Abstract
Malaria is one of the most severe public health problems in Ghana. In developing countries such as Ghana, with high of prevalence of malaria, the procedures for diagnoses and detection is limited in technological depth and for that reason most of these parasites; Plasmodium ovale, Plasmodium malariae, Plasmodium vivax and Plasmodium knowlesi are not routinely screened for; as such this study seeks to determine the prevalence of Plasmodium species among humans and monkeys at Mole National Park in Northern Ghana.
A total sample size of 217 comprising 214 human subjects and 3 baboons were recruited in this study conducted at Mole National Park. Data including; age, marital status, gender, occupation, knowledge on malaria and educational background information were collected. Blood samples were taken from both humans and baboons for the malaria testing using RDT, Microscopy and PCR to detect Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, Plasmodium vivax and Plasmodium knowlesi.
The mean age of the study participants was 24.2±15.2. All the 3 baboon subjects were negative on RDT but one (1) out of the three was positive on microscopy. That notwithstanding, the same microscopy positive baboon subject was Pan-Plasmodium positive on PCR but was negative for the tested Plasmodium falciparum, Plasmodium ovale and Plasmodium malariae.
The study recorded prevalence of plasmodium species using RDT and microscopy respectively to be 22.9% and 18.2%. The prevalence of Plasmodium falciparum, Plasmodium ovale and
Plasmodium malariae among the human participants from this study were 39.9%, 12.6% and 18.6% respectively. Mixed infections were identified in 7.5% of the human participants where 4.2% was Plasmodium falciparum/Plasmodium ovale, 2.8% was falciparum/malariae mixed infection and 0.5% malariae/ovale mixed infection.
Sociodemographics, knowledge on malaria and interaction with monkeys of study participants did not show any significant association with malaria infection.
Prevalence of malaria parasites was high among the participant, which poses threat to reduction of malaria in the country.
Keywords: Malaria; Ghana; Outpatient departments; Monkeys; Plasmodium ovale
Introduction
Malaria infection in humans is caused by five plasmodium species: Plasmodium falciparum, Plasmodium ovale, Plasmodium vivax, Plasmodium malariae and Plasmodium knowlesi. Among the five human plasmodium parasites, Plasmodium falciparum causes more than 90% of the world recorded malaria morbidity and mortality four of the plasmodium species: Plasmodium falciparum, Plasmodium ovale, Plasmodium vivax and Plasmodium malariae are transmitted from one person to another by the bite of an infected female anopheline mosquito [1]. The fifth malaria parasite, Plasmodium knowlesi is a simian malaria parasite predominantly found in Southeast Asia. Zoonotic transmission of Plasmodium knowlesi malaria occurs following the bite of an infected female anopheles mosquito (Anopheles Leucosphyrus and Anopheles latens) which results in severe diseases, high mortality in infant, children and in naïve adults Wesolowski et al., [2].
Ghana is a malaria endemic country with the entire population being at risk of malaria infection. Children below 5 years of age as well as pregnant women are at a higher risk of malaria infection due to lowered immunity. Presumptively, malaria cases reported at the Outpatient Departments (OPD) was 37.5% in 2015 [3]. Over the years, malaria morbidity and mortality have been reported to be declining in Ghana due to the implementation of various policies by the Ministry of Health and the Ghana Health Service including the use of Sulfadoxine-Pyrimethamine (IPTp-SP) as an intermittent preventive treatment in pregnant women, the free distribution of insecticide treated net and diagnostic based approach with RDT, microscopy or PCR analysis before patient treatment [4,5].
In Ghana, Plasmodium falciparum causes about 90% of all malaria infection followed by Plasmodium malariae which causes less than 10% and more rarely Plasmodium ovale which causes about 0.15%. Plasmodium vivax and Plasmodium knowlesi have been reported to be absent in the country [3]. Anopheline gambiae species complex and Anopheline funestus are the major types of female anopheline mosquitoes that transmit the plasmodium parasites. Anopheles melas is found in the mangrove swamps of the southwest while Anoheline arabiensis in savannah areas of northern Ghana [3]. Despite the availability of effective antimalarial agents Hommerich et al., [4], failure to make the correct diagnosis has resulted in inappropriate therapy leading to preventable deaths.
The use of molecular tools for the detection of plasmodium parasite has deepened the epidemiology of malaria and highlighted plasmodium parasite infection in humans. A retrospective study conducted by using Polymerase Chain Reaction (PCR) on already examined Plasmodium malariae slide showed that 97% of the slides tested positive for Plasmodium knowlesi with only one being positive for Plasmodium malariae. The use of molecular biological technique has significantly impacted and facilitated the identification of infections caused by Plasmodium falciparum, other plasmodium species and mixed infections.
Background
Globally, malaria remains a significant infectious disease causing about 584,000 deaths and leads to an estimated 198 million cases in humans annually [6]. Statistics from 2015 to 2017 shows no significant reduction in global malaria cases. 219 million cases and 435,000 related deaths were recorded in 2017 about half of the world populations live in malaria risk areas. The annual malaria associated mortality approaches 1 million globally, with two (2) children dying from the disease every minute [7].
The protozoan parasite belonging to the genus Plasmodium causes malaria in humans. More than 150 plasmodium species have been identified to cause malaria in mammals, birds and reptiles [8]. Malaria parasite tends to be host specific regardless of having large number of hosts.
The natural host for the four (4) Plasmodium species; Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae and Plasmodium ovale is human whereas long-tailed macaques (Macaca fascicularis) and pig-tailed macaque (Macaca nemestrina) are hosts for five (5) Plasmodium species: Plasmodium knowlesi, Plasmodium fieldi, Plasmodium coatneyi, Plasmodium cynomolgi, and Plasmodium inui.
Even though the four species of Plasmodium parasite exploit humans as intermediate hosts, at least more than twenty-six additional Non-Human Primate (NHP) parasites exist outside Homo sapiens hosts [9]. The principal record of NHP malaria parasite was reported by in an orangutan, Pongo pygmaeus. Plasmodium pitheci species was described a few years later, alongside Plasmodium inui and Plasmodium cynomolgi that infect sympatric monkey species, Macaca fascicularis and Macaca nemestrina, in Borneo [10].
Primate malaria discoveries continued as parasitologists investigated an increasing diversity of apes, monkeys, and lemurs from across the world. The infection of human with monkey malaria in 1965 by Chin et al., [11] initiated a resurgence of NHP malaria research focused on Southeast Asia, resulting in nine newly reported malaria species in Asian apes and monkeys a review paper by Vythilingam, [12] reported that is first discovered malaria parasite in long-tailed macaques. The parasite was again identified by Knowles and Das Gupta in long-tailed monkey that was migrated from Singapore to Calcutta [13].
Epidemiology of Plasmodium Species
Geographic distribution
Malaria is a mosquito borne disease in humans and animals following the bite of infected anopheline mosquito [14]. Parasitic protozoan of the genus Plasmodium causes malaria in human [15]. The mosquitoes, which act as vector for this disease, are female Anopheles funestus, Anopheles moucheti, Anopheles gambiae and Anopheles arabiensis [1].
Plasmodium knowlesi has been reported to be absent in Africa and for that matter Ghana as there are no investigations that have proof of endemic transmission but recently, increase in deforestation has cause many monkeys to have close contact with humans. In wild life reserved areas such as Mole National Park in Ghana, monkeys especially the baboon usually find food in neighboring human habitat which may lead to possible zoonotic transmission.
Reservoir hosts
Humans are the natural reservoir host of the four common malaria parasites: Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae and Plasmodium vivax while distinctive hosts of Plasmodium knowlesi identified were long-tailed (Macaca fascicularis) and pig-tailed (Macaca nemestrina) macaques from Singapore [13]. Zoonotic transmission occurs in humans living close to these macaques as reported in Southeast Asia [2,16-19].
Morbidity and mortality
As indicated by World Health Organization (WHO), malaria accounts for about 219 million cases and an expected 1 million deaths per annum a 2014 report by WHO Africa indicates that in every minute a child living in Africa dies from malaria infection [6]. In 2015, Ghana recorded about 10 million suspected malaria cases with 31% being children under 5 years of age [3]. Within that same period, 7% of all mortality were attributed to malaria with 48.4% deaths being children under 5 years. Meanwhile mortality have declined significantly from 12.3% since 2011 to 4.2% in 2016 [3] due to improved health systems, financial aids from international organizations and strategic plans such as the Presidents Malaria Initiative and National Malaria Control Program [3].
Transmission
Vectors and mode of transmission: Humans becomes infected with malaria parasite following the bite of infected female anopheline mosquitoes. More than 30 species of the mosquito vectors have been described to transmit malaria parasite [1]. These mosquitoes usually bite at dusk and at night which is the most active feeding times for the vectors the mosquito is infected following the bite of an infected human where it sucks the male and female gametocytes. The gametocytes continue the sexual phase of the cycle and the sporozoites fill the salivary glands of the infested mosquito humans can also be infected by contaminated blood in rare cases. Vertical transmission of plasmodium species can also occur during delivery (congenital malaria). Blood transfusion, organ transplant or shared use of needles can be a risk of malaria transmission since malaria parasite normally feed on red blood cells. [20].
Hypothetically, four modes of malaria transmission have been identified: from an infected monkey to another monkey, from an infected monkey to human, from an infected human to another human and from an infected human back to monkey.
Risk factors for Plasmodium species transmission: Genetic factors of an individual from birth pose a risk of malaria infection. Two genetic factors have been linked with the red blood cell, which are of epidemiological importance. People with sickle cell traits are relatively protected from falciparum malaria infection. The sickle cell traits are commonly found in Africa where malaria is endemic and is thought to provide protection from malaria infection [21]. Duffy antigen has been reported to be the invasion point for Plasmodium vivax and Plasmodium knowlesi in humans the presence of the Duffy antigen on the surface of the erythrocyte increases the susceptibility to vivax and knowlesi malaria and it is believed that Duffy antigen acts as a receptor for attachment of plasmodium merozoites majority of Africans especially West Africans are Duffy negative and therefore are resistant to Plasmodium vivax and Plasmodium knowlesi infection [21]. Previous repeated malaria infection may lead to the development of acquired immunity against malaria and subsequent malaria infection may not develop severe complications and may lack typical malaria symptoms [21]. Pregnancy increases susceptibility to malaria. Pregnant women who have had immunity against malaria may lose this immunity especially in their first or second pregnancy [21].
Human behavior may pose a risk to malaria infection. Forest exposures, poor rural population in endemic areas, uneducated individuals, travelers from non-endemic area to endemic area are factors associated with malaria infection. Human activities may create site for mosquito breeding such as standing waters in irrigation ditches, burrow pits, etc [21].
For all vector-borne infections, the transmission is highly based on the bionomics of the vector in question. For example, because Anopheline arabiensis is an outdoor resting dominant vector species Hay et al., [22] people who work outdoors are at risk of contracting malaria by this vector.
Life Cycle of Plasmodium Species
Malaria parasites life cycles in humans and other primates begin when an infected female anopheline mosquito inoculates sporozoites into the bloodstream of its host while feeding on the blood [8].
Sporozoite in the skin
When an infected female anopheline mosquito bite humans or primates, sporozoites are introduced with the saliva into the vascular tissues of the skin. Within one minute, the motile sporozoites pass through the capillary wall and enter the blood stream [23]. Whiles in circulation the sporozoites moves quickly to invade the parenchymal cells of the liver within 40 minutes [24].
Liver stage malaria
After a successful invasion into the hepatocyte, a single parasite undergoes mitosis to produce 10,000 to 20,000 merozoites within 7-10 days period [25]. The enlarged hepatocyte called schizonts burst to release its content (merozoites) into circulation [23]. The liver phase of the life cycle is not associated with clinical signs and symptoms of malaria, yet permits the parasite to replicate [23].
Asexual erythrocytic cycle
When the merozoites are out from hepatocytes, they invade Red Blood Cells (RBCs) and enter into the asexual erythrocytic cycle. At this point, the parasites (trophozoites) grow and utilize the RBC contents as food. The merozoites grows and develop into a ring or early trophozoite within the erythrocyte. The early or ring trophozoites now develop into a mature trophozoite that undergoes asexual multiplication to form numerous merozoites in the erythrocyte which is now called schizont. When the erythrocytic schizont ruptures, it releases the merozoites which then invade more erythrocytes, thereby completing the erythrocytic cycle. Some of the parasites also differentiates into male and female gametocytes, which are taken up by female anopheline mosquito during blood meal.
Transmission back to mosquitoes
Once the gametocyte is inside the mosquito midgut, the temperature increases and pH changes, initiating gametogenesis and fertilization leading to the formation of motile diploid ookinetes that leave the blood meal bolus and cross the midgut epithelium to become a sessile oocyst. More than 10-14 days old sporozoite develops inside the oocyst by means of mitosis, and these escape through an enzymatic process into the mosquito body cavity. The sporozoites circulate by means of the haemolymph and attaches onto the basal lamina of the mosquito salivary gland, prepared for presentation into the next host [23] (Figure 1).
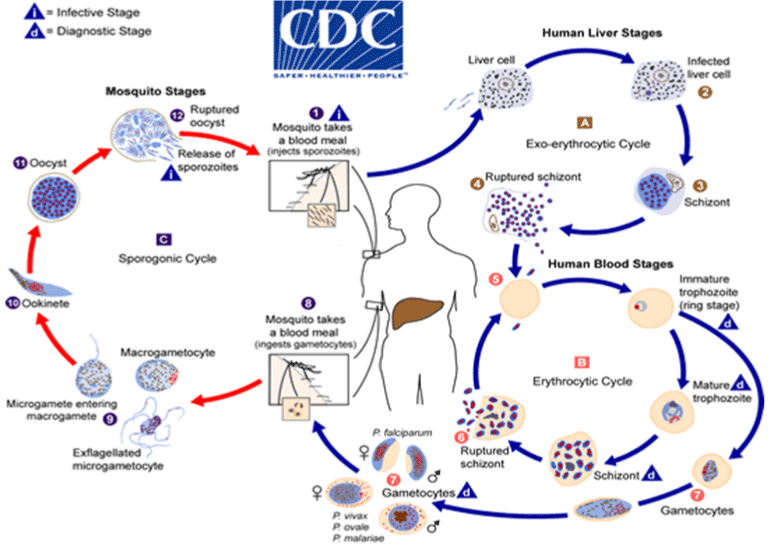
Figure 1: The life cycle of Plasmodium parasite.
Pathogenesis, Pathology and Symptomatology of Plasmodium Species
Pathogenesis
The pathogenesis of malaria infection is highly associated with the hemolysis of infected and uninfected red blood cell. The formation of malaria pigment occurs when the parasite releases its antigenic substance into the host, which elicits an immune response. Cytoadherence is an important virulent factor adapted by plasmodium parasite to cause severe complications. The process of cytoadherence is as a result of the expression on the surface of the parasitized red cell of strain specific-ligands, which stick to a particular receptor complex on the endothelial cell. Anemia is inversely proportional to the number of red blood cells in individuals with recurrent episodes of malaria infection. This indicates that non-infected red blood cell may become sensitized and destroyed [24].
Pathology
Hyperparasitaemia: Hyperparasitaemia remains one of the significant virulence features of Plasmodium species. Investigations by Chaudhury and Venkataramana [26], Cox-Singh et al., [17] recorded parasitemia of over 700,000 per μL of blood on account of the short erythrocytic cycle of some of the parasites particularly Plasmodium knowlesi. This parasitemia can build daily if uncontrolled.
Parasite sequestration: Parasite sequestration is an important factor in the development of cerebral malaria in falciparum infection and in a post mortem brain section of a fatal case of Plasmodium knowlesi reported by Cox-Singh et al., [27], sequestration of parasitized RBCs was evidenced. In another ex-vivo investigation by Chaudhury and Venkataramana [26], parasitized erythrocytes exhibited cytoadherence to the receptors of the human endothelial cells. Their findings point to a conceivable sequestration of Plasmodium species infected RBCs in the capillaries.
Symptomatology
Fever, chills and rigors accompanied by cerebral headache, myalgia, poor appetite, cough, abdominal pain and diarrhea are the major symptoms of malaria [26,28]. An experiment conducted by Chin et al., [11] shows that patients injected with infected blood through intravenous or intramuscular route, developed clinical signs after an average of 8 days (3-14 days).
Plasmodium Species Diagnosis and Treatment
Clinical diagnosis
The most widely recognized research discoveries reported for 107 prospectively studied malaria patients were tachypnea, fever, and tachycardia [28]. In two prospective study of 107 and 130 patient by Barber et al., [29]; Daneshvar et al., [28], hepatosplenomegaly was reported for in 24 to 40% and 15 to 26% of cases, respectively. Clinical indications of severe complications including low oxygen saturation, tachypnea, chest crackle (indicating acute respiratory distress or coexisting pneumonia), hypotension, and jaundice have been recorded [17,28,30].
Laboratory finding
Thrombocytopenia: The most commonly reported blood abnormality which appears to be almost universal in all malaria infection is thrombocytopenia in a study by Daneshvar et al., [28] at the Kapit hospital, 98% of 107 patients presented with thrombocytopenia, and 2% of patients also became thrombocytopenic within 24 hour of admission. In spite of the incredibly high extent of patients with thrombocytopenia, and a 3rd of these patients being severely thrombocytopenic (50,000 platelets per μL blood), bleeding complications were infrequently observed a Comparative studies carried out by Barber et al, shows that the severity and recurrence of thrombocytopenia were higher with knowlesi malaria than with falciparum, ovale, malariae and vivax malaria infection Barber et al., [29]. Willmann et al., [31] also described laboratory markers of severity in malaria infection and discovered thrombocytopenia (45,000 platelets per ul blood) to be associated with complication having sensitivity and positive predictive value of 71% and 22% respectively [31].
Severe Anemia: Severe malarial anemia is commonly accompanied with chronic and recurrent infections of malaria and can results in hypohaemoglobinaemia < 5g/dl (normal values are between 10-15g/dl for humans).
Low hemoglobin levels occurring during malarial infections can be attributed to sequestration of RBC during parasite multiplication as well as removal of infected RBCs as part of immune - mediated clearance mechanism. Also, hyperactivity of the immune system in the spleen could result in premature removal of uninfected RBC. The development of new RBCs in the process of erythropoiesis replaces the loss RBCs. However, haemozoin and anti-malarials produced by these plasmodium species alter the normal hematopoiesis Lamb [23] which can result in severe anemia.
Renal complications: Renal complication may develop during malaria infection and it is generally oliguric (<400ml/day) or anuric (<50ml/day), uncommonly nonoliguric and occasionally may need dialysis. In acute cases, tubular necrosis may result secondary to renal ischemia. ‘Blackwater fever’ resulting in hemoglobinuria may be temporal and unassociated with renal failure [32].
Metabolic acidosis: Metabolic acidosis is significantly associated with severe malaria infection. It is facilitated by the lack of RBC in circulation especially in patients with SMA as a result of hypoxemia and anaerobic metabolism. Also, in severe malaria, hypervolemia can enhance metabolic acidosis [23].
Pulmonary complications: Tachypnea and dyspnea are the characteristic symptoms of pulmonary edema accompanied by hypoxemia and respiratory failure due to intubation. Acute Respiratory Distress Syndrome (ARDS) and increase in pulmonary capillary permeability may develop as a result of pulmonary edema. A 5.6% and 10.7% frequencies were recorded on pulmonary edema for 107 and 130 knowlesi malaria cases studied respectively [28,29]. ARDS was seen in 52% cases with a crude mortality rate of 37% in severe cases of knowlesi malaria reported by [33]. Frequently associated complications were reported to be present (median 3), and in four patients, ARDS developed following admission, a pattern similar to that seen with falciparum malaria [33]. Statistical study undertaken by Barber et al., [29]; Daneshvar et al., [28] showed positive and independent association with hyperparasitaemia, neutrophilia and anemia at admission.
Hypoglycemia: Low blood sugar is a characteristic symptom in patients with severe malaria. Hypoglycemia may be induced by quinine or quinine-induced hyperinsulinemia; however, it may be found in patients with normal insulin levels [32].
Laboratory Diagnosis
Microscopy
Microscopy still remains an easy, economical and standard method for the diagnosis of malaria using thick and thin blood film stained with Giemsa, Wright’s, or Field’s stain. An ideal sample for malaria microscopy is blood obtained from the earlobe or finger. The reason being that, the density of developed trophozoites or schizonts is greater in blood from this capillary-rich area. Blood obtained by venipuncture collected in heparin or sequestrine (EDTA) anticoagulant-coated tubes should be immediately used to prevent modification in the morphology of White Blood Cells (WBC) and malaria parasites the four main Plasmodium species that cause malaria in humans, are identified widely by trained microscopists microscopic diagnosis of Plasmodium knowlesi is inadequate and unreliable due to morphological similarities it shares with Plasmodium falciparum and Plasmodium malariae [34]. In view of the possibly fatal result in Plasmodium knowlesi compared with the benign nature of Plasmodium malariae, it was recommended at a WHO consultation meeting on Plasmodium knowlesi that, microscopists should report all Plasmodium malariae positive outcomes as Plasmodium malariae / Plasmodium knowlesi.
Antigen detection test
Several Rapid Diagnostic Tests (RDTs) are available, which are fast, reliable and simple to use and can detect Plasmodium falciparum and non-falciparum infections or both [18]. RDTs are based on the detection of antigens derived from malaria patients in lysed blood, using immunochromatographic methods all the current commercially available RDTs were developed before it was discovered that Plasmodium knowlesi is a significant cause of human malaria.
Polymerase chain reaction (PCR)
Currently, molecular diagnostic assays specific for multicopy genes (such as small subunit rRNA or mitochondrial cytochrome b are actually considered the gold standard to detect unequivocally Plasmodium species malaria in humans [35].
Of the various gene targets which have been tried, the 18s Small- Subunit Ribonucleic Acid (SSUrRNA) has been used in most studies because of its genetic variation and hence for species differentiation [36]. The PCR can be used with the DNA from various samples like whole blood with anticoagulant, dried blood spots on filter paper and even scrapings from stained blood slides [17,26]. The conventional nested PCR is considered as “molecular gold standard” for malaria species detection [26]. However, this technique requires 5-6 separate PCR reactions to detect the 5 malaria parasites. This necessitates the use of multiple reagents and disposables increasing the chances of cross contamination. Moreover, it is cumbersome, time consuming and labour intensive. In view of the limitation of nested PCR, real time PCR has been employed for Plasmodium species detection. It is highly sensitive and can detect even very low parasitaemia of 1-2 parasites/ μL. Using a genome-mining approach, researchers from USA developed a new single-step PCR assay that targets a multicopy sequences for Plasmodium species and was apparently able to detect 1 parasite/μL with a specificity of 100% [37].
Loop mediated isothermal application (LAMP): A highly sensitive assays based on loop-mediated isothermal amplification (LAMP) which uses primers that detect the species-specific gene of Plasmodium species have been developed [38-40]. LAMP is highly sensitive, specific, rapid and does not require sophisticated instrumentation. However its assays need to be validated in large studies on the field [41].
In spite of these superior performances, the molecular detection methods are slower and more expensive than microscopy, and hence unlikely to replace routine microscopy in rural setting.
Treatment
Malaria is preventable and treatable but can be fatal if it’s left untreated. Quick and complete removal of the plasmodium parasite from the patient’s blood is the primary aim of treatment therapies. This is to prevent the development of uncomplicated malaria to severe infection that may lead to malaria related anemia and to reduce mortality. In the view of public health, treatment is intended to lessen the transmission of the infection to others, by reducing the infectious reservoir and to prevent the development and spread of resistance to antimalarial medicines [1]. Majority of the drugs used for the treatment of malaria are active against trophozoite stage of the parasite in the blood including chloroquine, atovaquone proguanil, artemether-lumefantrine, mefloquine, quinine, quinidine, doxycycline (used in combination with quinine), clindamycin (used in combination with quinine), artesunate. In addition, primaquine is active against the dormant parasite liver forms (hypnozoites) and prevents relapses. Primaquine should not be taken by pregnant women or by people who are G6PD (glucose-6 phosphate dehydrogenase) deficient. Patients should not take primaquine until a screening test has excluded G6PD deficiency [21].
Prevention and Control
Vector control is the ultimate intervention program to lessen the burden of malaria transmission at the community level. Personal protection against the bites of mosquitoes is the ultimate means of preventing malaria infection. Insecticide-Treated Mosquito Nets (ITNs) and Long-Lasting Insecticidal Nets (LLINs) are the two main effective vector control programmes established by the public health distribution program. WHO has recommended the distribution of nets to high risk persons and malaria endemic areas. The provision of free LLINs to individuals has been the effective means for everyone to sleeps under a LLIN every night. To effectively prevent malaria infection, Indoor Residual Spraying (IRS) with insecticides is an efficient way to minimize the spread of malaria. The potency of indoor spraying is 3-6 months, based on the surface on which it is sprayed and the type of insecticide used. Dicholoro Diphenyl Trichloroethane (DDT) can be effective for 9-12 months in some cases. Longer-lasting forms of existing Indoor Residual Spaying (IRS) insecticides, as well as new classes of insecticides for use in IRS programmes, are under development.
Malaria in Baboons
Initially discovered Plasmodium species in the blood of monkeys in Africa. Leveran also discovered similar malaria parasite in an Africa monkey and named it after Koch as Plasmodium. Following the discovery of malaria parasite in the blood of monkeys by Koch, malaria parasites have been reported as occurring in the monkeys of the New World, baboons, orang-utan, chimpanzee and gorilla [42].
In May 1922, microscopic analysis of a blood sample from a sick baboon found in Accra, Gold Coast, West Africa revealed the presence of hyperparasitemia in the baboon with plasmodium parasite [43].
According to the human-like structure of the baboon placenta and the cyto-adherent property of Plasmodium knowlesi have informed the choice for baboon-Plasmodium knowlesi model. The baboon model of placental malaria is therefore useful in understanding pathophysiology of malaria infection in humans.
Challenges in the Diagnosis of Plasmodium Species Infection
Timely diagnosis and treatment are crucial for reducing the risk of malaria complication [2,44]. In view of this, proper management and control strategies are imperative for the accurate identification for the Plasmodium species. Microscopy remains the ultimate standard method for diagnosing malaria infection in clinical practice and research but there are limitations in sensitivity and specificity [45].
According to Barber et al., [29], Lee et al., [34], the use of microscopy for the detection of plasmodium parasite cannot accurately and reliably distinguish Plasmodium knowlesi from Plasmodium falciparum, Plasmodium malariae and Plasmodium vivax. The developmental stages of Plasmodium knowlesi thus the early trophozoite stage shares similar features such as double chromatin dots, multiple-infected erythrocytes and appliqué forms with Plasmodium falciparum and Plasmodium malariae [34]. The advent of RDTs has enhanced the diagnosis of human malaria. The advantages range from being rapid; user friendly to its cost effectiveness According to Moody one of the greatest advantages of RDTs is its ability to detect parasitemia above 100 parasites per μL of blood. However, Plasmodium knowlesi specific RDTs are not commercially available and the existing formats of RDTs are found to perform sub optimally when used in Plasmodium knowlesi endemic regions [46]. When the sensitivity of pan malarial marker pALD for Plasmodium species was compared, the sensitivity of Plasmodium knowlesi was found to be lower than that of Plasmodium vivax and Plasmodium falciparum.
Taking into account the many challenges associated with microscopy of Plasmodium species and antigen detection tests, molecular diagnostic tools are currently the only reliable option for the definitive diagnosis of Plasmodium species malaria infections. Molecular technique is the accurate method that has made the discovery of Plasmodium knowlesi malaria possible.
The genomic targets of plasmodium being studied currently are the 18S Small-Subunit rRNA (ssu- rRNA), the circumsporozoite surface protein gene, a nuclear gene encoding a cysteine protease and the cytochrome [47]. The 18s ssu-rRNA is the mostly studied genetic targets for species characterization of the different plasmodia [37].
Studies by Eede et al., [48], Imwong et al., [49] Sulistyaningsih et al.,[50] shows that the Plasmodium knowlesi -primers developed by Balbir Singh and co-workers, demonstrate cross- hybridization with Plasmodium vivax-sequences giving false positive results. A real-time PCR give an optimal performance by binding specifically with a 30-base pair of a variable region of Plasmodium knowlesi which showed a 100% specificity and a high analytical sensitivity (detecting 3 parasites/L of blood) [51]. However, they are expensive to run and require a substantial initial financial investment. The availability of this technique is found in referral laboratories in developing countries and diagnostic laboratory in developed countries. PCR and other molecular methods such as Loop Mediated Isothermal Amplification (LAMP) will not replace microscopy in rural areas due to their relatively high cost.
Study Area
The study was conducted at the Mole National Park, which is the largest wildlife reserve in Ghana. The park hosts different species including fauna, grassland, trees, herbaceous plants, shrubs and over 93 mammal species, covering an area of 4840 km2 and lies between 9o42’N 1o50’W/9.700 oN 1.833oW. About 600 inhabitants of Mole include employees of the National Wildlife Division of the forestry commission of Ghana who reside in the park with their families. Baboons, Patas, Green vervet are the most common monkeys that come to the community every day for leftover food. The coexistence of these primates with the community makes zoonotic transmission a possibility.
Study site
Public health laboratory: The Public Health Laboratory (PHL) is located in the Tamale Metropolitan area and has WHO accredited malaria microscopists who assisted in the examination of the smears.
West African Centre for Cell Biology and Infectious Pathogens (WACCBIP): The West African Centre for Cell Biology and Infectious Pathogens (WACCBIP) is located at the University of Ghana, Legon, Accra. The institution is well equipped with modern and standard molecular assays for the conduct of this study.
Study Design
A cross-sectional study design was conducted from 29th January to 20th May 2019 at the Mole National Park.
Sample Size
Humans
The minimum sample size of humans that were screened for Plasmodium species was determined by the Yamane’s formula.
Where, n = the sample size
N = the size of the population e = the error 5% point
By using Yamane’s formula, a minimum sample size of 240 for humans was calculated from a population of 600 (according to National Population census, 2010).
Monkeys
Three monkeys (Olive Baboon) were sampled from Mole National Park for the identification of Plasmodium species.
Data Collection
A structured questionnaire was used for participant data collection. The socio-economic data collected include age, gender, marital status, occupation and educational information. Their knowledge on malaria and their interaction with the monkeys were also sampled.
Blood Sample Collection
Human sampling
An informed consent form was read and signed by the participant if satisfactory. Questionnaires were administered to the consented participant to fill. The informed consent was read and interpreted to participants who could not read. The questionnaires were then filled based on answers given by the participant. Consent was sought from parents or guardians for participants below 18 years of age. Tourniquet was applied to the upper arm of the participants to make the veins more prominent. The puncture site was cleansed with 70% ethanol and allow to air dry.
Blood samples were obtained by venipuncture. Approximately three milliliters (3 ml) of venous blood sample was drawn from human participants with a sterile dry plastic 5ml syringe and needle into a standard Ethylene-Diamine-Tetra acetic acid (EDTA) vacutainer tube. The tourniquet was released and a piece of dry cotton was pressed on the puncture site until bleeding stops. A volume of 75μL of the blood was spotted on a 3mm thick Whatmann filter Paper, allowed to dry and sealed in clean zip-lock transparent plastic bag with desiccant.
RDT for falciparum malaria
The Rapid Diagnostic Test (RDT) for malaria, CareStart HRP-2 (AccessBiolnc., New Jersey, USA) was used to test for Plasmodium falciparum parasite in all the samples. The RDT test was conducted base on manufacturer’s instructions by dropping 5μL of blood into a sample well (labeled “S”) on the cassette and 2 drops of the buffer dropped into the buffer well (labeled “A” on the cassette). The cassette was placed on a horizontal surface for 20 minutes. A positive result was indicated by the appearance of both the test (labeled “T”) and control (labeled “C”) bands and a negative result indicated by only the control band. All samples were labeled using unique codes generated at the point of collection from Mole National Park.
Capture and Immobilization of Non-Human Primates (NHPs)
Three ml of intravenous blood samples were taken from the femoral vein of the olive baboon (Papio anubis) species after they were carefully trailed and darted with a Dan Inject Dart Gun for immobilization using Ketamine 10% in combination with medetomidine as a tranquilizer.
Sampling from NHP
A volume of 75μL was spotted on a 3mm thick Whatmann filter paper, allowed to dry and sealed in clean zip-lock transparent plastic bag with desiccant. The Rapid Diagnostic Test (RDT) for malaria, CareStart HRP-2 (AccessBiolnc., New Jersey, USA) was used to test for Plasmodium falciparum parasite in all the samples. The RDT test was done following manufacturer’s instructions as previously described.
Sample storage and transport
The dry blood spot from both Humans and Baboons were stored at room temperature and transported to the West African Centre for Cell Biology of Infectious Pathogens (WACCBIP), University of Ghana for molecular detection of Plasmodium species.
Sample processing and microscopic analysis
A 2μL and 6μL of whole blood were used to prepare thin and thick films respectively on the same slide and labeled appropriately. The dried thin film was immediately fixed in absolute methanol while the thick film was allowed to air dry. The thick and thin films were stained with Giemsa stain (1:9 dilutions with buffer) and examined with immersion oil (x100) objective. For the thick film, at least 100 fields were observed for trophozoites, schizonts and gametocytes and were counted against at least 200 - 500 white blood cells. Thin film was observed for speciation of organism against white blood cell count and parasitized red blood cell. Stained slides were examined by 2 qualified microscopists from the Public Health Laboratory. The results were recorded in a Microsoft excel Spreadsheet.
Quantification
Parasite Density against Relative White Blood Cell (WBC) Count and relative Red Blood Cell count
Thick film: Thick film microscopic reporting of malaria parasite was done by counting 8000 WBC per uL of blood
Thin film: Thin film microscopic reporting of malaria parasite was done by counting 5,000,000 Red Blood Cell (RBCs) perμL of blood
Species Identification by Nested Polymerase Chain Reaction (PCR)
DNA Extraction
The Chelex DNA extraction method was used to extract DNA from the dry blood spot as described by [21]. DBS containing 75μL of blood were excised into 2 mL Eppendorf tube and soaked overnight in 1 mL of 0.5% saponin in 1X Phosphate Buffered Saline (PBS). The Eppendorf tubes were centrifuged at 14000 rpm for 2 minutes, Saponin suctioned, and the contents of the Eppendorf tubes were washed with 1 mL of 1X PBS and suctioned. The washing step was repeated until no haem or red colour was unseen on the filter papers. A volume of 50μL of 20% chelex and 100μL nuclease free grade water were added to the tubes, and incubated in a boiling water bath for 20 minutes. The tubes were centrifuged at 14000 rpm for 2 minutes and 100μL of the resulting supernatant was pipetted into a 96 well plate and stored at -20oC until used.
Plasmodium parasite detection by nested PCR
Malaria parasites were detected using nested PCR. Speciation and the identification of the plasmodium species were based on the amplification of the small subunit ribosomal genes with the second round PCR primers and amplicons sizes [52]. A 2μL of DNA was used in a 15μL reaction with outer forward primer rPlu6 and reverse primer Plasmo 2 for the first round PCR reaction using the Applied Biosystems Proflex PCR system of Thermo Fisher Scientific. The firstround PCR product was amplified in 15μL solution containing 7.5μL 2X Hotstar DreamTaq master mix, 0.3μL of 10μM rPlu6 Primer, 0.3μL of 10μM Plasmo2 Primer, 4.9μL of nuclease free grade Water and 2μL of genomic DNA. The PCR was performed following the standard thermycling conditions involving an initial denaturation period of 95oC for 2 minutes, denaturation for 30 seconds, annealing at 58oC for 30 seconds, extension at 72oC for 60 seconds and final extension of 72oC for 5 minutes. This first-round PCR was run for 35 cycles. For the second round PCR, 1μL of the first-round PCR product and species-specific primer for the four human malaria species were mixed in a 2 ml Eppendorf reaction tube. A total of 35 PCR cycles (95oC for 2 minutes, 95oC for 15 seconds, 58oC for minutes, 72oC for 30 seconds and 72oC for 5 minutes) were performed. The PCR product were resolved in 1.5% agarose gel electrophoresis and viewed under florescence light using the Amersham Imager 600, (GE Healthcare UK Limited Amersham Place Little Chalfont Buckinghamshire HP7 9NA United Kingdom).
Parasite Diagnosis by RDT, Microscopy and PCR for Human Samples
RDT and Microscopy
A total of 214 samples were tested with RDT and microscopy and confirmed by PCR analysis. Of the 214 samples tested by RDT, 22.9% (49/214) were positive for HRP2 antigens. On the other hand, 18.2% (39/214) were positive for malaria by blood films. Table 1, Figure 2 and 3 presents the results of RDT and microscopy analysis.
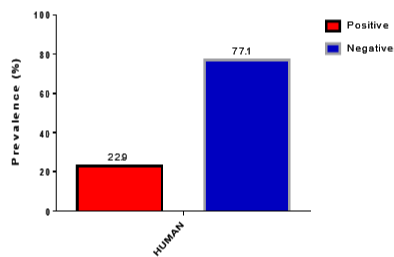
Figure 2: Graph Showing RDT Results.
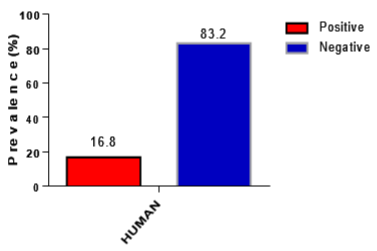
Figure 3: Graph showing microscopy Results.
RDT (%)
Microscopy (%)
Positive
49 (22.9)
39 (18.2)
Negatives
165 (77.1)
175 (81.8)
RDT and Microscopy results of human samples.
Table 1: Prevalence of malaria by RDT and microscopy.
Species identification by species-specific PCR for human samples
Species-specific PCR based on ribosomal RNA (rRNA) small subunit (SSU) detected 39.9% (79/198) of test PCR samples to be Plasmodium falciparum mono-infection, 12.6% (25/198) Plasmodium ovale mono-infection and 18.6% (36/198) were Plasmodium malariae mono- infection. Mixed infections were also observed in 16 samples (Table 3). There were 4.2% Plasmodium falciparum/plasmodium ovale coinfection, 2.8% falciparum/malariae coinfection and 0.5% malariae/ovale coinfection. The prevalence of plasmodium parasite by PCR analysis is presented in Table 2 and Figure 4.
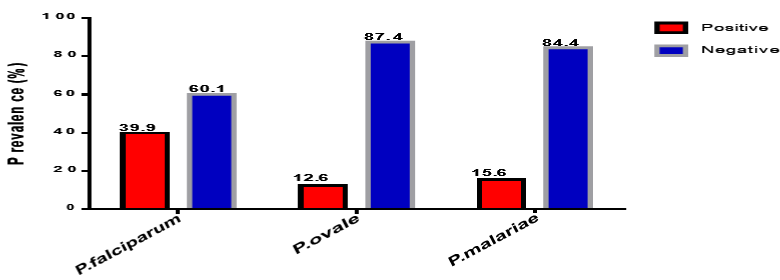
Figure 4: A graph showing the prevalence of PCR results for Plasmodium falciparum, Plasmodium ovale and Plasmodium malariae.
PCR
n=198 (%)
Pf
Po
Pm
POSITIVE
79 (39.9)
25 (12.6)
31 (18.6)
NEGATIVE
119 (60.1)
173 (87.4)
167 (96.3)
Pf: Plasmodium falciparum; Po: Plasmodium ovale; Pm: Plasmodium malariae.
Table 2: Plasmodium parasite identification by PCR analysis.
PCR (%)
Pf+Po
9 (4.2)
Pf+Pm
6 (2.8)
Pm+Po
1 (0.5)
Total
16 (7.5)
Pf: Plasmodium falciparum; Po: Plasmodium Ovale; Pm: Plasmodium Malariae.
Table 3: Mixed infection detected by PCR analysis.
Outcome of the NHP Sampling
All the three baboon monkeys that were captured for the malaria investigation were males with an average weight of 25.4 kg. One out of the three baboon samples was positive for microscopy with parasitemia of 3080 parasite/μL of blood. Details of the monkey sampling are presented in Table 4.
RDT (n)
MCROSCOPY(n)
PCR (n)
Parasitemia
Parasite/μLGenus-specific Plasmodium
Pf
Po
Pm
Positive
0
3080
1
0
0
0
Negative
3
2
3
3
3
Table 4: Plasmodium parasite detection of the diagnostic tools of NHP.
Siodemographic Characteristics of Participants
The average age of the study participants was 24.2±15.2 years with 116 (54.2%) male participants and 98 (45.8%) female participants. More than half of the participants were single 124 (57.9%) while 90 (42.1%) were married. Majority of the participants were educated 206 (96.3%).
140 (65.4%) were unemployed and 74 (34.6%) were employed (Table 5). Presents the frequency distribution of the Siodemographic characteristics of the participants of this study.
Variables
Frequency
Percent
Gender
Female
98
45.8
Male
116
54.2
Age
= 10
25
11.7
11-20
100
46.7
21-30
22
10.3
30+
67
31.3
Education
Uneducated
8
3.7
Educated
206
96.3
Marital Status
Married
124
57.9
Single
90
42.1
Occupation
Unemployed
140
65.4
Employed
74
34.6
Table 5: Frequency distribution of Sociodemographic Characteristics of participants.
Siodemographic Factors Assiated with Malaria Infection from Logistic Regression
The Siodemographic characteristics of the participant are reported in Table 6. The logistic regression model showed that the prevalence of malaria among participants had no significant difference (p>0.05) in their age, gender, educational status, marital status and cupation.
Variables
B
S.E.
Wald
Exp (B)
95% C.I. for EXP (B)
P- Value
Lower
Upper
Gender
Male
-0.001
0.338
0.000
0.999
0.515
1.937
0.997
Female
0b
Age
2.05
0.562
= 10
-1.056
0.847
1.554
0.348
0.066
1.83
0.213
11-20
-0.642
0.657
0.956
0.526
0.145
1.907
0.328
21-30
0.2
0.581
0.119
1.222
0.391
3.817
0.73
31+
0b
Education
1.282
0.864
Primary
-0.286
0.66
0.188
0.751
0.206
2.737
0.665
SHS
-0.586
0.554
1.117
0.557
0.188
1.65
0.291
JHS
-0.183
0.64
0.082
0.833
0.238
2.919
0.775
No School
-0.454
0.895
0.257
0.635
0.11
3.67
0.612
Tertiary
0b
Marital Status
Married
-0.353
0.51
0.479
0.703
0.258
1.91
0.489
Single
0b
Occupation
Employed
0.561
0.642
0.762
1.752
0.498
6.167
0.383
Unemployed
0b
P-value: Analyzed by binary logistic regression analyses and considered significant at < 0.05 (2-tailed); the reference category is: Dependent variable is PCR Plasmodium falciparum positive; b: Parameter considered redundant and set to zero; B: Regression coefficient; ExpB: Exponentiation of B; which is the same as AOR: Adjusted Odds Ratio; 95% CI: 95% Confidence Interval; S.E: Standard Error.
Table 6: Factors associated with malaria infection from logistic regression.
Knowledge of Participants on Malaria Assiated with Malaria Infection
The knowledge of the participants about malaria was assessed; out of the 214 participants, 207 (96.7%) have heard of malaria while 205 (95.8%) confirmed mosquito as the vector for malaria transmission. With regards to their knowledge of the four-malaria parasite: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae and Plasmodium ovale, 150 (70.1%) had no knowledge about the parasite while 64 (29.9%) had knowledge about the four malaria parasites. Majority of the participants 180 (84.1%) do not have knowledge about the fifth malaria parasite, Plasmodium knowlesi.
Their knowledge about the signs and symptoms of malaria were also assessed (multi-checked variable). Majority of the participants 177 (82.7%) responded having fever, 114 (48.6%) responded having loss of energy, 132 (53.3%) responded having headache while only 21(9.8%) responded having itches. In assessing their personal protection against malaria infection (multi- checked variables), 189 (88.3%) uses mosquito net, 70 (32.7%) uses mosquito spray, 65 (30.4%) uses mosquito coil, while 111 (57.9%) uses other forms of protection against the vector.
Majority of the participants 188 (87.9%) have mosquito net while 26 (12.1%) do not use mosquito net. More than half of the participants (60.7%) started using the mosquito net 3 months ago while 84 (39.3%) of the participants responded using the mosquito 36 month ago. Only 56 (26.2%) of the participants used mosquito net a night before filling this questionnaire. Additionally, participants interaction with monkeys were also recorded. None of the participants (100%) had monkey as a pet. All participants live or school at the tourist site (Mole national Park) (Table 7). Presents the knowledge of the participant about malaria infection.
Variables
Frequency
Percentage
Heard Of Malaria
No
7
3.3
Yes
207
96.7
Transmiting Vector
No
9
4.2
Mosquito
205
95.8
Symptoms Of Malaria*
Fever
177
82.7
Loss Of Energy
104
48.6
Vomiting
114
53.3
Sweating
76
35.5
Headache
132
61.7
Body Pain
113
52.8
Chill
91
42.5
Itching
21
9.8
Loss Of Appetite
115
53.7
Protection From Mosquito Bite*
Mosquito Net
189
88.3
Mosquito Spray
70
32.7
Coil
65
30.4
Others
111
51.9
Know Of Plasmodium Parasite
No
150
70.1
Yes
64
29.9
Know Of P. Knowlesi
No
180
84.1
Yes
34
15.9
Malaria Kills
No
19
8.9
Yes
195
91.1
Community Insecticde Spray
No
174
81.3
Yes
40
18.7
Uses Mosquito Net
No
26
12.1
Yes
188
87.9
Period Of Net Use
36 Months Ago,
84
39.3
3 Months Ago,
130
60.7
Use Of Treated Net
No
35
16.4
Yes
179
83.6
Used Net Yesterday
No
158
73.8
Yes
56
26.2
Monkey As Pet
Yes
0
0.0
No
214
100
*Multi-checked variable.
Table 7: Frequencies of Malaria knowledge factor of participants.
Logistic Regression of the Knowledge of Malaria Factors Assiated with Malaria Infection
Using binary regression model, the contribution on the knowledge of malaria of the participants were examined to predict the likelihood of participants being infected with malaria parasite. The prevalence of malaria was not statistically different (p>0.05) in those who have heard of malaria, the knowledge of the transmitting vector, knowledge of the four common plasmodium parasite, knowledge of Plasmodium knowlesi, whether there had been community insecticide spraying, the use of mosquito net, the use of treated net or the use of mosquito net yesterday (Table 8). Presents the logistic regression model on the knowledge of malaria factors Assiated with malaria infection.
B
Wald
S.E.
Exp(B)
95% C.I. for EXP(B)
P-
VALUELower
Upper
Heard of Malaria
Yes
-0.515
0.157
1.299
0.597
0.047
7.618
0.692
No
0b
Transmitting Vector
Yes
0.44
0.158
1.106
1.553
0.178
13.572
0.691
No
0b
Know of 4
Common PlasmodiaYes
-0.429
1.206
0.391
0.651
0.303
1.401
0.272
No
0b
Know of P. Knowlesi
Yes
0.739
1.871
0.540
2.093
0.726
6.031
0.171
No
0b
Community Mosquito
Insecticide SprayYes
0.501
1.296
0.440
1.651
0.696
3.913
0.255
No
0b
Use of Mosquito Net
Yes
0.616
1.651
0.480
1.852
0.723
4.740
0.199
No
0b
Period of Mosquito Net Use
Yes
-0.477
2.222
0.320
0.621
0.332
1.162
0.136
No
0b
Treated Net
Yes
-0.103
0.020
0.732
0.902
0.215
3.789
0.888
No
0b
Used Net Yesterday
Yes
-0.254
0.636
0.319
0.776
0.415
1.449
0.425
No
0b
P-value: Analyzed by binary logistic regression analyses and considered significant at < 0.05 (2-tailed); The reference category is: Dependent variable is PCR Plasmodium falciparum positive; b: Parameter considered redundant and set to zero; B: Regression coefficient; ExpB: Exponentiation of B; which is the same as AOR: Adjusted Odds Ratio; 95% CI: 95% Confidence Interval; S.E: Standard Error.
Table 8: Knowledge of malaria factors associated with malaria infection.
Discussions
The Ghana Health Service [3] annual report indicates that 25.6% of all in-patient malaria cases in the country were recorded from the Northern region of Ghana, which was the highest among other regions in the country. Ghana is divided into three malaria epidemiological zones: the Northern savannah; the tropical rainforest; and the coastal savannah and mangrove swamps. In the Northern Savannah zone, pronounced seasonal variation curs, which has a prolonged dry season from September to April. The normal duration of the intense malaria transmission season in the Northern part of the country is about seven months beginning in April/May and lasting through to September [3]. In endemic settings including the study site where humans coexist with wild animals in a typical forest, the prevalence of malaria infection could be predicted to be high in concurrent with a study conducted by Barber et al.,[29] which reported that “a greater proportion of malaria infected patients lived in or near forested areas”.
From this study, RDT and microscopy reported a malaria prevalence of 22.9% and 18.2% respectively, which is slightly below the malaria prevalence published in National survey of 34.3% and 26.4% for RDT and microscopy respectively [3]. The difference in prevalence of RDT may be attributed to false positives that were detected by RDT test kit [53]. A study done to ascertain the prevalence of falciparum and non-falciparum in symptomatic and asymptomatic participants revealed high malaria prevalence of 87.3% when microscopy was used as the test method Previous study showed higher prevalence of 59%, 21% and 39% respectively for RDT, microscopy and PCR analysis from four different hospitals at the Volta region [53].
Only three plasmodia species out of the 5 known plasmodium species mentioned in this study prevailed at Mole National Park when PCR analysis was done. Majority of the malaria infection detected by PCR results were falciparum infections (39.9%) which is lower than the 90-98% which account for all malaria infections in the country [54].
Although only 0.15% of all malaria cases in Ghana are caused by Plasmodium ovale, 12.5% prevalence was observed in our studies [3]. The difference could be due to the sensitivity of the test method used because PCR is more sensitive than microscopy and RDT [51,53,55]. In consistence with studies done elsewhere in the country, Plasmodium ovale has been found to circulate in the population of the Ashanti’s and in a foreigner who visited a tourist site in the northern part of the country [56]. Plasmodium malariae in this study showed high prevalence of 18.6% compared to the national prevalence of 2 - 9% [3]. These differences may be attributed to cohabitation of participants with NHP and climate of this forest ecological zone [57].
In most African countries and other part of South East Asia, increase in farming and forest activities have been known to increase malaria prevalence [30].
Out of the 214 samples that were tested on PCR, 7.5% were found to be mixed infected. Previous studies conducted by Dinko et al., [53] at Ahafo Ano South district of Ashanti Region showed substantial mixed infections in asymptomatic school children. They detected mixed infection of Plasmodium falciparum with either Plasmodium ovale or Plasmodium malariae that is consistence with our study. A study by Imwong et al., [58] affirms that anopheline mosquitoes may harbor more than one malaria parasite, which can be transferred to human during blood meal resulting in mixed infections or separate insulation from different anopheline mosquitoes.
A study conducted in Mali by Dolo et al., [59] showed simultaneous infection with more than one Plasmodium falciparum parasite (genotype) in asymptomatic school children. At the peak of the transmission season, asymptomatic participants had six to eight different parasites in their blood at the same time. A review of mixed malaria species infections in anopheline mosquitoes showed that, Africa has contributed lesser to the diagnosis of malaria mixed infections globally [58]. This may be due to less advanced methods for plasmodium detection or misdiagnosis.
The persistence of the pan-plasmodium positive identified in the NHP in this study is a cause for concern and warrants further investigation. A laboratory-based study done to ascertain Plasmodium knowlesi model of placenta malaria in baboons (Papio anubis) indicated that the baboons developed acute or severe malaria after day 7 of plasmodium parasite inulation in view of this, the panplasmodium positive baboon will be sequenced to identify the specific plasmodium species present.
Binary regression model of risks factor analysis of the demographic information of having malaria in asymptomatic participants showed that age, gender, marital status, education and cupation did not cause any significant increase or decrease in risks of having malaria even though 39.9% of participant showed high falciparum infection on PCR results. A study conducted by Diallo [59] showed that being single or having no formal education had a higher odd for malaria infection. Another study done to assess demographic risk factors for malaria infection showed that male gender or forest exposures are risk factors for malaria infection [60].
In this study, knowledge of participants on malaria did not show any risk for malaria infection in contrast to a study conducted in pregnant women; knowledge on malaria and the use of mosquito net were assiated with the reduction of malaria infection [21]. Even though most participants (83.6%) have treated mosquito net, only 26.2% sleep under it a night before the demographic data was collected (Table 5). This may be attributable to the hot weather conditions at the time of this cross-sectional study (January to May) which participants complained of discomfort in sleeping under mosquito net.
Conclusion
The prevalence of plasmodium parasite in human samples was 22.9% and 18.2% respectively for RDT and microscopy. A prevalence of 39.9% of Plasmodium falciparum, 18.6% of Plasmodium malariae and 12.6 % of Plasmodium ovale were detected in human samples using PCR. One out of the 3 baboon samples were pan-plasmodium positive but negative for Plasmodium falciparum, Plasmodium ovale and Plasmodium malariae on species-specific PCR. Mixed infections were identified in 7.5% of the human participants. Siodemographic, knowledge on malaria and interaction with monkeys of study participants did not show any significant association with malaria infection.
References
- World Health Organization, World Health Organization 2016. World Malaria Report. 2015.
- Wesolowski R, Wozniak A, Mila-Kierzenkowska C, Szewczyk-Golec K. Plasmodium knowlesi as a Threat to Global Public Health. Korean J. Parasitol. 2015; 53: 575-581.
- Fy-ghana-malaria-operational-plan. 2015. Ghs_Annual_Report_2016.
- Hommerich L, Von Oertzen C, Bedu-Addo G, Holmberg V, Acquah PA, Eggelte TA, et al. Decline of placental malaria in southern Ghana after the implementation of intermittent preventive treatment in pregnancy. Malar. J. 2007; 6: 144.
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2004; 2: CD000363.
- WHO World Malaria Report. 2014.
- Kantele A, Marti H, Felger I, Müller D, Jokiranta TS. Monkey Malaria in a European Traveler Returning from Malaysia. Emerg. Infect. Dis. 2008; 14: 1434-1436.
- Malaria Parasites and Other Haemosporidia PCC. Garnham. Blackwell, Oxford, England; Davis, Philadelphia. 1966: 1132.
- Liu Y, Hsiang MS, Zhou H, Wang W, Cao Y, Gosling RD, et al. Malaria in overseas labourers returning to China: an analysis of imported malaria in Jiangsu Province, 2001–2011. Malar. J. 2014: 13: 29.
- Faust C, Dobson AP. Primate Malarias: Diversity, distribution and insights for zoonotic Plasmodium. One Health. 2015; 1: 66-75.
- Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquited quotidian-type malaria in man transferable to monkeys. Science. 1965; 149: 865.
- Vythilingam I. Plasmodium knowlesi in humans: A review on the role of its vectors in Malaysia. 2010;27: 1-12.
- Knowles R, Gupta BMD. A Study of Monkey-Malaria, and Its Experimental Transmission to Man. Indian Med. Gaz. 1932; 67: 301-320.
- Fana SA, Bunza MDA, Anka SA, Imam AU, Nataala SU. Prevalence and risk factors associated with malaria infection among pregnant women in a semiurban community of north- western Nigeria. Infect. Dis. Poverty. 2015; 4: 24.
- Khanam S. Prevalence and Epidemiology of Malaria in Nigeria: A Review. 2017: 3.
- Cooper DJ, Rajahram GS, William T, Jelip J, Mohammad R, Benedict J, et al. Plasmodium knowlesi Malaria in Sabah, Malaysia, 2015–2017: Ongoing Increase in Incidence Despite Near-elimination of the Human-only Plasmodium Species. Clin. Infect. Dis. 2020; 70: 361-367.
- Cox-Singh J, Davis TME, Lee KS, Shamsul SSG, Matusop A, Ratnam S, et al. Plasmodium knowlesi Malaria in Humans Is Widely Distributed and Potentially Life Threatening. Clin. Infect. Dis. 2008; 46: 165-171.
- Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, et al. Severe malaria - a case of fatal Plasmodium knowlesi infection with postmortem findings: a case report. Malar. J. 2010; 9: 10.
- Vythilingam I, Tan CH, Asmad M, Chan ST, Lee KS, Singh B. Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans. R. Soc. Trop. Med. Hyg. 2006; 100: 1087-1088.
- Malaria, NIH: National Institute of Allergy and Infectious Diseases. 2019.
- Anabire NG, Aryee PA, Abdul-Karim A, Abdulai IB, Quaye O, Awandare GA, et al. Prevalence of malaria and hepatitis B among pregnant women in Northern Ghana: Comparing RDTs with PCR. PLOS ONE. 2019; 14: e0210365.
- Hay SI, Sinka ME, Okara RM, Kabaria CW, Mbithi PM, Tago CC, et al. Developing Global Maps of the Dominant Anopheles Vectors of Human Malaria. PLOS Med. 2010; 7: e1000209.
- Lamb T (Ed.). Immunity to Parasitic Infection, 1 edition. Ed. Wiley-Blackwell, Chichester, West Sussex, UK ; Hoboken, NJ. 2012.
- John DT. Markell and Voge’s Medical Parasitology, 9/E edition. Ed. Elsevier. 2016.
- Greenwood D, Slack RCB, Barer MR, Irving WL. Medical Microbiology E-Book: With STUDENTCONSULT online access. Elsevier Health Sciences. 2012.
- Chaudhury A, Venkataramana B. Plasmodium knowlesi: The fifth malaria parasite. J. Clin. Sci. Res. 2017; 6: 171.
- Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, et al. Severe malaria - a case of fatal Plasmodium knowlesi infection with postmortem findings: a case report. Malar. J. 2010; 9: 10.
- Daneshvar C, Davis TME, Cox-Singh J, Rafa’ee MZ, Zakaria SK, Divis PCS, et al. Clinical and Laboratory Features of Human Plasmodium knowlesi Infection. Clin. Infect. Dis. 2009; 49: 852-860.
- Barber BE, William T, Grigg MJ, Meno J, Auburn S, et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013; 56, 383-397.
- Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar. J. 2013; 12: 8.
- Willmann M, Ahmed A, Siner A, Wong IT, Woon LC, Singh B, et al. Laboratory markers of disease severity in Plasmodium knowlesi infection: A case control study. Malar. J. 2012; 11: 363.
- Rampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: Severe malaria. Crit. Care. 2003; 7: 315-323.
- Losert H, Schmid K, Wilfing A, Winkler S, Staudinger T, Kletzmayr J, et al. Experiences with severe P. falciparum malaria in the intensive care unit. Intensive Care Med. 2000; 26: 195-201.
- Lee K.S, Cox Singh J, Singh B. Morphological features and differential counts of Plasmodium knowles i parasites in naturally acquired human infections. Malar. J. 2009; 8: 73.
- Snounou G, Singh B. Nested PCR analysis of Plasmodium parasites. Methods Mol. Med. 2002; 72: 189-203.
- Lucchi NW, Oberstaller J, Kissinger JC, Udhayakumar V. Malaria Diagnostics and Surveillance in the Post-Genomic Era. Public Health Genomics. 2013; 16: 37-43.
- Lucchi NW, Poorak M, Oberstaller J, DeBarry J, Srinivasamoorthy G, Goldman I, et al. A New Single-Step PCR Assay for the Detection of the Zoonotic Malaria Parasite Plasmodium knowlesi. PLOS ONE. 2012; 7: e31848.
- Aninagyei E, Smith Graham S, Boye A, Egyir Yawson A, Acheampong DO. Evaluating 18s-rRNA LAMP and selective whole genome amplification (sWGA) assay in detecting asymptomatic Plasmodium falciparum infections in blood donors. Malar. J. 2019; 18: 214.
- Iseki H, Kawai S, Takahashi N, Hirai M, Tanabe K, Yokoyama N, et al. 2010. Evaluation of a loop-mediated isothermal amplification method as a tool for diagnosis of infection by the zoonotic simian malaria parasite Plasmodium knowlesi. J. Clin. Microbiol. 2010; 48: 2509-2514.
- Lau YL, Fong MY, Mahmud R, Chang PY, Palaeya V, Cheong FW, et al. Specific, sensitive and rapid detection of human plasmodium knowlesi infection by Loop-Mediated Isothermal Amplification (LAMP) in blood samples. Malar. J. 2011; 10: 197.
- Antinori S, Galimberti L, Milazzo L, Corbellino M. Biology of Human Malaria Plasmodia Including Plasmodium Knowlesi. Mediterr. J. Hematol. Infect. Dis. 2012; 4: e2012013.
- Green R. A malarial parasite of Malayan monkeys and its development in anopheline mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1932; 25: 455-477.
- MacFie JWS. A Malaria Infection of the Baboon Papio Sphinx. Proc. R. Soc. Med. 1928; 21: 467-471.
- Yusof R, Lau YL, Mahmud R, Fong MY, Jelip J, Ngian HU, et a. High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar. J. 2014; 13: 168.
- Foster D, Cox-Singh J, Mohamad DSA, Krishna S, Chin PP, Singh B. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar. J. 2014; 13: 60.
- Kawai S, Hirai M, Haruki K, Tanabe K, Chigusa Y. Cross-reactivity in rapid diagnostic tests between human malaria and zoonotic simian malaria parasite Plasmodium knowlesi infections. Parasitol. Int. 2009; 58: 300-302.
- Vythilingam I, NoorAzian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, et al. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit. Vectors. 2008; 1: 26.
- Eede PV den, Van HN, Van Overmeir C, Vythilingam I, Duc TN, Hung LX, et al. Human Plasmodium knowlesi infections in young children in central Vietnam. Malar. J. 2009; 8: 249.
- Imwong M, Tanomsing N, Pukrittayakamee S, Day NPJ, White NJ, Snounou G. Spurious Amplification of a Plasmodium vivax Small-Subunit RNA Gene by Use of Primers Currently Used To Detect P. knowlesi. J. Clin. Microbiol. 2009; 47: 4173-4175.
- Sulistyaningsih E, Fitri LE, Löscher T, Berens-Riha N. Diagnostic Difficulties with Plasmodium knowlesi Infection in Humans. Emerg. Infect. Dis. 2010; 16: 1033-1034.
- Divis PC, Shokoples SE, Singh B, Yanow SK. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar. J. 2010; 9: 344.
- Dinko B, Ayivor-Djanie R, Abugri J, Kye-Duodu G, Tagboto S, Tampuori J, et al. Comparison of malaria diagnostic methods in four hospitals in the Volta region of Ghana. 2016; 7: 5.
- Dinko B, Oguike MC, Larbi JA, Bousema T, Sutherland CJ. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian schoolchildren. Int. J. Parasitol. Drugs Drug Resist. 2013; 3: 45-50.
- Malaria-country-profiles. Malarial Parasites recorded from Monkeys of the Families Cercopithecoidea and Colobidae. Rec. Malar. Surv. India. 2011; 3: 381-444.
- Siwal N, Singh US, Dash M, Kar S, Rani S, Rawal C, et al. Malaria diagnosis by PCR revealed differential distribution of mono and mixed species infections by Plasmodium falciparum and P. vivax in India. PLOS ONE. 2018; 13: e0193046.
- Tordrup D, Virenfeldt J, Andersen FF, Petersen E. Variant Plasmodium ovale isolated from a patient infected in Ghana. Malar. J. 2011; 10: 15.
- Lindsay SW, Martens WJ. Malaria in the African highlands: Past, present and future. Bull.World Health Organ. 1998; 76: 33-45.
- Imwong M, Nakeesathit S, Day NPJ, White NJ. A review of mixed malaria species infections in anopheline mosquitoes. Malar. J. 2011; 10: 253.
- Dolo A, Masinde GL, Sagara I, Diallo M, Koita OA, Doumbo, OK, et al. False- Negative Rapid Diagnostic Tests for Malaria and Deletion of the Histidine- Rich Repeat Region of the hrp2 Gene. Am. J. Trop. Med. Hyg. 2012; 86: 194-198.
- Herdiana H, Cotter C, Coutrier FN, Zarlinda I, Zelman BW, Tirta YK, et al. Malaria risk factor assessment using active and passive surveillance data from Aceh Besar, Indonesia, a low endemic, malaria elimination setting with Plasmodium knowlesi, Plasmodium vivax, and Plasmodium falciparum. Malar. J. 2016; 15: 468.
