Abstract
The occurrence of the invasive snowflake coral, Carijoa riisei (Duchassaing & Michelotti, 1860) in Malaysian waters was reported after the species was first detected at Pulau Payar Marine Park in 2014. Its recent appearance in Pulau Payar highlights the need for baseline data concerning the distribution pattern and diversity of C. riisei within the Pulau Payar Marine Park and may enable effective remedial actions in controlling the overgrowth of this octocoral. The snowflake coral and other substrates were quantified over quadrats located randomly on a 50 m transects in three study sites, i.e. Coral Garden, Kaca Reef and Lembu Rock. The percent cover of each substrate category and diversity were determined using the Coral Point Count with Excel extensions (CPCe) software. The snowflake coral was most frequently observed at the depths of 10 to 20 m at each of the study sites. The area with highest coverage of this octocoral was in Kaca Reef at the depth of 20m. About one-third of the benthos and abiotic substrate at all sites was populated by C. riisei. Statistically, no significant differences were found between the distributions of C. riisei by sites. Using diversity indices, we were able to demonstrate the ability of this species to exploit a wide range of differing environments. This ability has allowed it to spread within this marine protected area. Based on this study, we suggested monitoring programs should be regularly conducted within Pulau Payar Marine Park, other reefs should be surveyed for its presence, and an effective mitigation program should be developed for the conservation of the marine ecosystems affected by this invasive species.
Keywords: Invasive species; Snowflake coral; Octocorallia; Marine protected area; Peninsular malaysia
Introduction
Invasive Alien Species (IAS) are one of the main threats to biodiversity, often adversely affecting ecosystems on a large scale. The spread of invasive alien species can have negative impacts on the environment, human health, animals and plants, and the economy [1]. However, information regarding the existence and impact of marine IAS from Malaysian waters is lacking. Recently, there have been 10 marine IAS documented from Malaysian waters. Of these, a single report of invasive coral species was recorded in Peninsular Malaysia [2].
Carijoa riisei (Duchassaing & Michelotti, 1860), globally known as snowflake coral, is a non-photosynthetic colonial octocoral native to the Indo-Pacific [3-6], although its original native distribution is still unknown [7]. Being a shade-loving species, this coral is commonly found on hard substrates, away from direct sunlight. It has been observed growing on rocks, on substrates covered with sediment and even on other organisms such as sponges, corals, and bivalves [5,8]. It also grows well on artificial hard surfaces such as concrete, metal, and plastic [9]. It has been reported on sunken ships, pier pilings [10] and artificial reefs visited by SCUBA divers [6].
The snowflake coral is a fast growing species with its linear branch growth rate exceeding one cm per week and its axial polyp extension reaching between 0.5 to 2.0 cm per week [8,10]. This species can reach sexual maturity in a few months once its branches reach 2.5 cm, and then it appears to reproduce continuously [11]. This species was observed to overgrow and successfully compete with black coral and other native invertebrate forms [8]. Because of its ability to dominate space and crowd out other marine organisms, snowflake coral is considered as a wide-spread invasive fouling species [7] and is listed in the database of invasive species of IUCN [6].
The first invasion of C. riisei was documented in Hawaii in 1972 [11]. Since then, it has invaded many countries in the Caribbean- Atlantic Ocean as well as the Indo-Pacific [3]. In South East Asia, C. riisei has reportedly spread to Indonesia [12], Vietnam [13], and allegedly, Thailand and the Philippines [8]. This highly invasive species was discovered for the first time in Malaysia in 2014, at a depth of more than 10m, in Pulau Payar [14]. Unfortunately, detailed data on the degree of its impact is lacking. The means of the snowflake coral’s invasion of Pulau Payar is still unknown. Since this marine park is in close proximity to Indonesia and Thailand, there is a likelihood of its larvae were transported by water currents from these neighboring countries.
Baseline data concerning the status of C. riisei in Pulau Payar Marine Park is necessary to enable effective remedial actions in controlling the spread of this invasive species. It is vital to know its distribution, its impact on native species, and its ecological preferences (depth, substrates, etc.). Therefore, the aim of this study was to assess the current distribution and quantify the density of the invasive C. riisei within this marine park waters.
Materials and Methods
Study area
Payar Archipelago is located about 28km off the coast of Kedah state, on the west coast of Peninsular Malaysia. It consists of four islands, the largest being Pulau Payar, and the other three islands being Pulau Kacha, Pulau Lembu and an offshore island, Pulau Segantang. Since 1994, it has been gazetted as the Pulau Payar Marine Park (PPMP) under the Malaysia’s Fisheries Act 1985 (Amended 1991). This archipelago is the only marine park situated in the west coast of Peninsular Malaysia and is the most biodiverse coral reef in the Straits of Malacca. The waters surrounding the islands support 38 genera of coral [15] and 48 species of coral reef fish [16]. This present study focused on the C. riisei population at three representative sites i.e. Coral Garden (CG) of Pulau Payar, Kaca Reef (KR) of Pulau Kacha, and Lembu Rock (LR) of Pulau Lembu (Figure 1).
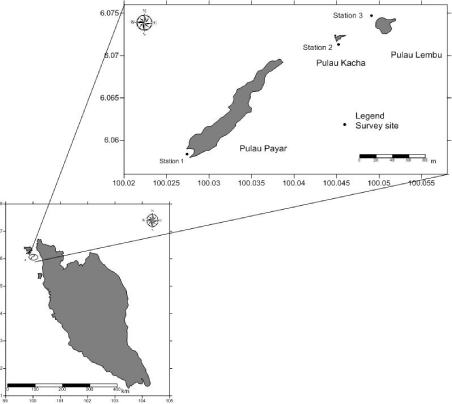
Figure 1: Sampling stations (Coral Garden (Station 1), Kaca Reef (Station 2) and Lembu Rock (Station 3)) at PPMP.
Survey method
An underwater survey was conducted from March to October 2015 using a photo-quadrat method. The photo quadrat method has been widely used in assessing marine organism community’s conditions, including coral habitats [17]. Four quadrats of 0.5m by 0.5m (0.25m2) were randomly placed and sampled along a 50m transect positioned parallel to three selected water depth contours (10, 15 and 20 m). The distance between each quadrat at the same depth was approximately 10m. Digital photo quadrats were captured using Canon G16 with underwater housing Canon WP-DC52 fixed to a PVC frame holding the camera approximately 60cm distance from the substrate below. Most identification of the substrates was determined at field. Some of the water’s physical characteristics, seabed features and native species visible at the site were also recorded.
Data analysis
The Coral Point Count with Excel extensions (CPCe) version 4.1 software was used to analyses the photo quadrat digital images [18]. A total of 100 random points were overlaid within the marked border boundary on the image. The estimation of relative cover on each image was then based on five major substrate categories; namely Carijoa riisei (CR), Hard Coral (HC), other Soft Coral (SC), other biotic organisms (sponges, hydrozoan, clams etc.) (O), and abiotic material (rubble, rock, sand) (RSS). The Excel spreadsheet was generated after the data had been collected. The spreadsheet contents included statistical parameters of each substrate type (mean and standard deviation) and diversity indices (Shannon-Weaver and Simpson). The result from the CPCe analyses for percentage coverage was used to assess the distribution of C. riisei and substrate category diversity and evenness were calculated for each site using the Shannon-Winer and Simpson’s indices. According to Magurran [19], the Shannon-Weaver Index (H) increases as both the richness and the evenness of the community increase with the typical values are generally between 1.5 and 3.5 in most ecological studies and the index is rarely greater than 4. While Simpson’s Index (1-D) takes into consideration the number of categories present, as well as the abundance of each category. The value of this index ranges between 0 and 1, with greater values representing greater sample diversity. The diversity index also increases with increasing evenness among the abundances of each substrate category.
Results and Discussion
Carijoa riisei was mostly found growing on the surface and crevices of rock substrates at depths of between 8 to 22 m (Table 1). This octocoral was not observed attached on any substrate deeper than 25m. In Indonesia, however, this octocoral was recorded to 30m depth [12]. At the depth of 10m, C. riisei was found more commonly on walls and boulders while the presence of this species at the depth more than 20 meter was usually in patches depending on type of substrates. This finding is in accordance with Sánchez and Ballesteros [10], where they recorded dense but sporadic stands of C. riise, at depths of up to 15m, in Colombia. In Ecuador, this snowflake coral typically occurred on vertical surfaces in depths between 5 and 17 m [5,20].
Description
Coral Garden
Kaca Reef
Lembu Rock
Site Characteristics
a) Bottom temperature (oC)
29 - 30
29 - 30
29 - 30
b) Current
Moderate
Moderate
Moderate
c) Distance visibility (m)
2 - 3
2 - 3
2 - 3
d) Water depth* (m)
10 - 22
8 - 20
10 - 20
e) Seabed features (In order of dominance)
Mostly boulders & bedrock; course gravel; sand
Mostly sand; boulders; coarse gravel; small stone
Mostly sand; boulders and bedrock; coarse gravel
Native Biota Recorded
a) Favites spp.
v
b) Montipora spp.
v
c) Porites spp.
v
d) Tubastrea spp.
v
v
v
e) Turbinaria spp.
v
f) Dendronephthya spp.
v
v
g) Juncella spp.
v
v
h) Sponges
v
v
i) Hydrozoans
v
v
j) Sea anemones
v
Note: *Water depth where C. riisei was observed.
Table 1: Sampling sites characteristic and native biota recorded with C. riisei during the survey.
The physical characteristics at the study sites were very similar consisting of boulders, and coarse gravels, as shown in Table 1. The ability to dominate large areas of reef under conditions of low light, moderate current flow and the presence of hard substrata has allowed this species to invade vast areas, ranging from the intertidal zone to depths of more than 100m [21].
Native species commonly found growing with C. riisei were also recorded. We observed the competition for spaces between the snowflake coral and a number of endemic species such as Tubastrea micrantha (black tree coral) and the delicate carnation coral, Dendronephthya spp. Abdullah [14] reported that the presence of C. riisei in PPMP was particularly conspicuous at CG site, where this species was found to be over-running Dendronepthya spp. However, no quantitative data was produced in his findings. Thus, no comparison of distribution from the first sighting at PPMP can be made with the current study. In Columbia and Ecuador, C. riisei was observed to outcompete other species of soft corals [10,20].
Carijoa riisei was also found growing on other stationary species including corals and shells (Figure 2). Sessile organisms like corals and shellfish were readily colonized wherever there was a firm surface on which C. riisei could attached it stolons (root-like structure) [9,11]. The ability of C. riisei to settle and grow on stationary organisms increases the chances of the successful dispersal of larvae leading to a wide range of distribution of this species [8]. Carijoa riisei was also found growing on open substrates (such as sand) and substrates covered with sediment, as shown in Figure 2.
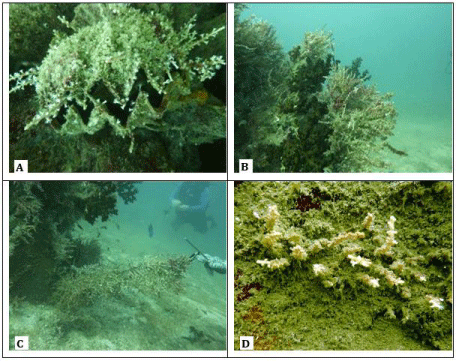
Figure 2: Colonization of the snowflake coral on different type of substrates,
i.e. zig zag oyster (A), black-tree coral Turbinaria sp. (B), sand (C), sedimentcovered
substrate (D).
Table 2 shows the coverage of substrates at each surveyed site. The highest coverage of C. riisei was observed at LR (39.72±22.76%), followed by CG (37.70±30.01%) and KR (34.57±23.22%). A Oneway ANOVA showed that there was no significantly difference in the distribution of C. riisei among these sites; F(2,27) = 0.0931<3.3541=F crit, p=0.9114 (a=0.05). The snowflake coral was equally distributed in CG, KR and LR indicating that there were few obstacles in these areas to its aggressive colonization. A similar result was recorded in Ecuador where the coverage of C. riisei was between 20.2% to 44.6% [5].
% Coverage (Mean ± SD)
CG (n = 12)
KR (n = 12)
LR (n = 12)
C. riisei
37.70 ± 30.01
34.57 ± 23.22
39.72 ± 22.76
Other corals
16.88 ± 19.38
5.83 ± 7.27
9.07 ± 10.61
Other biotic
8.22 ± 15.82
5.79 ± 8.79
1.46 ± 3.67
Abiotic
37.19 ± 24.13
53.82 ± 16.95
49.75 ± 19.78
Total
100
100
100
Note: n = number of samples.
Table 2: Mean coverage (%) of C. riisei and other benthos surveyed in PPMP.
About one-third (34-38%) of the substrate at all sites was populated by the snowflake coral, while cover for native species and abiotic substrates were about 10-25% and 37-54% respectively. The composition of native species (corals and other living substrates) and abiotic substrates in the previous study at PPMP were 23-34% and 66- 77% respectively [16]. The current study indicates that the presence of C. riisei may have continued to alter the composition ratio of the substrates since it was first reported. This octocoral is known, under favourable condition, to out compete other organisms and saturate the available space [11].
There were no significant differences in the C. riisei coverage between depths for sites CG and LR, as determined by the Oneway ANOVA, with the F value and p-value of F=1.810; p=0.218 and F=0.742; p=0.497 respectively. The snowflake coral was evenly distributed at all three depths. However, there was a significant difference in the C. riisei coverage among the three water depths at KR with the value of F=5.975; p=0.037<a=0.05 (Table 3). The result shows that the coverage of C. riisei at KR was unevenly distributed and became denser in the deeper area. The depth with highest coverage of C. riisei was at 20m. The differences in distribution of snowflake coral at KR could be indicative of differences in reef morphology, current regimes or other physical parameters. These will be looked into in future studies and compared with physical differences at other sites.
C. riisei % Coverage (Mean ± SD)
Sites
10m
15m
20m
F
p-Value
F crit
CG
28.37 ± 7.58
52.14 ± 40.94
13.75 ± 27.5
1.81
0.218
4.256
KR
3.00 ± 5.20
27.11 ± 15.63
50.54 ± 24.08
5.975
0.037
5.143
LR
30.28 ± 25.58
45.74 ± 29.79
27.24 ± 21.20
0.742
0.497
3.885
Table 3: Summary of statistical test results (One-way ANOVA) on significant differences for each site among the three water depths of 0 m, 15 m and 20 m.
All sites had negative correlations between the percentage coverage of C. riisei and substrate (abiotic component, RRS) (Figure 3). Carijoa riisei population distribution in the CG (Figure 3a) was scattered and had a weak negative correlation or almost no correlation between the C. riisei cover and the substrate, (r=-0.578). While C. riisei population distribution in the KR (Figure 3b) and LR (Figure 3c) were more uniform and had strong negative correlations, with RRS (r=-0.8976 and r=-0.8933 respectively). Correlation analysis found that, there was no statistically significant difference in correlations between the presence of the C. riisei and the coverage of the substrate in CG, as p=0.0801>alpha=0.05. However, the p-value for the other two sites, KR and LR showed a significant difference on the presence of this species and the distribution of the substrate existing in the area.
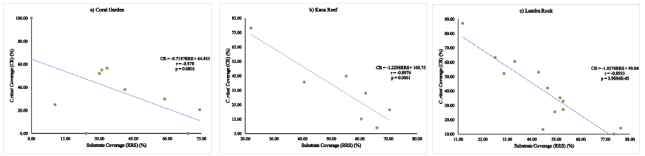
Figure 3: Correlation between percentage coverage of C. riisei and substrate (RRS) at each of the study site; a) CG b) KR and c) LR.
At each site, the relationships between C. riisei coverage and RSS coverage dominate the quadrates, i.e. either corals and other biota appear to be slow to colonize RSS and/or C. riisei is quick to occupy this “vacant space”. The availability of hard substrates such as boulders and walls provide space for this invasive species to grow and reproduce. We can see in Figure 2 that the percentage cover of C. riisei can increase to the point that little open space is available for other colonizers. While C. riisei did overgrow other biota, it appeared to more quickly dominate open areas without competition from other species. This invasive species was reported to “saturate” available habitats and reach densities of over 1,600 axial polyps per square meter in Hawaii [8].
Diversity indices are a valuable tool allowing us to describe community structure numerically [22]. Here we have used the Shannon-Weaver (H) and Simpson’s (1-D) Indices to describe the relative contribution made by each “biotic category” in increasing the diversity of an RSS environment at each of the study sites. With our richness limited to two categories, the absence of one category from a sample site causes a large reduction in diversity, e.g. the low diversity of “other biotics”. Alternatively, the wide-ranging distribution of the invasive C. riisei across the sample sites maximizes richness and increases evenness. The high diversity indices for C. riisei on high RSS sites reflect the ubiquitous nature of this aggressive invader. Table 4 shows the indices calculation, where the diversity of C. riisei and abiotic (RRS) found at each of the sites showed the highest average value (C. riisei: H=0.29, 1-D=0.20/RRS: H=0.30, 1-D=0.26). The diversity of other corals (hard coral and other soft coral) and other biotic organisms was much lower, in the average range of H=0.18, 1-D=0.03 and H=0.09, 1-D=0.01 respectively.
Site
CG
KR
LR
Average
Category
H
1-D
H
1-D
H
1-D
H
1-D
Carijoa riisei
0.27
0.22
0.3
0.21
0.3
0.17
0.29
0.2
Other corals
0.23
0.06
0.17
0.02
0.13
0.01
0.18
0.03
Other biotics
0.12
0.03
0.04
0
0.11
0.01
0.09
0.01
Table 4: The Shannon-Weaver (H) and Simpson’s (1-D) Indices for the C. riisei and other categories in the study sites.
Conclusion
The invasive species C. riisei, the snowflake coral, could pose a serious hazard to the coral reef environments in Malaysia. The occurrence of this invasive coral in PPMP is a great concern because of its aggressive nature. Although C. riisei has only been detected at 3 sites in PPMP, the rapid growth attributed to this species is likely to pose a serious threat to the native species that contribute to the rich marine biodiversity of PPMP. The information provided here would be important for monitoring purposes and surveys that are more extensive in the future. So much so, it is worth for another quantitative survey.
It is crucial to continue evaluating the possible spread of C. riisei in PPMP, in order to assess the threat of this species. Detailed surveys using permanent monitoring plots should be conducted across time in the affected area, to monitor the changes in the coral diversity. Plots must also be established to monitor newly colonized areas around the reefs. Regular and efficient monitoring programs are crucial in order to develop a mitigation plan to deal with this invasive species and reduce the risk to other nearby islands such as Langkawi Archipelago and Songsong Archipelago. Further studies on the distribution of C. riisei in other areas within Malaysia’s waters, historical-geographical relationships and the impact of this species on Malaysia’s marine ecosystem need to be carried out.
Acknowledgements
The authors wish to thank the Marine Park and Resource Management Division (formerly known as the Department of Marine Park Malaysia) of Department of Fisheries, Malaysia for making this project possible. The assistance of Dr. Ho Yuek Ming (Universiti Kebangsaan Malaysia) is greatly acknowledged. The staff of PPMP who have contributed to this research are also appreciated.
References
- National Committee on Invasive Alien Species Malaysia. Invasive alien species in Malaysia. Department of Agriculture Malaysia, KL, MY. 2018: 113.
- Department of Agriculture Malaysia. National action plan on invasive alien species 2021-2025. Department of Agriculture Malaysia, Putrajaya, MY. 2021: 74.
- Concepcion GT, Kahng SE, Crepeau MW, Franklin EC, Coles SL, Toonen RJ. Resolving natural ranges and marine invasions in a globally distributed octocoral (genus Carijoa). Mar. Ecol. Prog. Ser. 2010; 401: 113-127.
- Dhivya P, Sachithanandam V, Mohan PM. New record of Carijoa riisei at Wandoor-Mahatma Gandhi marine national park [MGMNP], Andaman and Nicobar Islands, India. Indian J. Mar. Sci. 2012; 41: 212-214.
- Cardenas-Calle M, Perez-Correa J, Uzca-Sornoza C, Bigatti G, Diez N, Lozada M, et al. Invasion and current distribution of the octocoral Carijoa riisei (Duchassaing & Michelotti, 1860) in the Ecuadorian coast (Eastern Tropical Pacific). Aquatic Invasions. 2021; 16: 62-76.
- Global Invasive Species Database. Species profile: Carijoa riisei. 2021.
- Salimi PA, Creed JC, Esch MM, Fenner D, Jaafar Z, Levesque JC, et al. A review of the diversity and impact of invasive non-native species in tropical marine ecosystems. Marine Biodiversity Records. 2021; 14: 11.
- Kahng SE, Grigg RW. Impact of an alien octocoral, Carijoa riisei, on black corals in Hawaii. Coral Reefs. 2005; 24: 556-562.
- Raghunathan C, Venkataraman K, Satyanarayana C, Rajkumar R. An invasion of snowflake coral Carijoa riisei (Duchassaing and Michelotti 1860) in Indian Seas: Threats to coral reef ccosystem. In: Venkataraman et al. (eds), Ecology and Conservation of Tropical Marine Faunal Communities. Springer-Verlag Berlin Heidelberg. 2013: 381-393.
- Sánchez JA, Ballesteros D. The invasive snowflake coral (Carijoa riisei) in the Tropical Eastern Pacific Colombia. Rev. Biol. Trop. 2014; 62: 199-208.
- Kahng SE, Benayahu Y, Wagner D, Rothe N. Sexual reproduction in the invasive octocoral Carijoa riisei in Hawaii. Bull. Mar. Sci. 2008; 82: 1-17.
- Calcinai B, Bavestrello G, Cerrano C. Dispersal and association of two alien species in the Indonesia coral reefs: the octocoral Carijoa riisei and the demosponge Desmapsamma anchorata. J. Mar. Biol. Ass. UK. 204; 84: 937-941.
- Nam NH, Huong NT, Hanh TTH, Thanh NV, Cuong NX, Thung DC, et al. Pregnane steroids from the Vietnamese octocoral Carijoa riisei. Natural Product Research. 2017; 31: 2435-2440.
- Abdullah MP. What we know about invasive species snowflake coral? In: Department of Marine Park Malaysia Research Seminar 2015 Abstract Book. DMPM, Putrajaya, MY. 2015: 16.
- Khodzori FA, Saad S, Mohammad-Noor N. Coral community structure in Payar Island Marine Park, Malaysia. Journal of Sustainability Science & Management. 2019; 14: 29-39.
- Department of Marine Park Malaysia. Marine biodiversity expedition report 2012: Northern Straits of Malacca - Payar & Songsong Islands Archipelago, Vol.1, DMPM, Putrajaya, MY. 2013: 110.
- Molloy PP, Evanson M, Nellas AC, Rist JL, Marcus JE, Koldewey HJ, et al. How much sampling does it take to detect trends in coral-reef habitat using photoquadrat surveys? Aquat. Conserv. Mar. Freshw. Ecosyst. 2013; 23: 820-837.
- Kohler KE, Gill SM. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006; 32: 1259-1269.
- Magurran AE. Measuring biological diversity. Blackwell Science Ltd, Oxford, UK. 2004: 256.
- Steiner SCC, Riegl B. Community structure of shallow water Alcyonacea (Anthozoa: Octocorallia) from the southern Tropical Eastern Pacific. Ecol. Res. 2018.
- Grigg RW. Invasion of a deep black coral bed by an alien species, Carijoa riisei, off Maui, Hawaii. Coral Reefs. 2003; 22: 121-122.
- Tabugo SRM, Manzanares DL, Malawani AD. Coral reef assessment and monitoring made easy using Coral Point Count with Excel extensions (CPCe) software in Calangahan, Lugait, Misamis Oriental, Philippines. Computational Ecology & Software. 2016; 6: 21-30.
