Abstract
The application of Municipal Solid Waste Incineration (MSWI) fly ash in asphalt mixtures is an efficient way to utilize fly ash. The feasibility of applying various kinds of dechlorinated fly ash in asphalt mixtures was comprehensively discussed in the study. The effects of types and amounts of dechlorinated fly ash on the performances of prepared asphalt mixtures were explored. Additionally, their environmental risks and economic values were further evaluated. The water absorption coefficient and porosity of fly ash-asphalt mixtures were respectively in the ranges of 0.15-0.5% and 3-4%, which met the application requirements of asphalt. The replacement of Raw Fly Ash (RFA) and Dechlorinated Fly Ash (DFA) improved Marshall stability, split strength, and Tensile Strength Ratio (TSR) of asphalt mixtures. DFA realized the more significant improvements. The leaching concentrations of heavy metals in all the prepared asphalt mixtures were lower than the detection limit, indicating that there was no risk of leaching toxicity.
Keywords: Dechlorinated MWSI fly ash; Asphalt mixture; Water stability; Leaching of heavy metals
Introduction
Due to the rapid economic development in China, the production of municipal solid wastes in China has increased year by year. Since 2015, China has replaced the USA and became the country with the largest waste production in the world [1,2]. Municipal Soil Waste Incineration (MSWI) technology with significant advantages, such as volume reduction, stabilization and resource utilization, has been developing rapidly in recent years in China. Municipal Soil Waste Incineration produces fly ash (MSWI fly ash), which is equivalent to 3 to 5% of the mass of original wastes [3-5]. Incinerated fly ash contains heavy metals (such as Zn, Cu, Pb, and Cr), a large amount of soluble salts (mainly chlorides), and persistent organic pollutants (such as dioxins). It is an internationally recognized hazardous waste [6-10]. In the HJ1134-2020 standard [11], the recommended fly ash treatment processes includes washing, curing/stabilization, molding technology, low-temperature thermal decomposition, high-temperature sintering, high-temperature melting, etc. and it is pointed out that the soluble chlorine content in fly ash treatment products should be controlled [11,12]. The most widely used dechlorination method is the washing process, which can not only remove soluble chlorides, but also bring heavy metals and other ions contained in fly ash into water to form intractable wastewater with high concentrations of salts [13,14]. In this study, the heat treatment method was used as the dechlorination pretreatment way of fly ash since it could largely remove soluble and insoluble chlorides and wrap heavy metals in a silicate-based glass matrix in order to achieve the purpose of stabilizing heavy metals [6].
In accordance with the JTG F40-2004 standard [15], it is recommended that fly ash, steel slag, ore slag, etc. can be used as fillers together with mineral powder in asphalt mixtures. Since physical and chemical properties of MSWI fly ash are close to those of coal fly ash and ore slags, some researchers have proposed to apply MSWI fly ash in asphalt mixtures in recent years [16-18]. In the study, the feasibility of the application of fly ash in asphalt mixtures was evaluated in two aspects: the pavement performance and leached heavy metals of asphalt mixtures.
Materials and Methods
Experimental materials
Raw Fly Ash (RFA) samples used in the experiment were from a grate furnace in an incineration plant in East China, where the process utilizing quicklime, activated carbon and bag filter was used for flue gas purification. RFA samples were placed in an oven at 105±5 °C and dried for 24h to remove water. After grinding and filtering through a 150-mesh sieve, pebbles, gravels and other impurities with larger particle diameter were removed. The chemical composition of RFA samples is shown in Table 1. Other physical and chemical properties of RFA samples were compared with mineral powder and dechlorinated fly ash in the following sections. In the experiment, natural basalt produced by Raofeng Stone Sale Company in Jinshan District, Shanghai was used as the aggregate and limestone ore powder (≥99.9%) was used as slag powder. In addition, SK 70# ordinary asphalt for pavement was used as asphalt.
Components
Contents (wt. %)
SiO2
16.88 ± 0.09
Fe2O3
19.37 ± 0.08
Al2O3
4.24 ± 0.09
CaO
33.53 ± 0.09
MgO
11.52 ± 0.21
ZnO
1.10 ± 0.05
CuO
0.23 ± 0.01
K2O
0.58 ± 0.03
Na2O
0.59 ± 0.07
TiO2
0.29 ± 0.00
PbO
0.28 ± 0.01
Cr2O3
0.25 ± 0.01
Others
1.12 ± 0.00
Table 1: Chemical composition of raw fly ash (wt. %).
In this study, all the chemical reagents used for fly ash dechlorination, leaching, and mixture production in the experiment were purchased from Sinopharm Chemical Reagent Co., Ltd. and mainly included iron powder (Fe), aluminum powder (Al), acetic acid (CH3COOH), nitric acid (HNO3), sulfuric acid (H2SO4), hydrogen peroxide (H2O2), sodium silicate (Na2SiO3), hydroxide sodium (NaOH), hydroxylamine (NH2OH•HCl), ammonium acetate (NH4Ac), and hydrochloric acid (HCl). All reagents are of analytical grade. Deionized water used in the experiment was produced by the DZG-303A deionized water production machine in our laboratory and stored in a bucket. N2 (purity 99.99%) used in the experiment was produced by Shanghai Weizheng Chemical Co., Ltd.
Thermal pretreatment dechlorination of fly ash
Figure 1 shows a schematic diagram of the experimental apparatus for fly ash heat treatment. It mainly consists of vertical tubular furnaces, superheated steam generator and exhaust processor. The tube furnace was heated by an electric heating wire to a preset temperature of 700°C according to a heating rate of 20°C/min. Steam was heated to 200°C in the superheated steam generator. Before heat treatment, fly ash samples and enhancement agent (Fe/Al) were put in the central reaction zone in a tube furnace. The proportion of the enhancement agent was 10%. Within heating time of 1h, steam was added into the reaction zone. Original fly ash and the three types of dechlorinated fly ash were named RFA, FA-700-1, FA-Fe-10, and FAAl- 10, respectively.
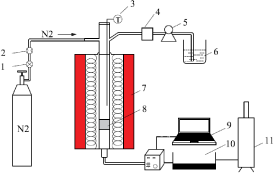
Figure 1: A flow diagram of the fly ash dechlorination apparatus. 1) Valve;
2) Flow meter; 3) Thermocouple; 4) Water vapor generator; 5) Water pump;
6) Deionized water; 7) Stainless steel vertical tube furnace; 8) Sample; 9)
Computer; 10) Dx4000 flue gas analyzer; 11) Exhaust burner.
Preparation of fly ash-asphalt mixtures
Asphalt mixtures were prepared according to the JTG E20-2011 standard [19]. The gradation type of fly ash-asphalt mixtures was AC- 13 and asphalt-aggregate ratio was 4.7%. Doping ratios of mineral powder in fly ash-asphalt mixtures in the experiment are shown in Table 2. The total doping ratio of filler was 3%. In the experiment, different ratios of fly ash (0%, 1%, 2% and 3%) was added to replace partial or all of limestone mineral powder. In the preparation process, according to the dry method, fly ash was added together with mineral powder. The other parameters were the same to those of the mixing process of hot-mix asphalt mixture.
Materials
Different particle sizes of basalt (mm)
Filler
10~15
5~10
3~5
0~3
Doping ratios /%
30
30
3
34
3
Table 2: Doping ratios of mineral powder in fly ash-asphalt mixtures.
Test methods
Pore structures (pore diameter and pore volume) and specific surface areas of fly ash and mineral powder were determined with Pore Diameter and Specific Surface Area Analyzer (3H-2000PS4, Beijing Beishide) and N2 as an adsorbent. The mineral compositions of fly ash samples were analyzed with X-ray diffractometer (XRD, BRUKER AXS D8-advance) under the conditions of Cu-Ka radiation (40kV, 40 mA), 20 = 10° to 70° and the scanning rate of 2°/min.
The elemental composition of fly ash was determined with X-ray fluorescence spectrometer (XRF, BRURER AXS SRS 3400). Fly ash samples were analyzed according to the 3050B [20] method of US Environmental Protection Agency, namely, HNO3/H2O2/HCl digestion method. According to the digestion method, heavy metals in RFA samples or dechlorinated fly ash samples were transferred into the solution for the subsequent analyses. Leaching toxicity of heavy metals was determined according to HJ/T300-2007 method [21]. Through the tank leaching tests in EA NEN 7375 standard [22], the release behaviors of heavy metals in the long-term leaching of asphalt block in the exposure scene was evaluated [23]. According to the NEN 7375 experimental method, the experimental period was divided into 8 stages (6h, 18h, 30h, 42h, 120h, 168h, 480h, and 672h respectively). After each stage, the extraction agent was replaced. The pH of the extraction agent was 3.8 and the liquid-solid ratio was 6. The species of heavy metals in fly ash were analyzed and determined by the BCR continuous extraction method proposed by the European Community Standards Agency. Through this method, heavy metals were divided into four species: acid-soluble species (F1), reducible species (F2), oxidizable species (F3) and residue species (F4). The concentrations of heavy metals (Cd, Pb, Zn, Cu, Ni, and Cr) in the fly ash digestion solution, leaching solution and BCR extraction solution were detected with an inductively coupled plasma-optical emission spectrometer (ICP-OES, Optima 2100DV).
The total chlorine and soluble chlorine in fly ashes were extracted respectively with nitric acid and deionized water according to the Japanese Industry Standard: Methods of Test for Chloride Ion Content in Hardened Concrete (JIS A1154: 2012). Then the Cl content in the extracted sample solution was measured with an ion chromatograph (ICS-1000, DIONEX, USA).
In the study, two water stability tests of fly ash-asphalt mixtures (freezing-thawing split-strength and Marshall stability) were performed to evaluate the influence of fly ash on the mechanical performances of mixtures. The freeze-thaw splitting test was performed in accordance with JTG E20-2011 test [19]. Marshall stability was measured with the stability tester (DF, Nanjing Tuoxing Instrument and Meter Research Institute).
Results and Discussion
Physicochemical properties of fly ash
Particle size distribution: The sieve analysis results of RFA and mineral powder are shown in Table 3. The particle size ranges of RFA and mineral powder were respectively 20 ~ 200 μm and 5 ~ 60 μm. The average particle size of RFA was slightly larger than that of mineral powder. Due to the higher purity of mineral powder (CaCO3 content >99.9%), its particle size distribution was more uniform.
Sieve aperture /um
1041
653
340
194.2
69.6
20.7
7.4
3.5
1.4
0.1
RFA
Pi/%
99.67
98.09
91.34
80.07
52.38
19.79
4.92
1.03
0
0
ai/%
0.33
1.58
6.75
11.27
27.69
32.6
14.87
3.89
1.03
0
Pi/%
100
100
100
99.95
95.44
57.37
23.82
11.72
4.38
0
ai/%
0
0
0
0.05
4.51
38.07
33.55
12.1
7.34
4.38
Notes: Pi refers to the passing rate; ai refers to the retained rate.
Table 3: Sieve analysis results of RFA and mineral powder.
Pore structure: Specific surface area, pore size, and pore volume of RFA were respectively 3.95 times, 1.77 times, and 6.94 times of corresponding values of mineral powder (Table 4). The three parameters of dechlorinated fly ash were smaller than corresponding values of RFA. The three parameters of FA-Fe-10 and FA-Al-10 fly ash samples were close to corresponding values of mineral powder.
Samples
Pore Size /nm
Pore Volume (cm3/g)
RFA
6.6393
10.12339
0.016803
FA-700-1
3.0211
5.90065
0.011163
1.3816
6.23799
0.005317
FA-Al-10
2.1945
5.97118
0.008551
Mineral powder
1.6797
5.76359
0.00242
Table 4: Comparison of specific surface area, pore size and pore volume of fly ash and mineral powder.
XRD patterns: Crystal compositions of RFA and dechlorinated fly ash are shown in XRD results (Figure 2). The peak with the highest intensity in the XRD pattern of RFA corresponded to calcite (CaCO3). The remaining calcium-rich crystalline phase was mainly anhydrite (CaSO4). The peak value of chlorides was also high and chlorides mainly included sodium salt (Halite, NaCl) and potassium salt (Sylvite, KCl). Compared with the materials in previous studies [24,25], RFA used in the study contained the lower content of quartz (SiO2).
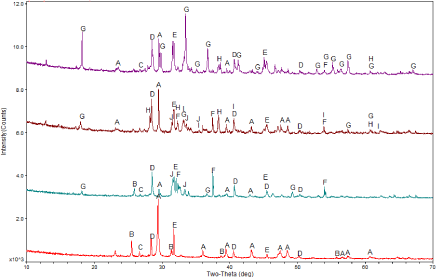
Figure 2: XRD patterns of fly ash samples: (a) RFA, (b) FA-700-1, (c) FAFe-
10, and (d) FA-Al-10. Compared with RFA, FA-700-1 had an obvious CaO
peak and a CaCO3 peak with the greatly reduced intensity, indicating that a
lot of CaCO3 was decomposed at 700°C to generate CaO. In addition, partial
Ca was combined with other elements to produce mayenite (Ca12Al14O33) and
calcium aluminum iron oxide (CaAl18Fe4O19). After dechlorination with added
Fe, an obvious peak of hematite (Fe2O3) were observed in XRD patterns of
fly ash. The addition of aluminum significantly promoted the formation of
mayenite (Ca12Al14O33).
Chlorine content: In RFA sample, total chlorine content and soluble chlorine content were respectively 10.08% and 7.12% (Table 5). Soluble chlorine accounted for 70.64% of total chlorine. Total chlorine contents in three dechlorinated samples (FA-700 -1, FAFe- 10, and FA-Al-10) were decreased significantly to 4.82%, 2.74%, and 2.03% and the soluble chlorine contents were also significantly decreased to 1.75%, 1.20%, and 1.48%, which respectively accounted for 36.31%, 43.80% and 72.91% of total chlorine contents of corresponding samples, indicating that the additions of steam and iron powder could reduce the percentage of soluble chlorine in total Cl chlorine and convert soluble chlorine into insoluble chlorine. However, added aluminum powder slightly promoted the conversion of insoluble chlorine to soluble chlorine. After dechlorination treatment, the soluble chlorine content in fly ash met the requirement in HJ 1134-2020 standard [11]. It was required that the soluble chlorine contents in treated products (high-temperature treatment products, fly ash obtained after washing treatment, etc.) should not exceed the standard limit of 2%.
Fly ash samples
RFA
FA-700-1
FA-Fe-10
FA-Al-10
Total Chlorine Content /%
10.08
4.82
2.74
2.03
Soluble Chlorine Content /%
7.12
1.75
1.20
1.48
Table 5: Chlorine contents of four kinds of fly ash.
Species of heavy metals: Figure 3 shows the species of heavy metals in four kinds of fly ash. The species of the majority of heavy metals, especially Cd, Zn and Cu, in FA-700-1 were more stable than those in RFA. The weak acid extracted species (F1) of Cd decreased from 38% to 20% and the acid-soluable species (F1) of Cr and Zn also significantly decreased because added superheated steam converted or volatilized the acid-soluble species of Zn. After dechlorination with added iron powder and aluminum powder, the species of heavy metals in fly ash became more stable. The weak acid extracted species (F1) and reducible species (F2) of most metals significantly decreased. Ni and Pb almost existed in residue species (F4). The above changes might be ascribed to the compounds generated in the reactions between heavy metals and reducing agents since these compounds had the enhanced ability to wrap heavy metals.
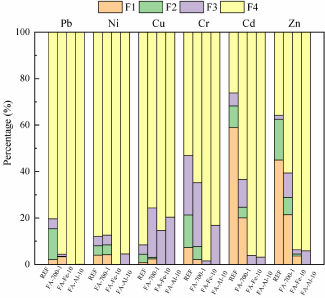
Figure 3: Distribution of species of heavy metals in four kinds of fly ash.
Leaching toxicity: In leaching results obtained according to HJ/T 300, after dechlorination with added superheated steam, leaching concentration of heavy metals from treated fly ash were lower than those from RFA. After adding Fe and Al powder, leaching concentration of heavy metals from dechlorinated fly ash were below corresponding detection limits, indicating that the addition of iron/ aluminum powder inhibited the leaching of heavy metals. Therefore, heavy metals reacted with iron in the heat treatment process to form iron-containing compounds and aluminum compounds, which had strong encapsulation properties.
Physical properties of fly ash-asphalt mixtures
The porosity of asphalt mixtures affect their durability, aging resistance and water damage resistance [26]. When the porosity of asphalt pavement is too large, water permeability of asphalt pavement is good and water easily enters the pavement structure. Under dynamic water conditions, water washes and destroys the bonding interface between the sealing layer and the asphalt surface layer, and water retained inside the pavement structure generates the pore water pressure [27]. If the temperature rises, the expansion force accelerates the flow of water, causes bonding failure, and decreases the viscous adhesion of asphalt itself and the mixture strength [28]. In addition, water penetration decreases the contact area between aggregates and asphalt, which is detached from the aggregate surface, thus reducing the mixture strength. Anti-shedding agents such as hydrated lime are generally added to improve the asphalt performance [29]. When the temperature decreases, according to the principle of freeze-thaw failures, the frost heaving force causes cracks and other defects on asphalt pavement [30].
Water absorption rates of all the fly ash-asphalt mixtures were between 0.15% and 0.5% and meet the requirements (less than 2%) in the JTG E20-2011 standard [19]. The porosity of all the fly ash-asphalt mixtures ranged from 3% to 4% and met the requirements (3% to 6%) in the JTG F40-2004 standard [15].
Marshall stability test
Marshall stability test is commonly used to determine the failure load and deformation capacity of asphalt mixture specimens [31,32]. In this study, Marshall stability test was performed according to T0709 method in the JTG E20-2011 test [19] in order to determine the optimum proportion of fly ash-asphalt mixtures. The mixtures was kept in constant temperature water bath at 60°C for 30-40 min to determine Marshall stability.
Marshall stability data of 4 types of fly ash-asphalt mixtures are shown in Figure 5. The Marshall stability value of the mineral powderasphalt mixture was 9.345kN. After mineral powder was replaced by RFA and dechlorinated fly ash, the strength of asphalt mixtures was increased to a certain degree. Except FA-700-1, the other three mixtures showed the increased Marshall stability and the increased proportion increased with the increase in the doping ratio of fly ash. When the doping ratios of RFA, FA-Fe-10, and FA-Al-10 were 3%, the Marshall stability values of corresponding asphalt mixtures were respectively 11.910kN, 11.580kN, and 13.345kN, which were respectively increased by 27.4%, 23.92%, and 42.80% compared to that of the mineral powder-asphalt mixture. As for FA-700-1 dechlorinated fly ash, with the increase in the doping ratio of fly ash, the Marshall stability value of the mixture firstly increased and then decreased. The combination of 1% FA-700-1 and 2% mineral powder corresponded to its highest Marshall stability value, 11.405kN.
Figure 4: Water absorption and porosity of asphalt mixture specimens.
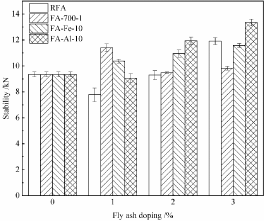
Figure 5: Marshall stability test results of different fly ash-asphalt mixtures.
Among the 4 alternative fillers, FA-Al-10 realized the most significant stability improvement effect, followed by FA-Fe-10. Slurry prepared with FA-Al-10 and FA-Fe-10 showed the high bonding strength with aggregates. Other scholars also reported that: due to the developed void structure, a large specific surface area, and a lot of highly active transition elements, fly ash strengthened the adsorption with the asphalt medium, thus improving the stability performance of mixtures [33].
According to JTG D50-2017 standard [34], the Marshall stability for expressways and first-class highways should be no less than 8kN and the Marshall stability of common road is no less than 5kN. All the above specimens met the standard.
Freezing-thawing split test
The freezing-thawing split-strength and not freezing-thawing split-strength and Tensile Strength Ratio (TSR) of different asphalt mixtures are shown in Figure 6 and Table 7. As for the asphalt mixtures of RFA, FA-700-1, and FA-Fe-10, the not freezing- thawing split-strength and freezing-thawing split-strength of the mixture specimens with fly ash contents of 1% and 2% were lower than the split-strength of the mineral powder-asphalt mixture. When the fly ash content increased to 3%, the not freezing-thawing split-strength and freezing-thawing split-strength of the fly ash-asphalt mixtures were higher than those of the mineral powder-asphalt mixture. When the fly ash content was 3%, the not freezing- thawing splitstrength values of the asphalt mixtures of RFA, FA-700-1, and FAFe- 10 were respectively increased by 11.30%, 8.79%, and 5.96% and the freezing-thawing split-strength values of the asphalt mixtures of RFA, FA-700-1, and FA-Fe-10 were respectively increased by 18.27%, 40.45%, and 50.05%. As for the asphalt mixture of FA-Al-10, the not freezing- thawing split-strength and freezing-thawing split-strength were greatly increased by 12.20%-19.00% and 64.39%-85.04%. In the freezing-thawing cycle, the split strength values of four fly ash-asphalt mixtures were decreased according to the following order: FA-Al- 10>FA-Fe-10>FA-700-1>RFA, indicating that dechlorinated fly ash could more significantly improve freezing-thawing split-strength than RFA.
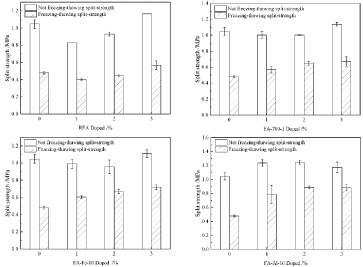
Figure 6: Freezing-Thawing Split results of different fly ash-asphalt mixtures.
Leaching method
Fly ash samples
Leaching concentration of heavy metals (mg/L)
Pb
Cd
Cu
Zn
Ni
Cr
HJ/T 300
RFA
5.69 ± 0.16
2.87 ± 0.13
6.61 ± 0.24
33.56 ± 1.45
0.20 ± 0.00
0.13 ± 0.00
FA-700-1
0.10 ± 0.00
0.16 ± 0.01
0.11 ± 0.01
0.17 ± 0.03
0.03 ± 0.00
ND
FA-Fe-10
ND
ND
ND
ND
ND
ND
FA-Al-10
ND
ND
ND
ND
ND
ND
Table 6: Leaching concentration of heavy metals from four kinds of fly ash.
Fly ash contents
RFA
FA-700-1
FA-Fe-10
FA-Al-10
0
45.86 ± 0.96
45.86 ± 0.96
45.86 ± 0.96
45.86 ± 0.96
1%
48.51 ± 1.25
57.27 ± 6.56
60.93 ± 1.59
63.29 ± 7.74
2%
47.82 ± 0.21
64.91 ± 2.11
70.63 ± 8.45
71.31 ±3.32
3%
48.68 ± 4.33
59.28 ± 6.64
65.09 ± 5.02
75.19 ± 0.95
Table 7: Tensile strength ratios (TSR) of different fly ash-asphalt mixtures.
Tensile Strength Ratios (TSR) of four fly ash-asphalt mixtures were significantly higher than that of the limestone-asphalt mixture, the blank group (Table 7). As for asphalt mixtures with 1-3% RFA, the TSR value was increased by 4.28%-6.15%. As for the two fly ashasphalt mixtures of FA-700-1 and FA-Fe-10, when the content of fly ash was not more than 2%, the TSR increased with the increase in the fly ash content. When the content of fly ash was 2%, the TSR values of the two mixtures were the largest and respectively reached 64.91% and 70.64%, which were respectively 41.54% and 54.02% higher than that of the control group (pure the limestone-asphalt mixture). When the content of fly ash exceeded 2%, the TSR values of asphalt mixtures decreased. In addition, the TSR value of asphalt mixture of FA-Al-10 increased with the increase in the content of fly ash. When the content of FA-Al-10 was 3%, the TSR of the mixture reached 75.20%. The above results showed that the incorporation of fly ash, especially dechlorinated fly ash, significantly improved the water stability of mixtures. The improvement might be interpreted as follows. Firstly, after highly alkaline fly ash was added, carboxylic acid in asphalt easily reacted with Ca-contained substances in fly ash to form alkaline salts, which had the developed adsorption performance and strengthened adhesion between aggregate and asphalt. Second, after fly ash was incorporated, the porosity of asphalt mixtures decreased and external moisture could not easily enter the internal structure of asphalt mixtures, thus enhancing its resistance to water erosion.
When the content of fly ash exceeded 2%, the water stability of the fly ash-asphalt mixture decreased. Qiao [35] also found that when the content of fly ash exceeded 2.5%, the water stability of the fly ash-asphalt mixture decreased. The result might be interpreted as follows. The hydrophilicity of fly ash was greater than that of limestone mineral powder. When the addition of fly ash was too large, too much water molecules were adsorbed in and around fly ash. Water molecules were polar substances and easily combined with aggregates, thus decreasing the adhesion of asphalt to aggregates. In addition, too much fly ash caused the too large specific surface area of the mineral material and reduced the thickness of the asphalt membrane. Therefore, water molecules more easily penetrated the asphalt membrane, caused asphalt to fall off the surface of aggregates and reduced the water stability [36].
Leaching Tests of Heavy Metals in Asphalt Mixtures
In the study, the leaching experiment was performed according to HJ/T 300 leaching procedure and EA NEN 7375 water tank leaching procedure. After leaching, the filtrate was analyzed by ICP for detecting heavy metals. It was found that the leaching concentrations of heavy metals in all the mixture samples were below corresponding detection limits. The result might be interpreted as follows. After heat treatment, heavy metals in fly ash were wrapped into the silicate-based matrix, thus stabilizing heavy metals. In addition, asphalt could also wrap heavy metals inside through adsorption [37,38]. Accordingly, dechlorinated fly ash applied in asphalt mixtures had no leaching risk of heavy metals.
Conclusion
In the study, the feasibility of applying dechlorinated fly ash in asphalt pavement was explored. The main conclusions are drawn as follows:
The physical properties (water absorption and porosity) of fly ash-asphalt mixtures met the related application requirements. The application of RFA and dechlorinated fly ash could improve the stability of asphalt mixtures to a certain degree and the improved stability exceeded 8kN specified in accordance with the JTG D50- 2017 standard [34]. The replacement of mineral powder by fly ash could increase the freezing-thawing split-strength and not freezing- thawing split-strength and tensile strength ratio of asphalt mixtures. In other words, the replacement of mineral powder by fly ash improved the water stability of asphalt mixtures. The leaching contents of heavy metals in intermittent and continuous leaching tests were below corresponding detection limits, indicating that fly ash-asphalt mixtures has no leaching toxicity risk of heavy metals.
Acknowledgment
The study was financially supported by National Key R&D Program of China (Grant number: 2018YFD1100600)
Availability of Data and Material
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Ma W, Chen D, Pan M, Gu T, Zhong L, Chen G, Yan B, Cheng Z. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: A comparative study. J. Environ. Manage. 2019; 247: 169-177.
- Liu DG, Ke Y, Min XB, Liang YJ, Jiang GH. Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System. Int. J. Env. Res. Pub. He. 2019; 16: 156.
- L Xu, Wu Q. Status and Development Trend of Harmless and Resourceful Disposal of Municipal Solid Waste Incineration Fly Ash. Adv. Environ. Pro. 2017; 7: 414-422.
- Linh HN, Tamura H, Komiya T, Saffarzadeh A, Shimaoka T. Simulating the impact of heavy rain on leaching behavior of municipal solid waste incineration bottom ash (MSWI BA) in semi-aerobic landfill. Waste Manage. 2020; 113: 280-293.
- Huang K, Fan X, Gan M, Ji Z. Use of Municipal Solid Waste Incinerator (MSWI) Fly Ash in Alkali Activated Slag Cement. Magnesium Technology. 2019; 19: 401-410.
- Zhao K, Hu Y, Wang Y, Chen D, Feng Y. Speciation and risk assessment of heavy metals in MSWI fly ash during thermal processing. Energ. Fuel. 2019; 33: 66-77.
- Aubert JE, Husson B, Sarramone N. Utilization of municipal solid waste incineration (MSWI) fly ash in blended cement Part 1: Processing and characterization of MSWI fly ash. J. Hazard. Mater. 2006; 136: 624-631.
- Lederer J, Trinkel V, Fellner J. Wide-scale utilization of MSWI fly ashes in cement production and its impact on average heavy metal contents in cements: The case of Austria. Waste Manage. 2016; 60: 247-258.
- Paula C, Pardo Bronte, Tilbrook, Erik, van, Ooijen, Abraham, Passmore. Wide-scale utilization of MSWI fly ashes in cement production and its impact on average heavy metal contents in cements: The case of Austria. Scientific Reports. 2019.
- Zheng L, Gao X, Wang W, Li Z, Zhang L, Cheng S. Utilization of MSWI fly ash as partial cement or sand substitute with focus on cementing efficiency and health risk assessment. Front. Env. Sci. Eng. 2020; 14: 87-97.
- MEEPRC. Technical specification for pollution control of fly-ash from municipal solid waste incineration. Ministry of Ecology and Environment of the People’s Republic of China. 2020.
- Du B, Li J, Fang W, Liu J. Comparison of long-term stability under natural ageing between cement solidified and chelator-stabilised MSWI fly ash. Environ. Pollut. 2019; 250: 68-78.
- Zhao K, Hu Y, Tian Y, Chen D, Feng Y. Chlorine removal from MSWI fly ash by thermal treatment: Effects of iron/aluminum additives. J. Environ. Eng. Sci. 2020; 88: 112-121.
- Chen WS, Chang FC, Shen YH, Tsai MS, Ko CH. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012: 237-238: 116-120.
- MOT. Technical Specification for Construction of Highway Asphalt Pavements. Ministry of Transport of the People’s Republic of China. 2004.
- Cho BH, Nam BH, An J, Youn H. Municipal Solid Waste Incineration (MSWI) Ashes as Construction Materials-A Review. Mater. 2020; 13: 3143.
- Romeo E, Mantovani L, Tribaudino M, Montepara A. Reuse of stabilized municipal solid waste incinerator fly ash in asphalt mixtures. J. Mater. Civ. Eng. 2018; 30: 04018157.1-04018157.10.
- Mirkovic K, Tosic N, Mladenovic G. Effect of Different Types of Fly Ash on Properties of Asphalt Mixtures. Adv. Civ. Eng. 2019: 1-12.
- MOT. Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. Ministry of Transport of the People’s Republic of China. 2011.
- EPA. Acid Digestion of Sediments, Sludges and Soils. Environmental Protection Agency. 1996.
- MEEPRC. Solid waste-Extraction procedure for leaching toxicity-Acetic acid buffer solution method. Ministry of Ecology Environment of the People’s Republic of China. 2007.
- EA. Leaching characteristics of moulded or monolithic building and waste materials. Determination of leaching of inorganic components with the diffusion Test. ‘The tank test’ based on the translation of the Netherlands Normalization Institute Standard. Environment Agency. 2004.
- Lampris C, Stegemann JA, Pellizon-Birelli M, Fowler GD, Cheeseman CR. Metal leaching from monolithic stabilised/solidified air pollution control residues. J. Hazard. Mater. 2011; 185: 1115-1123.
- Miao Q, Hao Z, Ping C, Hui XU, Yanxu G. Basic characteristics of municipal solid waste incineration fly ash in Hangzhou, China. J. Zhejiang. Sci-Tech. Univ. 2018.
- Long L, Jiang X, Lv G, Chen Q, Liu X, Chi Y, et al. Characteristics of fly ash from waste-to-energy plants adopting grate-type or circulating fluidized bed incinerators: a comparative study. Energ. Source. Part. A. 2018.
- Du, Ling X, Haibin D, Deyi D, Weidong L. Evaluation of thermal behavior and high-temperature performances of asphalt mixture containing fly ash cenosphere. Constr. Build. Mater. 2020; 245: 118429.
- Wang W, Wang L, Yan G, Zhou B. Evaluation on moisture sensitivity of asphalt mixture induced by dynamic pore water pressure. Int. J. Pav. Re. Te. 2020.
- Wang W, Wang L, Xiong H, Luo R. A review and perspective for research on moisture damage in asphalt pavement induced by dynamic pore water pressure. Constr. Build. Mater. 2019; 204: 631-642.
- Kanghun, Lee Manho, Kook Dongho, Ha Sooahn, Kwon Moonsup. Evaluation of Moisture Resistance of Hot-Mix Asphalt Mixture Containing Refined Air- Cooled Slag for Road Pavements. 2018.
- Xu G, Yu Y, Cai D, Xie G, Yang J. Multi-scale damage characterization of asphalt mixture subject to freeze-thaw cycles. Constr. Build. Mater. 2020; 240: 117947.
- Du Yinfei, Chen Jiaqi, Han Zheng, Liu Weizheng. A review on solutions for improving rutting resistance of asphalt pavement and test methods. Constr. Build. Mater. 2018; 168: 893-905.
- Istiar, Adi TJW, Sutikno, Rahmat, Kencanawati M, Siara I, et al. The effect of laterite stone as filler on marshall stability hot mix asphalt AC-WC. Matec Web of Conferences. 2019: 258.
- Ping WU, Wang XC. Study on performance of hot recycled asphalt mixture mixed with fly ash. Highway Tran. Inner. Mon. 2016.
- MOT. Specifications for Design of Highway Asphalt Pavement. Ministry of Transport of the People’s Republic of China 2017.
- Qiao J, Miaomiao H, Ying W, Can C, Zhigang LI. Pavement Performance of Municipal Solid Waste Incineration Fly Ash Asphalt Mixture. Journal of Materials ence and Engineering. 2019; 37: 291-295.
- Chen J, Yin X, Wang H, Ding Y. Evaluation of Durability and Functional Performance of Porous Polyurethane Mixture in Porous Pavement. J. Clean. Prod. 2018; 188: 12-19.
- Choi MJ, Kim YJ, Kim HJ, Lee JJ. Performance evaluation of the use of tirederived fuel fly ash as mineral filler in hot mix asphalt concrete. J. Traf. Trans. Eng. 2020; 7: 249-258.
- Zhao P, Jing M, Feng L, Min B. The heavy metal leaching property and cementitious material preparation by treating municipal solid waste incineration fly ash through the molten salt process. Waste Manage. Res. 2020; 38: 27-34.
