. Chile Forestal. 2005; 313: 46-49.
- Escobedo FJ, Wagner JE, Nowak DJ, De la Maza CL, Rodriguez M, Crane DE. Analyzing the cost effectiveness of Santiago, Chile’s policy of using urban forests to improve air quality. Journal of environmental management. 2008; 86: 148-157.
- Hernández J. La Situación del arbolado urbano en Santiago. Revista de Urbanismo. 2008; 18.
- Guerrero-Leiva N, Castro SA, Rubio MA, Ortiz C. Retention of Atmospheric Particulate by Three Woody Ornamental Species in Santiago, Chile. Water, Air, Soil Pollution. 2016; 227: 435.
- Muñoz D, Aguilar B, Fuentealba R, Préndez M. Environmental studies in two communes of Santiago de Chile by the analysis of magnetic properties of particulate matter deposited on leaves of roadside trees. Atmospheric Environment. 2017; 152: 617-627.
- Viecco M, Vera S, Jorquera H, Bustamante W, Gironás J, Dobbs C, et al. Potential of Particle Matter Dry Deposition on Green Roofs and Living Walls Vegetation for Mitigating Urban Atmospheric Pollution in Semiarid Climates. Sustainability. 2018; 10: 2431.
- Hernández J, Villaseñor NR. Urban Forestry & Urban Greening Twelve-year change in tree diversity and spatial segregation in the Mediterranean city of Santiago, Chile. Urban Forestry & Urban Greening. 2018; 29: 10-18.
- Préndez M, Alvarado G, Serey I. Some Guidelines to Improve Air Quality Management in Santiago, Chile: From Commune to Basin Level. In: Nicolás Mazzeo (Ed.) Air Quality Monitoring, Assessment and Management. IntechOpen. 2011.
- Dizeo de Strittmatter C. Nueva técnica de diafanización. Boletín de la Sociedad Argentina de Botánica. 1973; 15: 126-129.
- Dunlap J, Stettler R. Variation in leaf epidermal and stomatal traits of Populus trichocarpa from two transects across the Washington Cascades. Canadian Journal of Botany. 2001; 79: 528-536.
- Johansen DA. Plant microtechinique. New York: McGraw-Hill; 1940
- SINCA, Sistema de Información Nacional de Calidad del Aire, Gobierno de Chile. Estaciones de monitoreo de la calidad del aire.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2018.
- MMA. Ministerio del Medio Ambiente. Informe Final para la Gestión de Episodios Críticos de Contaminación Atmosférica por Material Particulado Respirable MP10, Periodo 2016. Secretaría Regional Ministerial del Medio Ambiente Región Metropolitana. 2016.
- Yáñez MA, Baettig R, Cornejo J, Zamudio F, Guajardo J, Fica R. Urban airborne matter in central and southern Chile: Effects of meteorological conditions on fine and coarse particulate matter. Atmospheric environment. 2017; 161: 221-234.
- Bruno G, Stiefkens L, Hadid M, Liscovsky I, Cosa MT, Dottori N. Efecto de la contaminación ambiental en la anatomía de la hoja de Ligustrum lucidum (Oleaceae). Boletín de la Sociedad Argentina de Botánica. 2007; 42: 231- 236.
- Pourkhabbaz A, Rastin N, Olbrich A, Langenfeld-Heyser R, Polle A. Influence of environmental pollution on Leaf properties of urban plane trees, Platanus orientalis L. Bulletin of environmental contamination and toxicology. 2010; 85: 251-255.
- Rai A, Kulshrestha K, Srivastava PK, Mohanty CS. Leaf surface structure alterations due to particulate pollution in some common plants. Environment. 2010; 30: 18-23.
- Kardel F, Wuyts K, Babanezhad M, Vitharana UW, Wuytack T, Potters G, et al. Assessing urban habitat quality based on specific leaf area and stomatal characteristics of Plantago lanceolata L. Environmental Pollution. 2010; 158: 788-794.
- Kardel F, Wuyts K, Khavaninzhadeh AR, Wuytack T, Babanezhad M, Samson R. Comparison of leaf saturation isothermal remanent magnetisation (SIRM) with anatomical, morphological and physiological tree leaf characteristics for assessing urban habitat quality. Environmental Pollution. 2013; 183: 96-103.
- Wuytack T, Wuyts K, Van Dongen S, Baeten L, Kardel F, Verheyen K, et al. The effect of air pollution and other environmental stressors on leaf fluctuating asymmetry and specific leaf area of Salix alba L. Environmental pollution. 2011; 159: 2405-2411.
- Wuytack T, Samson R, Wuyts K, Adriaenssens S, Kardel F, Verheyen K. Do Leaf Characteristics of White Willow (Salix alba L.), Northern Red Oak (Quercus rubra L.), and Scots Pine (Pinus sylvestris L.) Respond Differently to Ambient Air Pollution and Other Environmental Stressors? Water, Air, & Soil Pollution. 2013; 224: 1635.
- Rashidi F, Jalili A, Kafaki SB, Sagheb-Talebi K, Hodgson J, Talebi KS. Anatomical responses of leaves of Black Locust (Robinia pseudoacacia L.) to urban pollutant gases and climatic factors. Trees. 2012; 26: 363-375.
- Jochner S, Markevyvh I, Beck I, Traidl-hoffmann C, Heinrich J, Menzel A. The effects of short- and long-term air pollutants on plant phenology and leaf characteristics. Environmental Pollution. 2015; 206: 382-389.
- Singh H, Savita L, Sharma R, Sinha S, Kumar M, Kumar P, et al. Physiological functioning of Lagerstroemia speciosa L. under heavy roadside traffic: an approach to screen potential species for abatement of urban air pollution. 3Biotech. 2017; 7: 1-10.
- Cardoso-Gustavson P, Faia Fernández F, Domingos M, Paoletti E, da Cruz Centeno D, Ribeiro de Souza S. The role of secretory glands in plant sensitivity to ozone: an alternative route for uptake? In: The International Cooperative Programme on Effects of Air Pollution on Natural Vegetation and Crops, ARCHES-Conseils & International Union of Forest Research Organizations - Research Group 7.01. International Conference on Ozone and Plant Ecosystems. 2018; 63.
- Pellegrini E , Hoshika Y, Cotrozzi L, Lorenzini G, Nali C, Paoletti E, et al. Antioxidative responses of three oak species under ozone and water stress conditions. In: The International Cooperative Programme on Effects of Air Pollution on Natural Vegetation and Crops, ARCHES-Conseils & International Union of Forest Research Organizations - Research Group 7.01. International Conference on Ozone and Plant Ecosystems. 2018; p.71.
- Nicolas D, Le Thiec D, Olry JC, Vaultier MN, Jolivet Y. Poplar submitted to a succession of ozone and drought stresses: dynamic of stomatal responses. In: The International Cooperative Programme on Effects of Air Pollution on Natural Vegetation and Crops, ARCHES-Conseils & International Union of Forest Research Organizations - Research Group 7.01. International Conference on Ozone and Plant Ecosystems. 2018; 69.
- Dhir B. Air Pollutants and Photosynthetic Efficiency of Plants. In: Kulshrestha U, Saxena P. Plant Responses to Air Pollution. Springer Science+Business Media Singapore. 2016; 71-84.
- Egas C, Naulin PI, Préndez M. Contaminación Urbana por Material Particulado y su Efecto sobre las Características Morfo-Anatómicas de Cuatro Especies Arbóreas de Santiago de Chile. Información Tecnológica. 2018; 29: 111-118.
- Cape JN. Chapter 3 Plants as Accumulators of Atmospheric Emissions. Developments in Environmental Science. 2009; 9: 61-98.
- Burkhardt J, Grantz DA. Plants and atmospheric aerosols. Cánovas FM, Lüttge U, Matyssek R, editors. In: Progress in Botany. Springer International Publishing Switzerland. 2016; 78: 369-406.
- Emberson LD, Ashmore MR, Murray F, Kuylenstierna JCI, Percy KE, Izuta T, et al. Impacts of air pollutants on vegetation in developing countries. Water Air Soil Pollution. 2001; 130: 107-118.
- Brito CE, Bown HE, Fuentes JP, Franck N, Pérez-Quezada JF. Mesophyll conductance constrains photosynthesis in three common sclerophyllous species in Central Chile. Revista Chilena de Historia Natural. 2014; 87: 8.
- Correa-Galleguillos PC, De la Barrera F. Análisis de la estructura y de la composición del arbolado en parques del área metropolitana de Santiago. Chloris Chilensis. 2014; 17.
- Grote R, Samson R, Alonso R, Amorim JH, Cariñanos P, Churkina G, et al. Functional traits of urban trees: air pollution mitigation potential. Frontiers in Ecology and the Environment. 2016; 14: 543-550.
- Sjöman H, Hirons AD, Bassuk NL. Improving confidence in tree species selection for challenging urban sites: a role for leaf turgor loss. Urban Ecosystems. 2018; 21: 1171-1188.
