Abstract
A liquid chromatography-tandem mass spectrometry method has been developed for the simultaneous determination of metabolites of neonicotinoid insecticides with neonicotinoid insecticides and strobilurin fungicides in the particle phase fraction of atmospheric samples. Pressurized solvent extraction was used to extract the target analytes from particles collected on glass fiber filters, followed by a C18 SPE cleanup step. Recoveries of 85.9 to 108.3% and relative standard deviation <13% were obtained for all analytes. The method detection limit for metabolites of neonicotinoid insecticides were 0.5-3 ng/ mL (air concentrations of 0.44-2.66 pg/m³). Matrix effects were compensated for using standard addition calibration for quantitation. This paper provides the first detection of desmethyl-thiamethoxam which coincided with higher concentrations of thiamethoxam in atmospheric particles. Other neonicotinoid insecticides and strobilurin fungicides detected in the particle phase in the atmosphere included acetamiprid, clothianidin, imidacloprid, azoxystrobin, kresoxim-methyl, pyraclostrobin, and trifloxystrobin. This research highlights the potential of not only neonicotinoid insecticides and strobilurin fungicides moving in the atmosphere in the particle phase, but also a metabolite of thiamethoxam (desmethyl-thiamethoxam) in a region with orchards and vineyards where foliar applications with air blast sprayers dominate.
Keywords: Metabolites; Neonicotinoid insecticides; Strobilurin fungicides; Liquid chromatography-tandem mass spectrometry; Desmethyl-thiamethoxam; Atmospheric particles
Abbreviations
dSPE: Dispersive Solid Phase Extraction; GC-MS: Gas Chromatograph-Mass Spectrometry; LC-ESI+-MS/MS: Liquid Chromatography-Positive Electrospray-Tandem Mass Spectrometry; MDL: Method Detection Limit; QuEChERS: Quick, Easy, Cheap, Effective Rugged, and Safe; SPE: Solid Phase Extraction; US-EPA: United States Environmental Protection Agency.
Introduction
Neonicotinoid insecticides have been identified as a concern to the health of pollinators and aquatic insects [1-3]. Health Canada published three pollinator re-evaluations in April 2019 and subsequently cancelled the uses of three neonicotinoid insecticides (thiamethoxam, clothianidinm and imidacloprid) in Canada for foliar applications on crops including pome fruit and stone fruit, while stopping spraying on some other crops such as berries and fruiting vegetables, before and after bloom [4]. Further decisions and re-evaluations of these neonicotinoid insecticides in agriculture in Canada are still pending due to COVID-19 restriction delays and expected by 2022. In January 2020, the United States Environmental Protection Agency (US-EPA) also published their proposed interim decision for several neonicotinoids (thiamethoxam, clothiandin, imidacloprid, acetamiprid, and dinotefuran) which allows for continued usage in agricultural regions with label changes to reduce spray drift and runoff [5-9]. There were no specific changes to requirements for air blast sprayers which are commonly used in orchards and vineyards. Thiacloprid was canceled voluntarily by the registrant such that its registration review was cancelled in 2014. In 2018, the European Union also extended the ban on the use of neonicotinoid insecticides to all field crops as a result of growing evidence that these insecticides cause harm to pollinators such as honey bees [10]. Fungicides have also been identified as a potential concern to bee health and our previous study identified that some fungicides can be transported in the atmosphere in both the gas and particle phase [11-14].
A liquid chromatography-positive ion electrospray-tandem mass spectrometry method was developed for the simultaneous analysis of neonicotinoid insecticides and strobilurin fungicides in particles in the atmosphere at trace levels and provided the first detection of these active ingredients in particles in the atmosphere in an agricultural region with orchards and vineyards where appliations are typically using air blast sprayer [13]. The Okanagan Valley in Canada is in close proximity to the Okanogan County of Washington State in the United States with both regions having similar crops (orchards and vineyards dominant) and foliar spray applications of neonicotinoid insecticides and strobilurin fungicides. We aimed to assess whether with regulations and best management practices that existed in 2016 and 2018 if there were neonicotinoid insecticides and strobilurin fungicides as well as metabolites of neonicotinid insecticides present in particles in the atmosphere in this region of the United States.
Metabolites of neonicotinoid insecticides have been identified from plant and animal studies but measurements in environmental media have been very limited due to analytical method development changes [15-18]. Consequently, we screened for viable target metabolites of neonicotinoid insecticides from plant extract studies, water quality studies, or measurements in biological fluids, that could be analyzed with selective methods using Liquid Chromatography- Positive Electrospray Ionization Tandem Mass Spectrometry (LCESI+- MS/MS) to assess the feasibility for analysis of an environmental sample matrix at trace level [19]. Based on this review we included desmethyl-acetamiprid, desmethyl-Thiamethoxam (dm-THM), imidcloprid-urea, imidacloprid-olefin, and 6-chloronicotinic acid (a common metabolite of acetamiprid, clothianidin, dinotefuran, imidaclorpid, nitenpyram, thiacloprid, and thamethoxam) into the method development for a new method that could provide simultaneous analysis of metabolites of neonicotinoid insecticides with neonicotinoid insecticides and strobilurin fungicides and improve on the recovery of selected target analytes such as nitenpyram, a less commonly studied neonicotinoid insecticide with prior methods with recoveries as low as 60% in some sample matrices [13,20]. Metabolites of neonicotinoid insecticides are also more polar than their parent active ingredients and more prone to losses in sample preparation often requiring selective methods [19].
The neonicotinoid insecticides and strobilurin fungicides were identified in our studies as candidate active ingredients in formulations used in foliar applications in orchards and vineyards for transport in the atmosphere in the particle phase due to their low volatility. Metabolites were also of interest due to the large variation in concentrations of neonicotinoid insecticides observed in particles in the atmosphere in an adjacent agricultural region (Okanagan Valley in Canada) such that it was proposed that breakdown of the active ingredients may be occurring before or during atmospheric transport [13,19]. This study represents the first analysis of atmospheric particles for metabolites of neonicotinoid insecticides worldwide and was of interest to determine if metabolites of neonicotinoid insecticides were present in the atmosphere on particles in a region with expected foliar spray applications such that atmospheric transport could be a new pathway of transport in the environment. The Okanogan County and some surrounding counties of Washington state has both historical usage and usage of both neonicotinoid insecticides and strobilurin fungicides [21,22]. The target list of analytes was also expanded to include one chemical alternative to neonicotinoid insecticides, sulfoxaflor, which is a more recently registered sulfoximine insecticide and is currently registered for use in the United States [21]. Sulfoxaflor has four stereoisomers (2S,3S-sulfoxaflor, 2R,3Ssulfoxaflor, 2S,3R-sulfoxafor, and 2R, 3R-sufoxafor) and has not been included in other methods for neonicotinoid insecticides and in the analyses of food matrices has been analyzed in a selective method for only sufoxaflor or sufoxaflor and its two metabolites using either normal phase, reversed phase or chiral LC separations [23-27]. In addition, we also added picoxystrobin to the list of target analytes in the method as it is not commonly included in analyses of strobilurin fungicides as it can be more challenging to analyze with LC-MS/MS as compared to GC-MS methods which can be used for the analysis of strobilurin fungicides, but GC is not typically used for analysis of neonicotinoid insecticides [28-36].
Sample preparation methods for neonicotinoid insecticides and strobilurin fungicides have largely been developed for other matrices including honey, pollen, fruits and vegetables or soil often involving modified QuECHERS methods but these methods are not typically used for analysis of air samples [36]. Sampling for pesticides and other semi-volatiles in the atmosphere uses materials such as quartz fiber filters for the collection of particles with the pesticides most commonly extracted from these materials into an organic solvent with pressurized solvent-extraction followed by clean-up with solid phase extraction (SPE) or dispersive solid phase extraction (dSPE) methods. Materials used for air sampling have been shown not to contributed significantly to the matrix issues observed [13,14]. A uniquely aspect to atmospheric sample analyses is that the composition of the matrix can vary significantly over time (collection period during the agricultural season) and is often unknown due to the variety of other semi-volatile organics that can also be collected with high-volume air sampling with the concentration of these semi-volatile organics expecting to change with varying contributions from agricultural, industrial, and residential emission sources, and other combustion sources such as forest fires which have increased in occurrence in the study region. Consequently, calibration methods used for quantitation typically include the use of deuterated internal standards (internal standard calibration) or standard addition calibration rather than matrix matched calibration standards.
The goal of this study was to determine if metabolites of neonicotinoid insecticides, neonicotinoid insecticides, and strobilurin fungicides were present in particles in the atmosphere at Omak, WA. The sampling location was selected to be at Omak in Washington State as this county (Okanogan County) has a higher proportion of apple orchards and vineyards than the Okanagan Valley in Canada, which has more variation in stone and pome fruit trees. There is known usage of neonicotinoid insecticides and strobilurin fungicides regionally (Okanogan county and surrounding counties) in 2016 and 2018 [21,22].
Materials and Methods
Chemical and general details
Ethyl acetate, acetonitrile, and methanol were of pesticide grade and supplied by Fisher Scientific. Deionized water with resistivity <18MO cm was obtained from Nanopure Diamond system (Barnstead International, Dubuque, IA, USA). Formic acid with concentration >88.0% was obtained from VWR Scientific (West Chester, PA, USA). Solid Imidacloprid-d4 (IMI-d4), Clothianidin-d3 (CLO-d3), and Thiamethoxam-d3 (THM-d3) were obtained from C/D/N Isotopes Inc. (Pointe Claire, QC, Canada). Solids or stock solutions at 100μg/mL in acetonitrile or methanol of strobilurin fungicides (Azoxystrobin (AZOXY), Dimoxystrobin (DIMOXY), Fluoxastrobin (FLUOXA), Kresoxim-Methyl (KRES), Picoxystrobin (PICOXY), Pyraclostrobin (PYRA), and Trifloxystrobin (TRIFLOXY)) and neonicotinoid insecticides (Acetamiprid (ACE), Clothianidin (CLO), Dinotefuran (DIN), Imidacloprid (IMI), Nitenpyram (NIT), Sulfoxaflor (SULF), Thiamethoxam (THM), and Thiacloprid (THC)) and metabolites of neonicotinoid insecticides (6-Chloronicotinic Acid (CINA), Imidacloprid-olefin (IMI-olefin), Imidacloprid-urea (IMI-urea), Desmethyl Thiamethoxam (dm-THM), and Desmethyl Acetamiprid (dm-ACE) were purchased from Chem Service Inc. (West Chester, PA, USA).
Pesticide standards
Thiamethoxam-d3 (N-methyl-d3) (THM-d3) was used as a surrogate to evaluate recoveries in samples (SRMs 295.1→132.0 (cone voltage 20, collision energy 15); 295.1→184.0 (20, 22);. CLO-d3 (SRMs 253.1→172.1 (17, 15); 253.1→132.0 (15, 12)); was used to determine the final volume of an extract of the dried fraction F1 from the SPE cleanup step after the addition of internal standards and was approximately 1.0 mL. IMI-d4 (SRMs 260.1→179.0 (20, 14); 260.1→213.1 (20, 14)); was used as an internal standard for calibration purposes. Matrix effects for assessed for all samples collected in 2016 and selected samples in 2018 due to the expected potential influence of forest fires. The internal standard was not used in the determination of slopes from the solvent-based and standard addition calibration curves in the % ME calculation shown in Supplementary material, but was used in the standard addition calibration curves used for quantitation.
Individual stock solutions of pesticides in methanol were prepared by dissolving solids of individual pesticides (~1 mg) in 1 mL of methanol and stored at -4°C. Calibration standards were prepared from a stock solution containing a mixture of the standards at 1.000μg/mL in methanol. Internal Standard (IS), IMI-d4 at 75ng/ mL was used in calibration standards and all samples. Preparation of samples and calibration standards were carried out on the analysis day. The calibration range for solvent-based calibration curve was MDL -30ng/mL, but could be extended to 100ng/mL if required. The lowest prepared calibration standard was generally selected to be 0.5ng/mL. Standard addition calibration was completed with the dilution factor of the sample of ½ with standard concentrations added also to 30 ng/mL with IMI-d4 added as the internal standard at 75ng/mL.
Sample collection and preparation of particle extracts
Polyurethane Foam (PUF) air sampler (TE-1000BL, Tisch Environmental) was used to collect air samples at the Confederated Tribes of the Colville Reservation operated air monitoring site located at Omak within the Okanogan County. The PUF sampler motor operated at a flow rate of ~225 L/min with an air volume of approximately 350 to 370 m³ per day with most samples collected continuously over a 2-week sampling period. Air samples were collected from during 2016 from March 12 to August 30, and from May 23 to September 05, 2018. For the purposes of matrix evaluation, 3 samples from 2018 with higher atmospheric particle concentrations and a sample collected at the end of the agricultural season were used to assess potential additional matrix effects from wildfire sources to the atmosphere.
In the top portion of the sampling module a quartz microfiber filter (10.2cm diameter, Tisch Environmental) is inserted between two teflon rings. Filters are weighed in a glove bag under nitrogen atmosphere to ±0.00002g before and after sampling. The lower portion of the sampling module contains a glass cartridge with polyurethane foam (PUF, 12.7cm length X 7.3cm diameter) for gas phase concentrations of pesticides, which were not analyzed as part of this study. The Polyurethane Foam (PUF) was purchased from Tisch Environmental and certified to be flame retardant free and was pre-cleaned using pressurized solvent extraction with ethyl acetate as the extraction solvent using the same extraction procedures as sample extraction. The PUFs were air dried in the dark prior to use. The sampling modules are shipped to the sampling site or the materials are exchanged during instrument calibration visits. The sampling module is equipped with a PM2.5 cyclone designed such that particles <2.5μm are collected on filters. Particle concentrations (PM2.5) reported herein were determined by gravimetric analysis of filters obtained from the high-volume air sampler and ranged from 1.4 to 23.8 μg/m³ during 2016 and 10.2 to 75.7 μg/m³ during 2018 sampling.
Extraction and cleanup
The quartz filters were extracted according to Raina-Fulton method with modifications described herein briefly [13]. The filters were transferred to 30 mL extraction cells and extracted using an ASE100 pressurized solvent extraction system (Dionex, Sunnyvale, CA, USA) with ethyl acetate as the extraction solvent. The extraction procedure held the cell at 100°C and pressure of 1500 psi during the 30 min static mode followed by a 60% flush with ethyl acetate of the volume of the cell. Three static stages were used to ensure complete extraction of the target analytes from the particles collected on the quartz filter. A second extraction showed no detectable levels of target analytes. At the end of the extraction, the extraction cell is purged with nitrogen for 600s. The total extraction volume is approximately 70 mL. To this extract 1 mL of 2-propanol added as a keeper for the drying step.
This extract from pressurized solvent extraction s concentrated to <2mL, transferred to 15 mL vials, and dried again to ~1mL at 0.5mL/ hr with a slight vacuum <1kPa. All extracts are stored at -4°C until sample cleanup. Sample cleanup of the extract was completed using C18 SPE (6mL, 1000mg, Canadian Life Science, Peterborough, ON, Canada) and the cleanup was modified from the prior method13 to improve recoveries of metabolites of neonicotinoids. C18 SPE cartridges were conditioned with 6 mL of methanol and 6 mL of water. The sample extract (500μL) and 3μL of 1μg/mL THM-d3 were loaded onto the preconditioned SPE tube. This was followed by loading of 450μL of water, which was eluted into the F0 fraction and contained no target pesticides or metabolites. The pesticides of interest (neonicotinoid insecticides, metabolites of neonicotinoid insecticides, and strobilurin fungicides) were eluted into a fraction, F1, with 5mL of 100% methanol. A volume of 50μL 2-propanol was added to the F1 extract prior to drying. The eluted extracts from SPE were concentrated to ~1mL at 0.5mL/h with a slight vacuum <1kPa with an additional 50μL of 2-propanol added when the volume was reduced to 2.5mL and 1.5mL. The final volume of this fraction was measured by adding 20μL of 10μg/mL clothianidin-d3 (volume check standard) and ranged from 0.5-1.1 mL. Extracts were then generally diluted with methanol at a dilution factor of 1/2 prior to LC-MS/MS analysis with internal standard, imidacloprid-d4, added at 75ng/mL. A second fraction eluted from the SPE cartridge shown no detectable levels of target analytes or THM-d3. The amount of pesticides measured in the extract was divided by the total volume of air sampled during each sampling period to determine concentrations of pesticides (pg/m3) in the atmosphere in the particle phase.
LC-MS/MS analysis
A Waters LC system consisting of a 1525μ binary pump and column heater was utilized to conduct LC analysis. A LEAP Technologies autosampler (Carrboro, NC, USA) was used for 10μL injections at 100μL/s. In order to minimize the sample carry-over, two pre- and post-cleans with ethyl acetate followed by methanol were carried out. A guard column (4×2.0 mm, C18 Gemini) was connected to phenyl-hexyl column, 50×2.00 mm i.d., 2.5μm (Phenomenex, Torrance, CA, USA). It was then placed in a column heater at 30°C. Initial mobile phase was 40 v% acetonitrile containing 0.1 v% formic acid and 60 v% water with 3 v% methanol, 2 v% 2-propanol, 0.05 v% formic acid at a flow rate of 0.15mL/min. The following linear gradual change in mobile phase gradient of acetonitrile with 0.1 v% formic acid was applied: 0.0 min, 15 v%;1.5 min, 30 v%; 3 min, 33 v%; 7 min, 40 v%; 10 min, 50 v%; 12 min, 55 v%; 15 min, 70 v%, held for 3 min. The elution of all analytes was completed in less than 18.0 min. A pre-injection of 10μL of 2-propanol with a 20 min elution time at initial mobile phase conditions was also used to minimize carry-over issues as some samples exhibited high matrix interferences and this was found to improve retention time stability (±0.1 min) and column performance over time.
The Waters LC system was attached to a Quattro Premier (Milford, MA, USA) triple quadrupole with electrospray ionization in positive ion mode (ESI+). The temperature of the source was set to 120°C, desolvation temperature of 300°C, desolvation gas at 750 L/h, and cone gas at 150 L/h. The optimized settings for ESI were: extractor voltage of 4 V, capillary voltage of 3.50 kV, and RF lens of 0.1 V. The collision gas used for SRM was argon (UHP) at 0.15mL/ min or 9.3×10-4 mbar. Infusion experiments were performed for the new target analytes to determine the SRM conditions in ESI+ with a syringe pump flow rate of 50μL/min. Quantitative and confirmation SRMs used for all target analytes and the retention times of the target analytes with the optimized gradient program (Table 1).
Compound
Quantitative SRM, Confirmation SRMs (Cone Voltage (V), Collision Energy (eV))
MDL (ng/mL)
Recoveries±% RSD at 10, and 50 ng/mL
r2
Retention Time (min)
Newly added target analytes to LC-ESI+-MS/MS method
CINA
158.0à112.0 (27, 25)
158.0à122.0 (27, 25)2.0
2.0107.7±11.6; 93.1±5.2
0.993
0.995.22
IMI-urea
212.0à128.1 (25, 20)
212.0à98.9 (25, 20)
212.0à126.0 (25, 20)2.0
2.0
2.093.8±3.1; 97.6±6.4
0.986
0.992
0.9915.39
IMI-olefin
254.0à205.2 (15, 15)
254.0à236.0 (15, 8)1.0
3.0103.4±7.1; 100.6±10.4
0.978
0.9995.39
dm-ACE
209.1à126.0 (25, 15)
209.1à167.6 (25, 10)1.0
1.0101.9±5.4; 94.6±7.7
0.998
0.9985.95
dm-THM
278.1à132.1 (17, 17)
278. à167.0 (17, 25)0.5
0.592.1±6.3; 93.9±2.9
0.997
0.9956.97
Sulfoxaflor
278.0à174.4 (15, 10)
278.0à154.1 (15, 30)1.0
1.092.8±6.9; 92.2±5.8
0.997
0.9977.65*
Picoxystrobin
368.1à205.2 (20, 10)
368.1à145.1 (20, 15)2.0
1.089.5±5.3; 87.8±8.7
0.992
0.99116.31
Other Neonicotinoid Insecticides
Dinotefuran
203.1à157.2 (15, 7)
203.1à129.2 (15, 13)
203.1à113.0 (15, 12)1.0
1.0
0.5111.3±1.9; 108.3±6.5
0.999
0.999
0.9983.69
Nitenpyram
271.2à99.0 (25, 17)
271.2à56.0 (25, 30)
271.2à189.1 (25, 10)0.5
0.5
0.586.1±5.5; 85. 9±5.9
0.998
0.999
0.9984.71
Thiamethoxam
292.2à211.4 (19, 12)
292.2à181.3 (19, 27)1.0
1.096.4±7.7; 101.8±3.9
0.988
0.9855.39
Clothianidin
250.1à169.3 (17, 13)
250.1à132.0 (17, 15)1.0
2.097.1±5.3; 91.0±6.5
0.98
0.9825.9
Imidacloprid
256.1à175.0 (20, 15)
256.1à209.5 (20, 15)0.5
0.597.4±4.9; 94.0±4.5
0.996 0.998
6.63
Acetamiprid
223.1à90.0 (20, 30)
223.1à126.1 (20, 15)
223.0à56.0 (20, 15)2.0
0.5
2102.2±12.9; 92.6±5.2
0.993
0.997
0.9947.31
Thiacloprid
253. à186.2 (25, 20)
253.1à126.0 (25, 22)1.0
1.0100.1±9.8; 88.3±5.6
0.995
0.9989.22
Other Strobilurin Fungicides
Azoxystrobin
404. à327.4 (20, 20)
404.1à329.4 (20, 13)0.5
0.5102.6±4.7; 94.7±8.3
0.997
1.00015/05
Dimoxystrobin
327.6à205.3 (15, 10)
327.6à238.5 (15, 10)0.5
1.0108.7±5.1; 91.1±8.7
0.998
0.98215.36
Kresoxim-methyl
314.1à206.3 (15, 15)
314.1à116.3 (15, 15)
314.1à267.4 (15, 5)0.5
1.0
1.097.3±5.6; 85.4±2.5
1.000
0.999
1.00016.1
Fluoxastrobin
459.2à188.2 (25, 30)
459.2à427.4 (25, 15)1.0
0.5103.8±10.3; 92.8±5.4
0.998
0.9916.21
Pyraclostrobin
388.1à163.5 (20, 22)
388.1à194.5 (20, 11)0.5
0.596.4±5.7; 87.4±5.8
0.994
0.99416.73
Trifloxystrobin
409.1à186.4 (20, 20)
409.1à206.4 (20, 20)1.0
0.599.9±5.4; 87.7±2.8
0.998
0.99917.15
*Two peak observed with partial resolution intergrated together for quantitation
Table 1: Recoveries and Method Detection Limits of Metabolites and Selected Target Analytes.
Results and Discussion
Solid Phase Extration (SPE) method
C18 SPE was used to cleanup the pre-concentrated organic extracts from pressurized solvent extraction of filters containing particles from air sampling. C18 is the most common SPE sorbent used for air extracts for a large variety of pesticides [13,14]. The prior SPE method used for cleanup of neonicotinoid insecticides and strobilurin fungicides was modified to provide acceptable recoveries for metabolites of neonicotinoid insecticides (Table 1) and to accommodate a larger loading volume of sample extract (500μL). Adjustments during loading included only the addition of the surrogate (prepared in methanol) and water rather than 20/80 v/v% methanol/water to minimize the loss of metabolites of neonicotinoid insecticides during the loading and wash steps. The fraction eluted from the C18 SPE tube during loading and wash steps contained no target analytes. An elution volume of 5.0mL of methanol was adequate for elution of neonicotinoid insecticides and strobilurin fungicides in prior method [13]. This volume also provided acceptable recoveries for metabolites of neonicotinoids, sulfoxaflor, picoxystrobin, and THM-d3. To minimize loss of target analytes particularly CINA, dinotefuran, nitenpyram, and sulfoxaflor which have higher volatility than other neonicotinoid insecticides, a small amount of 2-propanol was added (50μL at 5, 2,5, and 1.5mL volume) as a keeper during the drying/preconcentration step required after SPE cleanup to reduce the eluted fraction volume from 5.0mL to 0.5-1.1 mL. Reducing the volume below 0.5 mL lead to loss of metabolites and nitenpyram. Strobilurin fungicides, in general, have lower volatility than neonicotinoid insecticides such that they are less prone to loss during drying. Recoveries for all target analytes were between 85.9 to 108.3% with the new SPE method (Table 1). The average recoveries for THM-d3 for particle air extracts in samples were 102.1±8.0 %. This is a significant improvement for recovery of more volatile target analytes such as nitenpyram (vapour pressure 10-4 Pa, recovert 85.9%) which have been prone to loss in prior methods [13,15-17]. Available QuEChERS methods for metabolites of neonicotinoid insecticides or neonicotinoid insecticides have shown that better recoveries can be obtained by careful selection of the salt used in the salting-out extraction of the target analytes in the acetonitrile layer and conditions for extraction are matrix dependent which makes optimized of conditions more challenging [2,5]. Some metabolites such as CINA can also bind strongly to dSPE sorbents such that C18 SPE rather than dSPE was used to improve recoveries [15]. Even with C18 SPE used after QuEChERS, the used of an additive, 2 v% trimethylamine, in acetonitrile (elution solvent) was necessary to obtain acceptable recoveries for more strongly bound analytes such as CINA [15]. The method herein was able to recover all metabolites of neonicotinoid insecticides including CINA with the use of methanol rather than acetonitrile as the elution solvent which is beneficial as a larger fraction of more nonpolar matrix would be retained on the C18 SPE. The use of methanol as the elution solvent also aids the drying step required for preconcentration of target analytes after cleanup as the drying conditions can be selected to minimize loss of more volatile target analytes.
LC-MS/MS gradient elution conditions
Further optimization of mobile phase conditions for inclusion of metabolites in the analysis largely focused on improving the peak shapes and separation conditions for early eluting target analytes, which included the metabolites of neonicotinoid insecticides and neonicotinoid insecticides. The mass spectrometric response in LCESI+- MS/MS of clothianidin, imidacloprid, and imidacloprid-d4 improves when methanol rather than acetonitrile is used as the organic modifier in gradient elution [13]. However, the strobilurin fungicides have strong retention with reversed-phase liquid chromatography even on C6-phenyl stationary phase as used herein such that acetonitrile is necessary to obtain analysis times <20 minutes. Similarly, selected analysis of sulfoxaflor and its two main metabolites has been accomplished using a gradient with acetonitrile with 0.1 v% formic acid when a C18 stationary phase was used and mobile phase conditions can be adjusted to reduce the chiral separation [23-27]. The four stereoisomers of sulfoxaflor can only be separated when a chiral column is used with detection limits of 0.5 ng/mL with ESI- rather than ESI+ [27]. Under ESI+ conditions formic acid is required in the mobile phase. To account for the addition of metabolites of neonicotinoid insecticides to the new method which elute early in a reversed phase separation the initial % of acetonitrile had to be reduced to 15% from 40% acetronitrile in the prior method which results in better retention and resolution of the metabolites of neonicotinoids and neonicotinoid insecticides [13]. However to further improve detection limits the additives used in the aqueous mobile phase were further optimized particularly for their ability to improve peak shapes rather than altering retention times. The use of 2-5 mM ammonium acetate has been used in the aqueous mobile phase for other methods for the separation of neonicotinoids [13,15], however peak shapes were distorted for early eluting analytes. Consequently, a comparison of detection limits was completed for different aqueous based mobile phases (using the same acetronitrile gradient) including 5 mM ammonium acetate, a small percentage of methanol added in the aqueous mobile phase that was optimized to be 3 v% for resulting improvements in the MS sensitivity of all target analytes, and 2 v% 2-propanol and 3 v% methanol added to the aqueous mobile phase (Figure 1). Improvements in peak shapes for early eluting peaks resulted in lower method detection limit for all analytes except IMI-olefin when the additives in the aqueous mobile phase were 2 v% of 2-propanol, 3 v% methanol, and 0.1 v% formic acid (Figure 1). Formic acid is necessary for optimal MS sensitivity of the target analytes in the mobile phase as previously observed when ESI+ is used with the percentage during gradient elution varying from 0.06% to 0.09 v%. Some studies have suggested that addition of ammonium acetate can improve MS sensitivity for dm-ACE and IMI-urea [35], however we found that similar or better sensitivity within 10% error of repeat analysis was obtained with the use of 2 v% 2-propanol and 3 v% methanol in the aqueous mobile phase when formic acid was present in the mobile phase. The advantage of using methanol in the aqueous mobile phase over ammonium acetate is that good MS sensitivities can also be obtained for CINA as was observed under the separation conditions used herein. Prior methods for analysis of CINA in urine used only formic acid (0.1 v%) in the aqueous and acetrontile mobile phases [17,19]. The gradient conditions were further optimized using the aqueous mobile phase containing 0.1 v% formic acid, 2 v% 2-propanol, and 3 v% methanol in water to obtain the detection limits shown in (Table 1).
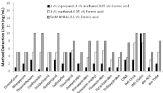
Figure 1: Method detection limits for target analytes as a function of aqueous
mobile phase additives used in gradient elution. Aqueous mobile phase
additives included 3 v% methanol and 0.05 v% formic acid; 3 v% methanol,
2 v% 2-propanol, 0.05 v% formic acid; and 5mM ammonium acetate, 0.1 v%
formic acid. Organic mobile phase used was acetonitrile with 0.1% v formic
acid.
The separation is a reversed-phase LC separation with more polar metabolites such as IM-urea and IMI-olefin co-eluting (retention time of 5.39 min) before IMI, but they can be detected by their unique SRMs, which are 212.0>128.10 m/z and 254.0>205.2 m/z, respectively (Table 1). The metabolite CINA is more polar than its parent compounds that contain the chloropyridinyl moiety such as ACE, IMI, THC and dm-ACE is more polar than acetamiprid and both of these metabolites elute before their parent compounds. However, dm-THM elutes after THM and NIT elutes before CINA, which may be related to the different selectivity of phenyl-hexyl stationary phase compared to other more common alkylsilane stationary phases such as C18. Kresoxim-methyl and fluoxastrobin partially co-elute, but are also distinguished by their unique SRMs (the quantitative SRM for kresoxim-methyl and fluoxastrobin are 314.1>116.3 m/z and 459.2>188.2 m/z, respectively). Deuterated standards (CLO-d3, IMI-d4, and THM-d3) also had unique SRMs and gave no response at the SRM used for monitoring CLO, IMI, or THM at the concentration used in these studies. Partial separation of the stereoisomers was observed for sulfoxflor with phenyl-hexyl stationary phase (Figure 1) as also observed with other reversed phase separations and these two peaks were intergrated together for quantiation. No sulfoxaflor was detected in atmospheric samples and no reported usage within the Okanogan county [21,22].
Method Detection Limit (MDLs) of metabolites and additional analytes
Method Detection Limits (MDLs) were determined by the lowest standard concentration within <25% deviation of the bestfit regression line of the calibration curve. MDLs for metabolites of neonicotinoids were 0.5-3 ng/mL. MDLs as air concentrations equate to 0.44-2.66 pg/m3. Although the MDLs for some metabolites were higher than their parent compounds (Table 1) they are within the range of other metabolite methods often targeting only a few metabolites [15-17]. This method provides better recoveries and MDL for CINA as compared to other methods with IMI-metabolites [15]. The poor recovery of CINA in earlier methods although attributed to strong retention on SPE sorbents may have been also due to loss during drying steps necessary to preconcentrate the target analytes prior to LC-MS/MS analysis.
Calibration curves and matrix effects
Solvent-based calibration curves (methanol as solvent) when imidacloprid-d4 was used as the internal standard showed good linearity with correlation coefficient (r²) >0.99. Similar linearity was also obtained for standard addition calibration curves over the same calibration range. Matrix effects in these particle phase atmospheric samples collected over the agricultural season were generally variable and moderate to severe such that standard addition calibration was used for quantitation (Figure I (Supplementary materials)). There were only two neonicotinoid insecticides (nitenpyram and clothianidin) and no metabolites of neonicotinoids that showed more than 40% of samples with soft matrix effects (Figure I). Metabolites of neonicotinoid insecticides were more prone to matrix effects than their parent compounds with between 8-25 % of samples exhibiting soft matrix effect even with C18 SPE cleanup. With the exception of the SRM 459→427 used for monitoring the confirmation response for fluoxastrobin, the SRM response of strobilurin fungicides was not impacted as much by matrix as compared to metabolites of neonicotinoids or neonicotinoid insecticides and these analytes tend to be more nonpolar (elute later in the separation) with higher mass SRMs. There were no apparent trends in the magnitude of matrix effects with retention time. The magnitude of moderate and strong matrix effects for quantitative and confirmation SRM varied the most for IMI-urea, IMI-olefin such that the 2nd most sensitive SRM transition was generally used for quantitation as more samples exhibited soft or moderate matrix effects at these SRMs.
The SRM response for target analytes from selected particle extract samples (3 samples) in 2018 with atmospheric particle concentrations from 10-42 μg/m3 during sampling periods where emissions from forest fires were present showed a much lower portion of analytes with soft matrix effects as compared to samples collected in 2016 when particle levels and incidence of forest fires were lower. Strong matrix effects were observed for ACE, DIN, IMI, SULF, THC, CINA, dm-THM, KRES, FLUOXA, PICOXY, and TRIFLOXY in these three particle air samples collected during wildfires in Washington State and British Columbia. Wildfires have been shown to increase the concentration of polycyclic aromatic hydrocarbons in the atmosphere, which are also collected with the high-volume air sampler and would not be fully removed with C18 SPE cleanup [37]. Although strong matrix effects were observed good linearity of, the standard addition calibration curves were obtained (r2 from 0.96-0.99).
The SRM chromatographs for dm-THM for an extract of a particle sample collected during June 28-July 5, 2016 at Omak, WA which was determined to have 2.72 pg/m3 of dm-THM (Figure 2). Although concetrations of dm-THM are low, this represents the first detection of a metabolite of any neonicotinoid in the atmosphere worlwide. There are no interferences observed in the chromatographs for either the quantitative or confirmation SRM near the retention times of dm-THM (6.97 min). When dm-THM was detected in particles in the atmosphere that concentrations of thiamethoxam were also higher (Figure 3). There was more frequent detection of thiamethoxam, imidaclorprid, and clothianidin in particles in the atmosphere in May or June in 2016 and 2018, whereas acetamiprid was more frequently detected later in the agricultural season (August) particularly in 2018. Concentrations of neonicotinoid insecticides were lower in particles in the atmosphere at Omak, WA than previously detected in the atmosphere in the Canadian Okanagan Valley when neonicotinoids were still permitted for use in the region [13]. Although neonicotinoids were still permitted for use in agricultural in the United States, the atmospheric concentrations may be influenced by measures to reduce pesticide usage in Washington State with the introduction of the Management Pollinator Protection Plan by Washington State Department of Agriculture in April 2018 which provides a framework of recommendations for beekeeper hive management and pesticide practices for farmers that are beneficial to the protection of pollinators [38]. Acetamiprid, clothianidin, imidacloprid, and thiamethoxam were used in Washington state in 2016 and 2018 [21]. Imidacloprid and acetamiprid were more heavily used in the Okanogan County and surrounding counties than thiamethoxam. Within the Okanogan County usage of imidacloprid and most surrounding counties declined, however usage of acetamiprid and thiamethoxam was approximately doubled in 2018 as compared to 2016 in the Okanogan County. Although there was no reported usage of clothianidin in the Okanogan county where Omak is located, it was used in agriculture at significant amounts in Grant and Franklin counties of Washington State. There were no reported usages of nitenpyram and sulfoxaflor for agricultural applications and these target analytes were not detected in samples [21,22].
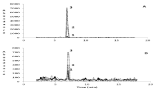
Figure 2: Selected Reaction Monitoring Chromatographs for Sample Extract
and Sample Extract with Standard Added. A, quantitative SRM 278→132 for
dm-THM; B, confirmation SRM 278→167 for dm-THM. Chromatographs: 1,
no standard added with sample extract diluted ½; 2, 2 ng/mL dm-THM added
to sample extract (diluted ½); 3, 5 ng/mL dm-THM added to sample extract
(diluted ½).
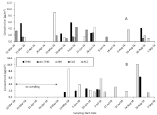
Figure 3: Concentrations of des-methyl thiamethoxam and selected
neonicotinoid insecticides detected in particles in the atmosphere at Omak,
WA. A, 2016; B, 2018. Note no samples were collected prior to May 3 in
2018. Sampling periods extend from start date noted to next start date.
Thiamethoxam (THM), Desmethyl-Thiamethoxam (dm-THM), Imidacloprid
(IMI), Clothianidin (CLO), and Acetamiprid (ACE).
The most frequently detected strobilurin fungicides in 2016 and 2018 were pyraclostrobin, trifloxystrobin, and azoxystrobin as shown in figure 4. Dimoxystrobin, fluoxystrobin, and picoxystrobin were not detected in 2016 or 2018 and usage is very low or not reported in Okanogan county and surrounding counties in 2016 or 2018 [21,22]. Kresoxim-methyl was only detected in one atmospheric particle sample in 2016. There was no trend in concentrations of strobilurin fungicides with concentrations of PM2.5 in the atmosphere and similar concentration ranges of the three most frequently detected strobilurin fungicides were observed in 2016 and 2018. Pyraclostrobin and trifloxystrobin were the most heavily used strobilurin fungicides in the Okanogan County and surrounding counties with similar usage of trifloxystrobin in 2016 and 2018 and high usage of pyrclostrobin in 2018 than 2016 in the Okanogan county although surrounding counties used similar or lower amounts in 2018 than 2016 [21,22]. Azoxystrobin usage is significantly lower than pyraclostrobin or trifloxystrobin with usage of azoxystrobin in the Okanogan County lower in 2018 than 2016 [15,16]. The relative high detection of azoxystrobin in particles in the atmosphere indicated a more regional to long-range atmospheric transport source as usage of azoxystrobin is significantly lower in the Okanogan County than some of the other counties in Washington state (Grant, Benton, Franklin, Walla Walla).
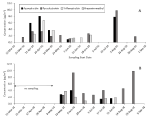
Figure 4: Concentrations of strobilurin fungicides detected in particles in the
atmosphere at Omak, WA. A, 2016; B, 2018. Note no samples were collected
prior to May 3 in 2018. Sampling periods extend from start date noted to next start date.
References
- Auteri D, Arena M, Barmaz, S, Ippolito A, Linguadoca L, Molnar T, et al. Neonicotinoids and bees: The case of the European regulatory risk assessment. Sci. Total Environ. 2017; 579: 966-971.
- Freidi A, Williams GR, Bruckner S, Neumann P, Straub L. The weakest link: Haploid bees are more susceptible to neonicotinoid insecticides. Chemosphere. 2020; 242: 125145.
- Sgolastra F, Medrzycki P, Bortolotti L, Maini S, Porrini C, Simon-Delso N, et al. Bees and pesticide regulation: Lessons from neonicotinoid experience. Biological Conservation. 2020; 241: 108356.
- Health Canada. Update on the neonicotinoid pesticides. 2020.
- United States Environmental Protection Agency. Pestiide Regristration Review; Proposed interim Decisions for Several Neonicotinoid Pesticides; Notice of Availability. EPA. 2020.
- United States Environmental Protection Agency. Dinotefuran, Proposed Interim Registration Review Decision Case Number 7441. EPA. 2020.
- United States Environmental Protection Agency. Imidacloprid, Proposed Interim Registration Review Decision Case Number 7605. EPA. 2020.
- United States Environmental Protection Agency. Clothianidin and Thiamethoxam, Proposed Interim Registration Review Decision Case Numbers 7620 and 7614. EPA. 2020.
- United States Environmental Protection Agency. Acetamiprid, Proposed Interim Registration Review Decision Case Number 7617. EPA. 2020.
- EPSA (European Food Safety Authority). Neonicotinoids: risk to bees confirmed. 2018.
- Domingues CEC, Inoue LVB, Silva-Zacarin ECM, Malaspina O. Foragers of Africanized honeybee are more sensitive to fungicide pyraclostrobin than newly emerged bees. Environ Pollut. 2020; 266: 115267.
- Batista AC, Domingues CEC, Costa MJ, Silva-Zacarin ECM. Is a strobilurin fungicide capable of inducing histopathogical effects on the midgut and Malpighian tubules of honey bees? J Apicultural Res. 2020; 59: 834-843.
- Raina-Fulton R. Determination of neonicotinoidinsecticides and strobilurin fungicides in particle phase atmospheric samples by liquid chromatographytandem mass spectrometry. J Agric Food Chem. 2015; 63: 5152-5162.
- Raina R, Belzer W, Jones K. Atmosphere concentrations of captan and folpet in the Lower Fraser Valley agricultural region of Canada. Air, Soil, and Water Research. 2009; 2: 41-49.
- Kamel A. Refined methodology for the determination of neonicotinoid pesticides and their metabolites in honey bees and bee products by liquid chromatography tandem mass spectrometry (LC-MS/MS). J Agric Food Chem. 2010; 58: 5926-5931.
- Gbylik-Sikorska M, Sniegocki T, Posynaik A. Determination of neonicotinoid insecticides and their metabolites in honey bee and honey by liquid chromatography tandem mass spectrometry. J Chromatogr B. 2015; 990: 132-140.
- Taira K, Fujioka K, Aoyama Y. Qualitative profiling and quantification of neonicotinoid metabolites in human urine by liquid chromatography coupled with mass spectrometry. PLoS One. 2013; 8: e80332.
- Raina-Fulton R. Chapter 2, Neonicotinoid Insecticides: Environmental Occurrence in Soil, Water and Atmospheric Particles. In Pesticides. Avid Sciences. 2016.
- Raina-Fulton R, Behdarvandan A. Liquid Chromatography-Mass Spectrometry for the Determination of neonicotinoid insecticides and their metabolites in biological, environmental, and food commodity matrices. Trends in Chromatography. 2016; 10: 51-79.
- Tanner G, Czerwenka C. LC-MS/MS analysis of neonicotinoid insecticides in honey: methodology and residue findings in Austrian honeys. J Agric Food Chem. 2011; 59: 12271-12277.
- US. Department of Interior, US. Geological Survey, National Synthesis Project, maps of pesticide usage data. 2020.
- Baker NT, Stone WW. Estimated annual agricultural pesticide use for counties of the conterminous United States, 2008-12: U.S. Geological Survey Data Series. 2015; 907.
- Kim S, Rahman MM, El-Aty AMA, Kabir MH, Na TW, Choi J, et al. Simultaneous detection of sulfoxaflor and its metabolites, X11719474 and X11721061, in lettuce using a modified QuEChERS extraction method and liquid chromatography-tandem mass spectrometry. J Biomedical Chromatogr. 2017: 31: e3885.
- Chen Z, Dong F, Xu J, Liu X, Cheng Y, Liu N, et al. Stereoselective separation and pharmacokinetic dissipation of the chiral neonicotinoid sulfoxaflor in soil by ultraperformance convergence chromatography/tandem mass spectrometry. Analytical and Bioanalytical Chemistry. 2014; 406: 6677-6690.
- Chen Z, Dong F, Xu J, Liu X, Cheng Y, Liu N, et al. Stereoseective determination of a novel chiral insecticide, sulfoxaflor in brown rice, cucumber, and apple by normal-phase high-performance liquid chromatography. Chirality. 2014; 26: 114-120.
- Zhang X, Li T, Zhang L, Hu T, Fu Y, Guo Z. Simutaneous determination of sulfoxaflor in 14 daily foods using LC-MS/MS. International J Envornmental Analytical Chemistry. 2019: 99: 557-567.
- Liu H, Jiang M, Li Q. Nonstereoselective dissipation of sulfoxaflor in defferent Puer tea processing. Food Sci Nutr. 2020; 8: 4929-4935.
- Kwon H, Kim C, Park B, Kim IH, Hong S, Son K, et al. Development of analytical method for picoxystrobin in agricultural commodities using GC/ ECD and GC/MS. Korean J Environ Agriculture. 2012; 31: 146-151.
- Kang D, Zhang H, Wang F, Shi L, Hu D, Zhang K. Simultaneous determination of difenconazole, trifloxystrobin and its metabolite trifloxystrobin acid residues in watermelon under field conditions by GC-MS/MS. Biomedical Chromatography. 2017; 31: e3987.
- Raina-Fulton R, Dunn N, Xie Z. Pesticides and their degradation products including metabolites: chromatography-mass spectrometry methods. Aliofkhazraei M editor. In Mass Spectrometry, Intech, Rijeka, Croatia. 2017.
- Raina-Fulton R, Behdarvandan A, Mohamad AA. The Challenges of Fungicide Analyses and Liquid Chromatography-Mass Spectrometry Methods. Austin Environ Sci. 2018; 3: 1031.
- Rutkowska E, Lozowicka B, Kaczynski P. Compensation of matrix effects in seed matrices followed by gas chromatography-tandem mass spectrometry analysis of pesticide residues. J Chromatogr. 2020; 1614: 460738.
- Kwon H, Lehotay SJ, Geis-Asteggiante L. Variability of matrix effects in liquid and gas chromatography-mass spectrometry analysis of pesticide residues after QuEChERS sample preparation of different food crops. J Chromatogr. 2012; 1270: 235-245.
- Lopez-Blanco R, Nortes-Mendez R, Robles-Molina J, Moreno-Gonzalez D, Gilbert-Lopez B, Garcia-Reyes J, et al. Evaluation of different cleanup sorbents for multiresidue pesticide analysis in fatty vegetable matrices by liquid chromatography tandem mass spectrometry. J Chromatogr. 2016; 1456: 89-108.
- Xiao Z, Yang Y, Li Y, Fan X, Ding S. Determination of neonicotinoid insecticides residues in eels using subcritical water extraction and ultraperformance liquid chromatography–tandem mass spectrometry. Analytica Chimica Acta. 2013; 777: 32-40.
- Raina-Fulton R, Xie Z. Sample preparation methods for pesticide analysis in food commodities, biological and environmental matrices. Stauffer MT editor. In ideas and applications toward sample preparation for food and beverage analysis. Intech, Rijeka, Croatia. 2017.
- Compendium Method TO-13A. Determination of Polycyclic Aromatic Hydrocarbons in Ambient Air Using Gas Chromatography/Mass Spectrometry. US Environmental Protection Agency, Office of Research and Development, Cincinnati, Ohio. 1999.
- Washington State Department of Agriculture (WSDA) Managed Pollinator Protection Plan. AGR Pub 101-681 (N/4/18). 2018.
