Abstract
The use of hydrogen as an energy vector has been considered as one promising way to attend society decarbonization. Hydrogen can be used as a chemical to store electricity and as a fuel to electric fuel cell mobility. This work makes hydrogen production potential economical evaluation of 5 real solar photovoltaic installations intended primarily for self-consumption. The surplus electrical energy can be used to produce hydrogen, which will be used later as a form of energy, potentially in an application. That provides greater economic value. Hydrogen serves as an important career to the storage of energy and can be more interesting and competitive than a battery-based solution. The results show that the use of hydrogen is only economically viable for mediumsized installations, greater than 300MW and for the production of hydrogen for mobility.
Keywords: Hydrogen vector; Energy economic analysis
Introduction
Currently, humanity is confronting a major environmental problem that demands scientific and innovative solutions: the rise in the average temperature of the planet. This problem has resulted due to the rise of carbon dioxide emissions in the last 200 years due to the massive use of fossil fuels, and therefore it is imperative to develop new sources and ways for energy and fuel production that can be sustainable and simultaneously with neutral emissions of carbon dioxide. The previous problem requires persistent work in scientific research focused on the implementation of innovative solutions that can be sustainable for the environment, economy, and society. Nowadays, hydrogen is an important intermediate in the chemical industry and refineries. Renewable hydrogen is seen as an important secondary energy carrier of the future and could be used directly as fuel and feedstock for further syntheses as well as for the generation and storage of electricity. Generally, hydrogen production processes can be classified into three categories: electrochemical, biological, and thermochemical methods [1]. All of these methods can be realized on a renewable base. In the case of electrochemical methods, electricity must be generated by sustainable energy sources. Biological processes are a promising alternative approach for production of hydrogen from low cost, renewable, and environment-friendly resources [2]. In this process microorganisms convert organic substrates and water molecules into hydrogen by catalytic activity of two main enzymes as hydrogenase and nitrogenase [3]. Bio-hydrogen can be produced through different processes including photo-fermentation, darkfermentation, CO gas-fermentation, and photolysis. Among these processes, dark fermentation and photo-fermentation are considered as the most promising processes [4]. Dinesh et al. [5] performed an economic analysis of bio hydrogen production from food waste using dark fermentation method and reach a low hydrogen production cost of 3.20$/kg. However, as production rate of the fermentation processes is very low, required size of reactor would be high and hence installation cost is high. This is the key challenge of fermentation processes is the low production capacity per unit of capital investment [6]. Thermochemical hydrogen production process produces hydrogen from synthesis gas which is obtained from different processes. This technology mainly constitutes pyrolysis and gasification of biomass processes where a gas mixture mainly comprising hydrogen, carbon monoxide, methane, and carbon dioxide is obtained [7]. This gas mixture needs to be further processed to hydrogen gas by steam reactions and water gas shift reaction [8]. However, the use of thermochemical methods for hydrogen production is very expensive. Gholkar et al. [9] performed a technoeconomic assessment of hydrogen and methane production from thermochemical conversion of microalgae and conclude that the process is only viable if the market price of hydrogen is as high as $10/ kg. Sara et al. [10] performed a techno-economic analysis of hydrogen production from fluidized bed gasification of lignocellulosic biomass on a small-scale system and found out even a greater hydrogen production cost of 12.75€/kg. In terms of electrochemical hydrogen production processes, electrolysis processes are those with the highest degree of maturity and highest yields [8]. The electrolysis of water consists of the decomposition of water into oxygen and hydrogen by the effect of the passage of a continuous electric current through the water in a device called an electrolyzer. Hydrogen and oxygen are produced from water through redox reactions. The electrolyzer is a device that combines oxidation and reduction reactions to produce hydrogen and oxygen from water. A typical electrolysis process can use three different types of electrolytes: liquid electrolyte, solid polymeric electrolyte in the form of a Proton-Conducting Membrane (PEM), or oxygen ion-conducting membrane [1]. Grimm et al. [11] performed a techno-economic analysis of two solar assisted hydrogen production technologies: A photoelectrochemical system and its major competitor, a photovoltaic system connected to a conventional water electrolyzer. The production cost of hydrogen resulted in 6.22$/kg for the photovoltaic-electrolyzer system and in 8.43$/kg for the photoelectrochemical system. Pinaud et al. [12] found a production cost of hydrogen even it higher in 10.40$/kg for the photoelectrochemical system. Since alkaline electrolysis is the most mature electrolysis technology and also most widely used [13]. An alkaline solution, which normally consists of 20-40% Potassium Hydroxide (KOH), is used as an electrolyte to increase the ionic conductivity of cells [14]. The main disadvantage of alkaline electrolysis is that the liquid alkaline solutions used are corrosive. In recent years, major developments have focused on reducing operating costs associated with electricity consumption, thereby improving efficiency. However, the current density has been increased, thereby reducing investment costs [15]. New materials are also being tested to replace the asbestos used in the diaphragm. These include membranes based on polymers of antimony impregnated with polymers, porous composites consisting of a matrix of polysulfone and ZrO2, known as Zirfon, and separators based on polyphenol sulfide. With regard to PEM electrolyzers, the main difference compared to alkaline electrolyzers is the use of electrolytes. PEM electrolysis employs a solid polymeric membrane as an electrolyte instead of the corrosive liquid electrolyte used in the alkaline electrolysis process. However, high-purity deionized water is required for this electrolysis process [16]. At the anode, water is oxidized to produce oxygen, electrons, and protons. Protons pass through the membrane to the cathode side while the electrons pass to the cathode side through an external circuit. At the cathode, protons are reduced to generate hydrogen. The PEM electrolyzer is more suitable for working with variable energy sources such as renewable energies. This is due to the transport of protons across the membrane which is facilitated by floating energy sources. Currently, the main drawback of PEM electrolyzers is the high cost of production, so the development of these types of demonstration projects on a pilot-scale contributes positively to the growth of these technologies that allow the energy storage and the production of fuels and raw materials with practically environmental null impact. The aim of this work is to evaluate the potential for hydrogen production in 5 real solar photovoltaic installations intended primarily for selfconsumption. Currently, all energy that exceeds the facility’s own consumption is either injected into the public utility grid, with a very low economic value or is simply wasted. The alternative that this study proposes is that this surplus electrical energy can be used to produce hydrogen, which will be used later as a form of energy, potentially in an application that provides greater economic value. Hydrogen thus serves an important function of storing electrical energy and can be more interesting and competitive than a battery-based solution. Its later use will be made essentially as thermal fuel, but it can also be used to produce electricity again through fuel cells either to produce electrical energy to inject into the grid or in hydrogenelectric mobility. This function is sometimes described as an energy vector, since it is not a primary source of energy, but it allows the transformation to other forms of energy in other applications.
Methodology
Five practical cases of units in Portugal that have renewable production systems for photovoltaic electricity were studied, namely.
Case A: Services Operational Center Facilities in évora.
Case B: Pharmaceutics facilities in Lumiar/Lisbon.
Case C: Services Operational Center Facilities in Porto Salvo/Oeiras.
Case D: Services Operational Center Facilities in Queluz/Sintra
Case E: Car Stand Facilities in Abrunheira/Sintra.
The choice of locations for the case studies fell on the technical conditions in terms of electricity consumption, power level, and consumption profiles, as well as the characteristics of the location and the available area of exposure to solar radiation. In addition to the technical framework, the choice of locations was linked to the existing hydrogen consumption potential, in order to be used as an energy vector, as well as its location within or close to industrial parks. In all cases, the Energy Audit carried out proposed and designed photovoltaic installations for Self-consumption. In some cases, it was necessary to resize the photovoltaic solar installation in order to guarantee a surplus of energy necessary for the production of hydrogen. Table 1 shows the global values obtained during 2017 and Figure 1 shows the curves of energy consumption, electricity produced by the photovoltaic system and hydrogen production in a typical summer week. Most of the installations presents most of its electricity consumption at night, so during the day the production of electrical energy by means of photovoltaics ends up generating excess electrical energy that can be stored. Case A is an operational center of a large company, located in évora. Since this type is a typical industrial park facility, we chose to include it in this study. The installation presents most of its electricity consumption at night, so during the day, the production of electrical energy by means of photovoltaics ends up generating surplus electrical energy that can be stored. Case B is an installation corresponds to an office building of an industrial company and is located in a technological park in Lisbon. The company’s laboratories are located in the contiguous building. Thus, it appears interesting to include the analysis of this installation in the present study. As it is an office, most of its electricity consumption occurs during the day, so that during the day, the production of electric energy by means of photovoltaics does not generate a significant surplus of electricity unless the production installation is slightly over-sized. This surplus of electrical energy is used to produce electrolytic hydrogen in the sense that it can be stored. In relation do Case C, the facility corresponds to a logistics center (offices, warehouses and workshops) of a large company and is located on a campus in Porto Salvo, municipality of Oeiras. Most of its electricity consumption occurs at night, so during the day, the production of electric energy through photovoltaics ends up generating surpluses. Case D is an operational center of a service company, which is located in Queluz, in the municipality of Sintra. This type of facility is typical of industrial parks, so it is also included in this study. This installation presents most of its electricity consumption at night, so during the day, the production of electrical energy by means of photovoltaics ends up generating surpluses. This surplus of electrical energy is sent to the electrolysis device for hydrogen production and storage. Finally, Case E is a facility corresponds to a large logistics center (offices, warehouses, sales stand, training centers and workshops) of a large automobile and heavy vehicle and bus company. It is located on a campus in Abrunheira, municipality of Oeiras. The installation presents the majority of its electric energy consumption during the day, so that during the day, the production of electrical energy by means of photovoltaics does not generate significant surpluses unless the production installation is slightly over-sized.
Case
A
B
C
D
E
Energy [kWh/year]
Total Installation consumption
153.141
198.409
94.677
170.663
210.942
power produced by the plant
90.642
123.242
51.935
32.467
102.644
Energy for self-consumption
23.487
86.802
16.742
26.559
75.422
Rations
Energy Produced/ Energy Consumed
59%
62%
55%
19%
49%
Self-consumption energy/energy produced
25.90%
70.40%
32.20%
81.80%
73.50%
Surplus/Energy Produced
74.10%
29.60%
67.80%
18.20%
26.50%
H2 Production
Surplus Energy (kWh)
67.156
36.44
35.193
5.908
27.223
Specific energy consumption (kWh/Nm3)
4.5-7.5
25%
25%
25%
25%
H2 Production Potential (Nm3)
16.789
9.11
8.798
1.477
6.806
Table 1: Summary of global values obtained during 2017.
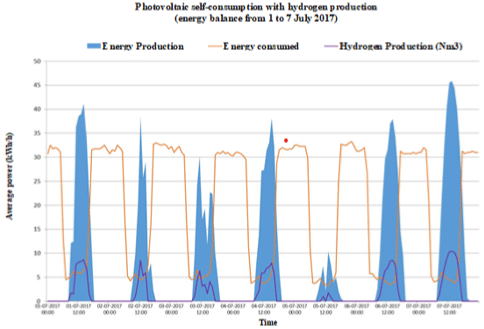
Figure 1a:
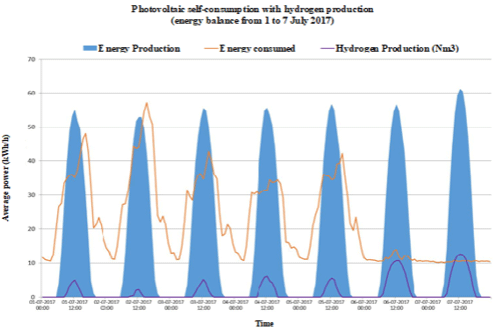
Figure 1b:
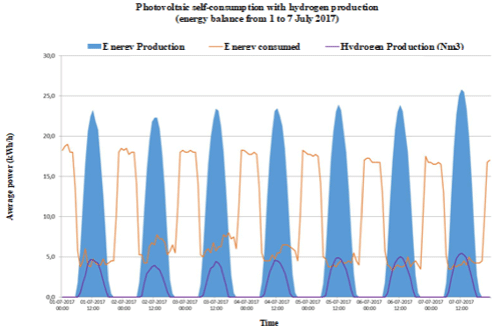
Figure 1c:
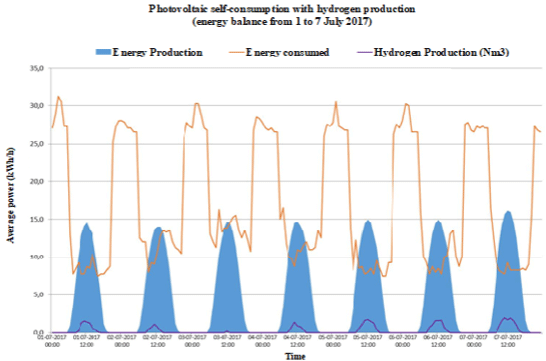
Figure 1:
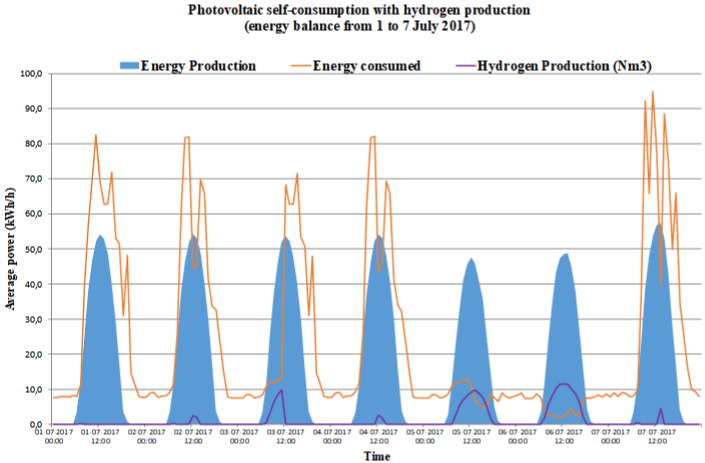
Figure 1e: Energy consumption, photovoltaic electric energy and hydrogen production in a typical summer week: a) Case A; b) Case B; c) Case C; d) Case D and
e) Case E.
Economic Analysis
The economic evaluation of the system was carried out based on the estimated forecast cash-flows and calculation of the Net Present Value (NPV), Internal Rate of Return (IRR) and Payback Period (PP) for the various case studies presented and for six scenarios as follows.
Scenario A: Use of excess energy for self-consumption with energy storage via electrolytic hydrogen production; energy production would be carried out based on fuel cells and would be injected into the unit’s internal network during periods when energy is needed; for the purposes of economic evaluation, it was considered that the energy injected into the internal network would have a profit equivalent to the cost that the company pays for that energy; in this situation, electrolyzers and PEM fuel cells would be used and hydrogen storage under pressure.
Scenario B: This situation is identical to situation A, but considering that there is a possibility of having a scale factor of 10 times higher. That is, assuming that in the industrial park there would be 10 entities with identical energy profiles and that could work together with clear benefits in terms of investment and operation costs.
Scenario C: A second scenario involves the use of excess energy from the unit to produce hydrogen with a high degree of purity for sale in hydrogen supply stations (Hydrogen Refueling Stations - HRS) for application in hydrogen electric vehicles or industrial applications; the use of light and heavy hydrogen electric vehicles has been increasing with different demonstration projects already at relatively high scales, both in Japan, the United States, as well as in the European Union, with Portugal at an early stage of this process considering that the existence of HRS’s is fundamental for the development of the energy vector under analysis.
Scenario D: This situation is identical to situation C, but considering that there is a possibility of having a scale factor of 10 times higher. That is, assuming that in the industrial park there would be 10 entities with identical energy profiles and that could work together with clear benefits in terms of investment and operation costs.
Scenario E: In this situation, we will evaluate the prospect of an effective reduction in the price of technology in the next 10 years, taking into account the developments that have been taking place, and in the medium term it may allow for a favorable economic evaluation of chemical energy storage solutions via hydrogen. This scenario uses equipment cost forecasts presented in different studies, in particular the one developed by FCH-JU [17]. This scenario should be compare with scenario A.
Scenario F: Possibility of using different electrolysis technologies, namely, PEM technology or Alkaline technology, since both are mature and have different costs and longevity; alkaline electrolyzers are more economical, but have less longevity than PEM. This economic assessment is carried out taking into account the marketable values of hydrogen and electricity presented in Table 2. The price of electricity was determined based on the market price in Portugal considering a bi-hourly situation. As for the price of hydrogen, it was estimated based on the principle of the competitiveness of the price of hydrogen compared to the current price of diesel. Considering a vehicle that has an average consumption of around 5.5 liters of diesel/100km and the current price of diesel on the order of 1.5€L, as well as the average consumption of a hydrogen vehicle in the order of 1kg of hydrogen per 100 km, a competitive price for the current sale of hydrogen will be in the order of 8€/kg.
Energy
Hydrogen (€/kg)
4.5
Hydrogen (€/Nm3)
0.4
Electricity (€/kWh)
0.18
Table 2: Marketable energy prices.
Equipment
Table 3 shows the values considered in terms of equipment for the different scenarios addressed. The investment values considered, operating costs and lifetime of the cells took into account market values and references taken from the literature [18,19].
Scenario
A
B
C
D
E
F
Cell type
PEM
Alkaline
Year
2020
2020
2030
2020
Power
Elec.
Elec.
H2
H2
Elec.
H2
Power
36
360
36
360
36
36
Electrolyzer
Electrolyzer cost + Compressor [€/kW]
1200
600
1200
600
804
1000
Lifetime (hours)
32000
32000
32000
32000
48000
60000
Maintenance and operation [% investiment]
1%
1%
1%
1%
1%
1%
Replacement cost [€/kW]
400
250
400
250
200
300
Storage tank
Tank cost [€/kg]
470
400
470
400
315
470
Lifetime [years]
25
25
25
25
25
25
Maintenance and operation [% investiment]
2.0
2.0
2.0
2.0
2.0
2.0
Fuel Cell
Fuel cell cost [€/kW]
1600
800
Fuel cell lifetime [years]
15
15
Maintenance and operation [% investiment]
1.0
1.0
Replacement cost [% investiment]
50
50
Table 3: Replacement Cost.
Operative parameters
The Table 4 shows the operating parameters used in this study. It was considered that the electrolyzer would have a power that would guarantee an 80% utilization of the maximum available peak and that it would have an efficiency of 70% [19]. The energy storage capacity was set for one week in order to allow normal fluctuations in production and consumption. In terms of electric energy production based on fuel cells, the use of PEMFC was assumed with a yield of 60% [20]. Considering that we have neither a constant production nor a constant load, and with an interest in being able to store as much energy as possible, observing the experimental data, a 7 days storage capacity allows to have flexibility in the system. Considering the parameters defined in the previous table and the energy data for one year of the various case studies, the following basic characteristics were defined for each of them Table 5.
Parameter
Electrolyzer
Peak utilization (%)
80%
Electrolyzer efficiency (%)
70%
Cell voltage (V)
1.5
Tank
Storage capacity (days)
7
Fuel cell
Fuel cell efficiency (%)
60%
Table 4: Global operative parameters.
Parameter
Case Study
A
B
C
D
E
Maximum storage power (kW)
45
57
25
10
54
Average power in production (kW)
22
18
11
4
21
Electrolyzer power (kW)
36
46
20
8
43
Operating time per year (hours)
3093
1997
3195
1500
1310
Maximum hydrogen production (kg/h)
0.63
0.79
0.35
0.15
0.76
Fuel cell power (kW)
2.68
1.46
1.41
0.24
1.09
Table 5: Storage system characteristics.
The power of the fuel cell was determined assuming that the production of electrical energy is processed regularly for about 2/3 of the day, a period in which there is no capacity for energy storage, a similar situation in all case studies. Based on the assumptions defined above, the five installations were studied, with the following NPV and the following IRR. The economic evaluation of each system combination was done by the determination of two economic indicators: the NPV and the payback period. The formula that was adopted for the calculation of NPV (€) is defined in equation 1 [21]:
CFn is the net incremental cash flow per year expressed in € (i.e. the difference between energy profits obtained from the system combination and the operating costs), n the year under focus, t the total lifetime presumed for the combination (assumed to be 15 years in all cases [22], i the discount rate (equal to 10%(23)) and CIC the initial investment applied in the equipment (€). In fact, CFn is constant for all the life period due to the fact that both energy profits and operating costs are assumed to be the same in every year. Since the first term of equation [1] is a geometric progression with a ratio of (1+i)-1, it can be rewritten in the form of equation [2] for a faster calculation of NPV:
A positive result for NPV indicates that the system combination is economically feasible during the life period of the equipment [22]. For all the solutions that presented economic feasibility the PP was also determined for each case based on the study of the accumulated cash flows that were foreseen over time. The accumulated cash power is given by equation 3:
Where all the variables have the same meanings as described before. The first year presenting a positive value for ACFn corresponds to the wanted PP when the initial investment and succeeding costs are completely returned through the energy profits. Table 6 shows the results obtained. The first observation that the results allow to obtain is a confirmation of our assumption that the larger the size of the photovoltaic electric energy-producing unit, the greater the potential for energy storage via hydrogen, and the more economically attractive a storage unit becomes. Larger units effectively reduce investment costs per unit of energy produced [19]. The results also show that small units in terms of photovoltaic production do not allow energy storage in economic terms. Considering the reference value for the sale of hydrogen, that is the current value of diesel, the hydrogen production could be economically viable. On the other hand, the results show that a medium-sized photovoltaic production unit, such as those studied in this work, is only economically viable if we invest in the production of hydrogen as a fuel. Finally, alkaline electrolyzers, although less efficient, allow better economic evaluations to be obtained at this stage than PEM electrolyzers.
Scenario
A
B
C
D
E
F
Cell type
PEM
Alkaline
Year
2020
2020
2030
2020
Power
Elec.
Elec.
H2
H2
Elec.
H2
Power
36
360
36
360
36
36
Case Study – A
NPV (€)
-5 499
326 586
49 354
833 682
12 871
57 444
PP (Year)
>15
6
6
3
9
5
IRR (%)
1.20%
18.30%
16.40%
38.20%
8.60%
20.90%
Case Study – B
NPV (€)
-33 913
6 570
-4 040
270 298
-11 869
6 171
PP (Year)
>15
15
>15
8
>15
13
IRR (%)
-6.80%
3.30%
2.00%
13.70%
-1.50%
4.70%
Case Study – C
NPV (€)
-4 649
163 786
23 962
427 686
5 462
28 436
PP (Year)
>15
6
7
3
9
5
IRR (%)
0.20%
17.20%
15.00%
36.00%
7.40%
19.40%
Case Study – D
NPV (€)
-6 854
-5 466
-1 974
37 190
-2 833
-103
PP (Year)
>15
>15
>15
9
>15
>15
IRR (%)
-8.30%
1.50%
0.30%
11.30%
-3.10%
2.80%
Case Study – E
NPV (€)
-38 598
-58 991
-16 563
130 671
-17 753
-6 825
PP (Year)
>15
>15
>15
10
>15
>15
IRR (%)
-9.80%
-0.20%
-1.60%
8.80%
-4.70%
0.90%
Table 6: Summary of the economic analysis.
Conclusions
An economic analysis of the hydrogen storage in five real solar photovoltaic installations intended primarily for self-consumption is made. The results obtained allow us to verify that there is interesting economic potential in the use of hydrogen as a chemical energy storage system. Results show that in economic terms the viability only happens for larger units and when the output is the production of hydrogen for fuel cell mobility. Results also show that alkaline electrolyzers, although less efficient, allow better economic evaluations to be obtained at this stage than PEM electrolyzers.
Acknowledgment
Work financed under the project H2SE - Hydrogen and Energy Sustainability - POCI-02-0853-FEDER-016230, Portugal.
References
- Holladay J, Hu J, King D, Wang Y. An overview of hydrogen production technologies. Catal Today. 2009; 139: 244-260.
- Srivastava N, Srivastava M, Malhotra BD, Guptad VK, Ramteke PW, Silva RN. Nanoengineered cellulosic biohydrogen production via dark fermentation: a novel approach. Biotechnol Adv. 2019; 37: 107384.
- Silva JS, Mendes JS, Correia JAC, Rocha MVP, Micoli L. Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J Biotechnol. 2018; 286: 71-78.
- Sen U, Shakdwipee M, Banerjee R. Status of biological hydrogen production. J Sci Ind Res. 2008; 67: 980-993.
- Dinesh GK, Chauhan R, Chakma S. Influence and strategies for enhanced bio hydrogen production from food waste. Renew. Sustain. Energy Rev. 2018; 92: 807-822.
- Dincer I, Acar C. A review on clean energy solutions for better sustainability. Int J Energy Res. 2015; 39: 585-606.
- Couto N, Rouboa A, Silva V, Monteiro E, Bouziane K. Influence of the biomass gasification processes on the final composition of syngas. Energy Procedia. 2013; 36: 596-606.
- Kannah RY, Kavitha S, Preethi, Karthikeyan OP, Kumar G, Dai-Viet NV, et al. Techno-economic assessment of various hydrogen production methods – A review. Bioresour Technol. 2021; 319: 124175.
- Gholkar P, Shastri Y, Tanksale A. Renewable hydrogen and methane production from microalgae: A techno-economic and life cycle assessment study. J Cleaner Production. 2021; 279: 123726.
- Sara HR, Enrico B, Mauro V, Andrea DC, Vincenzo N. Techno-economic analysis of hydrogen production using biomass gasification - a small scale power plant study. Energy Procedia. 2016; 101: 806-813.
- Grimm A, Jong WA, Kramer GJ. Renewable hydrogen production: A techno economic comparison of photo electrochemical cells and photovoltaicelectrolysis. Int J Hydrogen Energy. 2020; 45: 22545-22555.
- Pinaud BA, Benck JD, Seitz LC, Forman AJ, Chen Z, Deutsch TG, et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelecrochemistry. Energy Environ Sci. 2013; 6: 1983-2002.
- Zhang F, Zhao P, Niu M, Maddy J. The survey of key technologies in hydrogen energy storage. Int J Hydrogen Energy. 2016; 41: 14535-14552.
- Saraswat SK, Pant K. Ni-Cu-Zn/mcm-22 catalysts for simultaneous production of hydrogen and multiwall carbon nanotubes via thermo-catalytic decomposition of methane. Int J Hydrogen Energy. 2011; 36: 13352-13360.
- Ursua A, Gandia LM, Sanchis P. Hydrogen production from water electrolysis: current status and future trends. Proc IEEE. 2012; 100: 410-426.
- Turner J, Sverdrup G, Mann MK, Maness P-C, Kroposki B, Ghirardi M, et al. Renewable hydrogen production. Int J Energy Res. 2008; 32: 379-407.
- “Development of Business Cases for Fuel Cells and Hydrogen Applications for European Regions and Cities” commissioned by the Fuel Cells and Hydrogen 2 Joint Undertaking (FCH2JU), N°FCH/OP/contract 180. Reference Number FCH JU2017 D4259.
- Schmidt O, Gambhir A, Staffell I, Hawkes A, Nelson J, Few S. Future cost and performance of water electrolysis: An expert elicitation study. Int J Hydrogen Energy. 2017; 42: 30470-30492.
- Proost J. State-of-the art CAPEX data for water electrolysers, and their impact on renewable hydrogen price settings. Int J Hydrogen Energy. 2019; 44: 4406-4413.
- International Energy Agency (IEA), “Technology Roadmap: Hydrogen and Fuel Cells”, Paris, France, OECD/IEA. 2015.
- Luz FC, Rocha MH, Lora EES, Venturini OJ, Andrade RV, Leme MMV, et al. Techno-economic analysis of municipal solid waste gasification for electricity generation in Brazil. Energy Convers Manage. 2015; 103: 321-337.
- Hou P, Enevoldsen P, Eichman J, Hu W, Jacobson MZ, Chen Z. Optimizing investments in coupled offshore wind -electrolytic hydrogen storage systems in Denmark. J Power Sources 2017; 359: 186-197.
- Loisel R, Baranger L, Chemouri N, Spinu S, Pardo S. Economic evaluation of hybrid off-shore wind power and hydrogen storage system. Int J Hydrogen Energy. 2015; 40: 6727-6739.
