Abstract
Airborne microorganisms have potential to cause infections and respiratory diseases like asthma. It is necessary to reduce the human exposure to such pathogens. This study was based on the determination of indoor environmental quality and the quantification of microbial load in a university premises in Karachi, Pakistan. Seven different locations with high human occupancy during office hours were sample din winter and summer. Culturable microorganisms i.e. bacteria and fungi were collected on selective media by Spin Air V2 sampler. The average bacteria and fungi concentration sin winter were133 ± 26colony forming unit per cubic meter (CFU.m-3) and 199 ± 59 CFU.m-3 respectively. In summer counts were higher than winter with bacterial and fungal count of 205 ± 39 and 306 ± 102 CFU.m-3. There was strong correlation (r = 0.8, p = 0.032) between fungal count and humidity, and between bacterial and fungal count (r = 0.802, p = 0.030). The most common culturable airborne fungi in both seasons were Aspergillus, Penicillium, Cladosporium, Fusarium, Alternaria, Stachybotrys, Candida, and Rhodoturola. Resultsindicate that the indoor environment in university premises harbours high concentrations of microorganism and needs regular indoor air quality monitoring and establish local guidelines to prevent diseases.
Keywords: Airborne; Indoor environmental quality; Microbial load; Bacteria; Fungi; Humidity
Introduction
Microorganisms are inevitable in enclosed environment and air due to their presence in nature [1-3]. Indoor work places are continuously challanged by microorganisms including bacteria and fungi which can detriorate the indoor environmental quality. Alternaria, Aspergillus, Penicillium and Cladosporium are common filamentous fungi (Mold) found in indoor environment [4]. They are known as allergen fungi and isolated from patients having chronic sinusitis [5]. The standard for indoor environment stated that pathogenic and toxigenic fungi such as Aspergillus fumigatus and Stachybotrys atra are not permitted in indoor environment [6]. According to WHO [6] if single specie present at a concentration of > 50 CFU.m-3 investigation should be carried out properly. In the case of multiple species, 150 CFU.m-3 is acceptable, while if only common phylloplane fungi are present in indoor environment such as Cladosporium then 500 CFU.m-3 is acceptable.
Common bacteria of indoor environment are members of Propionibacterineae, Xanthomonadaceae, Micrococcineae, Enterobacteriaceae and Corynebacterineae [7]. Sphingomonas, Caenibacterium, Staphylococcus [7] and Streptococcus species are also found [8]. Gram-positive bacteria found in indoor environment are members of Phylum Firmicutes, while Gram-negative includes the family Oxalobacteraceae, Comamonadaceae, Neisseriaceae and Rhizobiaceae [8].
It is evident from previous studies that presence of toxigenic fungi [9-11] and bacteria [12] is significantly influenced with environmental factors such as dampness and humidity. Dampness, ventilation, heating and cooling systems [13] can cause Sick Building Syndrome (SBS), organic dust toxic syndrome and respiratory infections i.e. Legionellosis, humidifier fever, and hypersensitivity pneumonitis [14]. These infections can be transmitted through the air, affecting people working in this environment or associated with the building [15].
Dampness is measured as relative humidity in indoor environment. The USA Environmental Protection Agency allows 30- 50% relative humidity [16] while the International Energy Agency allows up to 80% [17]. An association has been reported not only between dampness and mold growth in indoor environment [4], but also between dampness, mold growth and respiratory symptoms [18,19]. Relationship was found between dampness in buildings and asthma [20] and sinusitis [21]. Prolonged exposure with mold in damp building can result in bronchial obstruction [22-23]. Indoor humidity and mold are associated with increased exacerbation, dyspnea, wheeze, cough, allergic rhinitis, eczema, and upper respiratory tract symptoms [12].
Temperature in indoor environment occurs in wide range which is favourable for the growth of microorganisms specially fungi. Most indoor fungi grow best between 10-35°C and cause considerable health effect such as hypersensitivity pneumonitis [13]. Human occupancy causes an increase in bacterial load [15]. Another important factor which determines the indoor microbial communities is ventilation which increases air borne bacteria even when there is no human occupant [24]. Good ventilation in indoor environment in university buildings has positive impacts on human health and productivity [24]. Improper ventilation can cause tiredness, headache, immunity weakness and sick building syndrome. It can affect the building structure and associate with mold growth [25]. Temperature, wind speed and air pressure have impacts on ventilation in indoor environment [25]. Presence of fungi in indoor environment is found to be positively associated with temperature and air exchange rate [26]. Increased wind speed resulted in decrease CO2 concentrations in indoor environment. Well design and well maintained buildings are necessary for the prevention of microbial growth (WHO, 2009).
Seasons with high microbial concentration can cause more infections [26]. Concentration and type of fungi were found to be higher in winter [27]. Cladosporium was dominant in summer in indoor environment while in winter Penicillium and Aspergillus were common [27]. Study of indoor environment of homes indicated significant seasonal variation in fungal count; high in summer and low in winter, while indoor bacteria were highest in spring and lowest in summer [26]. Increase in fungal spore count due to season can result in increased asthma in children [28]. Firmicutes were dominant in winter while proteobacteria were more common in summer [8].
Three diffferent ventilation systems viz. natural ventilation, mechanical ventilation and mixed model ventilation are normally utilised in commercial and non-residential building [29]. In natural ventilation, windows, doors, skylights and roof ventilators supplies an ample amount of air for for building. In mechanical ventilation, air is supplied, conditioned and thermally regulated with Heating and Verntilating Air Conditioning system (HVAC). The HVAC system intake outodoor air followed by filteration, heats/cools, dehumdifies/ humdifies and distribute through duct network to air vents located in the building. In mixed model ventliation very common in Pakistan, combining natural and mechanical ventilation system through use of window unit type air conditioners in high use spaces but with a substaintial and highly variable natural ventilation component through opening windows and doors. Presence of bacteria and fungi in the indoor environments such as schools, offices and residences increases the chances of exposure to harmful bio-aerosols and thus becomes a public health concern [30]. There is a need for understanding of the likely association between indoor air pollutants, environmental factors and human health, regular monitoring and quantification of exposure to airborne microorganisms.
In Pakistan, especially in highly populated city like Karachi, indoor air quality is a growing concern and published literature regarding indoor air pollution is limited [31]. Karachi has a warm humid climate with short spell of winter (December to February). Karachi air quality is relatively low compared to the rest of the World. The main contributing source of Karachi’s air pollution is fossil fuel combustion, specifically vehicle and industrial exhaust. Also, postharvest open burning of agricultural fields in winter can cause severe pollution events for a few days in a year. The ambient indoor air quality across Karachi has not been studied in the literature and the contribution of outdoor air pollutants to indoor environment had not been described by previous studies. The aim of this bio-aerosol sampling was the quantitative evaluation of the viable airborne bacteria and fungi in university premises in winter and summer seasons in Karachi and to determine the relationship between concentration of microorganism and different environmental factors. The hypothesis of this study was that the environmental factors such as humidity and temperature are the major reasons for the increased microbial count in indoor environment in university premises. Besides the standard enumeration of culturable microbes as CFU.m-3, this study attempted to analyse various environmental factors including temperature, relative humidity, dew point, barometric pressure and wind-speed to find the relationship.
Materials and Methods
Sampling Llocation
The bio-aerosols samples were collected from public sector university premises having more than 10,000 students and staff. Most of the students and staff come from all over the city. Seven different categories of indoor areas with mix and mixed model ventilation system were selected for airborne microorganisms and microclimatic parameters monitoring. These include class rooms, laboratories, offices, canteens, common rooms, libraries and computer labs. Twenty-one different locations (n=3 for each category) were sampled in duplicate on two different days in NED University of Engineering and Technology, Karachi in winter and summer seasons during months of December and January for winter sampling and April and May 2019 for summer. Attributes of the sampled locations are given in Table 1. In order to maintain the uniformity, all samples for microclimatic and bio-aerosol analysis were collected on the same day. That is, a total of 21 samples (3 from each category) were collected on the same day in summer and then it was repeated in winter.
Categories
Sampling points
Material
Floor type
Ventilation type
Surface area (m2)
Estimated population density (persons/10 m2)
Class rooms
3
Brick
Tiled
Natural
67
60
Laboratories
3
Brick
Mixed model ventilation
89.2
35
Offices
3
Brick
Tiled
Natural
22
10
Canteens
3
Brick
Tiled
Natural
150
100
Common rooms
3
Brick
Tiled
Mixed model ventilation
110
100
Libraries
3
Brick
Tiled
Mixed model ventilation
250
100
Computer labs
3
Brick
Timber
Mixed model ventilation
150
50
Table 1: Attributes of the sampled locations.
All bio-aerosol samples were collected in working hours using the Spin Air V2 (IUL, USA) sampler to assess the indoor airborne concentration of cultivable bacteria and fungi. The sampling height which was approximated to human breathing zone and at the center of the room. For bio-aerosol analysis, 300 litres of air were sampled using the Spin Air V2 sampler at a rate of 60 L.min-1 on selected agar medium onthe culture plate [32]. For uniform air impact on sampled agar media, the plate was rotated at 2 rpm. Before or after each sampling, the sampler surface was disinfected with a 70% ethanol solution to avoid the contamination.
The microclimatic parameters; wind speed, temperature, relative humidity, dew point and ambient pressure were measured using the 4000NV, Kestrel, Pocket Weather Tracker. All samples were collected during working hours or when human occupancy was involved (8:30 am – 2:30 pm). Samples were collected in duplicate in centre of the room well above the ground level (approximated to human breathing zone).
Enumeration of Bacteria and Fungi
Total culturable bacteria and fungi were impacted onto Tryptone soya agar (TSA, Merck) [33] and Malt extract agar (MEA, Merck) [34], respectively. TSA plates were incubated at 37oC for 24 h and MEA plates were incubated at 25°C for 72 h for the development of individual colonies. Colonies were counted by colony counter and colony forming units per cubic meter of air (CFU. m-3) were calculated using the volume of air sampled for both bacteria and fungi. Relative microbial abundances were calculated by the formula (number of colonies of a genus x 100/ number of colonies of all genera [35]. Microscopically, fungal genera were identified to genus level based on growth characteristics, colony morphology, and pigmentation on agar media and examination of colonies by lectophenol cotton blue for microscopic morphology and spore pattern. Published material was used for the identification of fungal genera [36-37].
Statistical Analysis
Statistical analysis was carried out by using Minitab 16. Pearson’s correlation (a=0.05) was calculated between bacterial and fungal concentrations and meteorological parameters in indoors environment to determine the relationships between two factors (bacterial concentration vs. humidity, fungal concentration vs. humidity, etc.).Two sample t-test (a=0.05) was performed to determine the difference between studied parameter during winter and summer seasons. All statistical tests were based on 95% confidence interval.
Results and Discussion
Bacterial Count in Indoor Environment in Summer and Winter
Table 2 shows the bacterial counts from seven different places in winter and summer seasons in the university premises. As can be seen in the table, in the winter season, common rooms are the places with highest number of bacterial counts, followed by class rooms and teacher offices. Also, as shown in Table 2, laboratories, libraries and computer labs have similar microbial counts. It is interesting to observe that the canteens, despite having high population density, have lowest number of bacteria. Canteen owners might be following the good hygiene rules and cleaning the places regularly which resulted in low bacterial count as compare to other places in University premises. The canteens surfaces are cleaned regularly multiple times in a day as compare to library, offices and other places where surfaces are cleaned only once during a working day.
Sampling points
Bacterial count on TSA (CFU.m-3±SD)
Fungal count on MEA (CFU.m-3±SD)
Winter
Summer
Winter
Summer
Class room
162±30
232±11
276±61
412±52
Laboratory
112±1
214±48
274±52
424±87
Office
151±76
253±121
208±46
351±149
Canteen
107±25
188±24
150±18
281±79
Common room
168±67
231±57
174±32
319±35
Library
114±19
143±13
193±20
189±48
Computer lab
118±26
170±44
120±27
162±30
TSA: Tryptic soya agar, MEA: Malt extract agar, CFU: Colony forming unit
Table 2: Bacteria and fungi on selective media during winter and summer seasons from different locations in University (n=21 sets).
In summer, as shown in Table 2, the highest average bacterial count of 253±121CFU.m-3 was observed in faculty offices. A vast difference in microbial count was also observed among these offices, which is indicated by the relatively high Standard Deviation (SD) values. The lowest count in offices was 113±7.0 CFU.m-3 while the highest count observed was 493±18.1CFU.m-3. Several environmental conditions such as ventilation, human occupancy and dampness might be the reasons for this variable count. Proper ventilation can reduce the microbial count in indoor environment. On the other hand, human occupancy [15] and dampness [4] can increase the microbial load. Table 2 also shows that the classrooms and common rooms have almost similar bacterial counts. High human occupancy might be a reason for the increase microbial count in these areas of University and both of there are cleaned once a week. Moreover, after finishing the working hours, the rooms were locked and opened again on next day.
Table 3 shows the morphological identification of bacteria in the indoor environment. As indicated in the table the Gram-negative short rods were the most dominant group in the university indoor environment and their relative abundance was 45.2%.Relative abundance of bacteria with respect to total bacteria indicates that the bacilli in chain and filamentous bacteria are also common in indoor places; 16.7 and 11.9%, respectively. Outdoor air might be the reason of their presence in indoor air. Several studies indicate that the outdoor air determines the composition of indoor air [24,38-39].
Organisms
Organisms Morphology
Relative abundance
Gram positive
Bacilli in chain
16.7%
Filamentous / branching bacteria (Actinomycetes)
11.9%
Diplococci / tetrads
9.5%
Cocci in bunches
7.1%
Coccobacilli
4.8%
Diptheroid rods
4.8%
Gram negative
Scattered short rods
45.2%
Table 3: Relative abundance of bacteria in indoor environment.
Fungal Count in Indoor Environment in Summer and Winter
Fungi growth on Malt extract agar in winter season showed that fungal count was highest in class rooms (276±61 CFU.m-3) followed by laboratories and offices (Table 2). Lowest numbers of fungi (120±27 CFU.m-3) were isolated from computer labs which have mixed model ventliation systems for cooling. Low temperature due to air conditioners might be the reason of low fungal count as same was the case in summer season when lowest count was observed in computer labs which was 162±30 CFU.m-3(Table 2). On the other hand, the high fungal count in summer season was found in laboratories, classrooms and offices which were 424±87, 412±52 and 351±149 CFU.m-3, respectively. This indicates that the presence of fungi was a continuous issue in these places irrespective of seasons. Contrarily to this, bacterial count was variable in sampling places in both seasons. Mean values for temperature, humidity, barometric pressure, dew point and wind speed are presented in (Table 4).
Sampling points
Temperature (oC)
Humidity (%)
Dew point (°C)
Barometric Pressure (psi)
Wind Speed (mph)
Winter
Summer
Winter
Summer
Winter
Summer
Winter
Summer
Winter
Summer
Class room
22±0.6
30.7±0.9
55.0±1.2
37.5±9.1
18.6±0.5
14.0±4.2
14.5±0.01
14.6±0.02
1.7±0.1
1.7±0.5
Laboratory
21.8±0.6
30.2±0.5
59.9±3.2
39.2±7.4
19.9±0.6
13.9±2.5
14.5±0.03
14.6±0.0
1.1±0.2
2.6±0.7
Office
21.2±0.4
30.1±0.3
53.8±2.4
32.1±0.9
18.3±0.9
14.3±2.3
14.6± 0.0
14.6±0.03
1.1±0.3
2± 0.8
Canteen
21.7±0.3
32±1.1
38.9±9.7
56.8±1.9
13.1±5.1
22.3±1.4
14.6±0.03
14.6±0.03
1.3±0.1
1.0± 0.2
Common room
21.3±0.4
31.6±1.4
28.2±2.3
57.4±2.5
8.0±1.7
22.0±1.0
14.7±0.1
14.6±0.0
2.2±0.6
2±1.0
Library
21.2±0.4
31.7±1.1
40.2±1.3
47.9±1.7
13.5±0.9
19.2±0.6
14.6±0.0
14.5±0.03
2.7±0.7
1.4±0.4
Computer lab
21.3±0.3
29.1±1.4
38.4±7.6
39.5±8.5
13.5±2.5
12.4±3.3
14.6±0.03
14.6±0.03
1.4±0.3
0.9±0.1
Table 4: Temperature, humidity, dew point, barometric pressure and wind speed (Mean ± SEM) at different locations in University premises.
Morphological analysis indicates that among the fungal groups grown on the MEA media, 78.4% were molds (filamentous fungi) while only 21.6% were yeast colonies (Table 5). Thus the mycelial molds were the most dominant group of fungi in indoor environment in university premises. Morphological study for the identification of fungi genus showed the presence of Aspergillus, Penicillium, Cladosporium, Fusarium, Alternaria, Stachybotrys, Candida, and Rhodoturola species. Molds were isolated from all sampling places while yeast was found in 61.90% of the sampling areas. Among molds, Cladosporium species were the most dominant mold and were isolated in 88.1% of samples. It was followed by Penicillium and Aspergillusspecies which were isolated from 83.3% and 76.2% of the samples, respectively. Isolation of Stachybotrys, Alternaria and Fusarium species were relatively low and they were present in 14.3%, 4.8% and 2.4% of samples, respectively. Candida species were present in 61.9% samples while Rhodoturola species were found in 23.8% samples (Figure 1). Relative abundance of each fungus count with respect to total fungi count isolated from different sampling sites from University premises was as follow; Cladosporium sp.= 30.7%, Aspergillus sp.= 22.3%, Penicillium sp. = 22%, Stachybotrys sp.= 2.6%, Alternaria sp.= 0.7%, Fusarium sp.= 0.03%, Candida sp. = 21% and Rhodoturola sp. = 0.6% (Table 5).
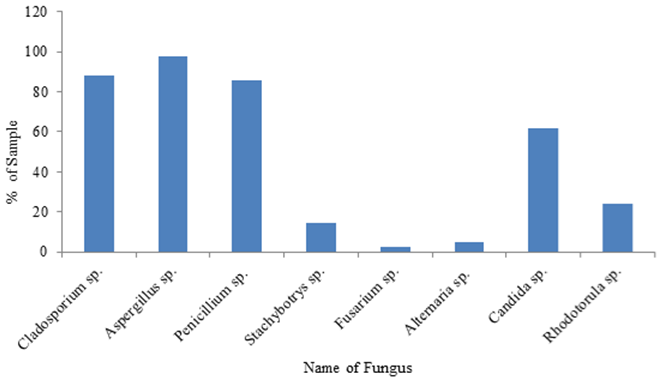
Figure 1: Distribution of fungi in total samples.
Organisms
Relative abundance
Mold
Alternaria
0.7%
Aspergillus
22.3%
Cladosporium
30.7%
Fusarium
0.03%
Penicillium
22%
Stachybotrys
2.6%
Yeast
Candida
21%
Rhodoturola
0.6%
Table 5: Relative abundance of fungi (mold and yeast) in indoor environment.
Bacteria presence in respect to area indicated that Grampositive bacilli and Gram-negative short rods were dominant in university indoor environment as they were present in all sampling sites except computer lab (Table 6). Coccobacilli and diptheroid rods were another dominant group found in offices, canteens and computer labs. Diplococci and bunches were found in 2 sampling sites only. Offices are the places where maximum seven varieties of bacteria were found. Fungal presence indicated that Aspergillus, Penicillium, Candida and Rhodoturola were dominant and present in all seven sampling sites (Table 6). Cladosporium was isolated from six sampling sites. Stachybotrys was present in offices, canteens and computer labs. Alternariawas found in laboratories and offices while Fusarium was present in computer labs only. According to WHO standard, Aspergillus and Stachybotrys species are not allowed in indoor environment [6]. Their presence in sampling sites might be harmful for human occupants.
Statistical analysis by two sample t-test (p = 0.05) indicated that there were significant differences in mean temperatures (p = 0.00), bacterial counts (p = 0.002) and fungal counts (p = 0.035) in winter and summer seasons. Mean bacterial and fungal counts were higher in summer (205 ± 39 and 306 ± 102 CFU.m-3) compare to winter season (133 ± 26 and 199 ± 59 CFU.m-3). Temperature might be a reason for this elevated count as mean temperature was high in summer (30.8 ±0.4°C), while it was 21.5 ± 0.1°C in winter. There was no significant difference in wind speed, barometric pressure, dew point and humidity (p>0.05). Correlation analysis by Pearson’s correlation test (p< 0.05) indicated that there were significant correlations between bacterial and fungal count in summer (r = 0.802, p = 0.030), wind speed and fungal count in summer (r = 0.77, p = 0.043) and between humidity and fungal count in winter (r = 0.79, p= 0.03). These results were consistent with previous studies where a relationship was reported between dampness and microbial count [9-10]. Not only fungal growth Adams et al. [11], but bacterial growth can also be higher in moist environment [12]. Several fungal genera have been reported in indoor environment including Acremonium, Alternaria, Aspergillus, Chaetomium, Cladosporium, Mucor, Paecilomyces, Penicillium, Stachybotrys, Ulocladium and Verticillium [40,-42].
Previous epidemiological studies showed that the dampness is associated with increase rate of respiratory illnesses including cough, asthma, bronchitis and eczema [12]. Poor indoor air quality can have resulted in less productivity of occupants [43-44]. Toxic reactions, hypersensitivity pneumonitis and other health effects may occur due to the exposure to airborne microorganisms containing aerosol [38]. Additionally, moisture content and humidity can determine the level and type of microorganisms in indoor environment.
Conclusion
In the current study, the presence of elevated concentration of fungi and its significant relationship with moisture in indoor environment indicates that the occupants of these indoor places in university premises are at a risk of respiratory infections. They include students, teaching faculty, and office staff who work for long hours in university. The potential of indoor microorganism especially the molds to cause infections indicates that there is a need of detailed epidemiological study and both indoor and outdoor air quality monitoring in the city and educational institutions with high public occupancy. Furthermore, there is a need of indoor air quality management for the prevention of epidemic of respiratory diseases in occupants of indoor environment.
References
- Mancinelli RL, Shulls WA. Airborne Bacteria in an Urban Environment. Applied and Environmental Microbiology. 1978; 35: 1095-1101.
- Brief RS, Bernath T. Indoor pollution: Guidelines for prevention and control of microbiological respiratory hazards associated with air conditioning and ventilation systems. Appl Ind Hyg. 1988; 3: 5–10.
- Robertson LD. Monitoring viable fungal and bacterial bioaerosol concentrations to identify acceptable levels for common indoor environments. Institute of Environmental Sciences and Technology, Contamination Control Proceedings of the 44th Annual Technical Meeting, Phoenix, Arizona, USA. 1998; 73-79.
- Dales RE, Miller JD, Mc Mullen ED. Indoor air quality and health: Validity and determination of reported home dampness and mould. Int J Epidemiol. 1997; 26: 120–125.
- Shin S, Ye M, Lee Y. Fungus Culture of the Nasal Secretion of Chronic Rhinosinusitis Patients: Seasonal Variations in Daegu, Korea. American Journal of Rhinology & Allergy. 2007; 21: 556-559.
- World Health Organization WHO: Regional Office for Europe. Regional Publications European Series, No. 31: Indoor air quality: biological contaminants: report on a WHO meeting, Rautavaara, 29th August- 2nd September 1988 Copenhagen, Denmark. http://who.int/iris/ handle/10665/260557(1998). Accessed 17 June 2021.
- Kelley ST, Gilbert JA. Studying the microbiology of the indoor environment. Genome Biology. 2013; 14: 202.
- Rintala H, Pitkäranta M, Toivola M, Paulin L, Nevalainen A. Diversity and seasonal dynamics of bacterial community in indoor environment. BMC Microbiology. 2007; 8: 56.
- Andersson MA, Nikulin M, Koljag U, Andersson MC, Rainey F, et al. Bacteria, molds and toxins in water damaged materials. Appl Env Microbiol. 2008; 63: 387–393.
- Etzel RA, Montana E, Sotenson WG, Kullman GJ, Allen AT, et al. Acute pulmonary haemorrhage in infants associated with Stachybotrysatra and other fungi. Arch Pediat Adol Med. 1998; 152: 757–762.
- Adams RI, Bhangar S, Dannemiller KC, Eisen JA, Fierer N, et al. Ten questions concerning the microbiomes of buildings. Build Env. 2016; 109: 224–234.
- Dedesko S, Siegel JA. Moisture parameters and fungal communities associated with gypsum drywall in buildings. Microbiome. 2015; 3.
- World Health Organization WHO: WHO guidelines for indoor air quality: dampness and mould. WHO Regional Office for Europe, Denmark. Edited by: Elisabeth H, Jerome R. 2009; 1-248.
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environmental Health Perspectives. 2011; 119: 748-756.
- Prussin AJ, Marr LC. Sources of airborne microorganisms in the built environment. Microbiome. 2015; 3.
- Sheldon LS, Sparacino CM, Pellizzari ED. Review of analytical methods for volatile organic compounds in the indoor environment. Indoor Air and Human Health. 1985; 335-350.
- Hospodsky D, Qian J, Nazaroff WW, Yamamoto N, Bibby K, Rismani-Yazdi H, et al. Human Occupancy as a Source of Indoor Airborne Bacteria. PLoS ONE. 2012; 7.
- Environmental Protection Agency EPA: A brief guide to mold, moisture, and your home. United States Environmental Protection Agency. 2010.
- IEA: Condensation and Energy: Source Book Report Annex XIV. 1991. http:// ecbcs.org/annexes/annex14.htm (1991). Accessed 16 June 2021.
- Brunekreef B. Damp housing and adult respiratory symptoms. Allergy. 1992; 47: 498-502.
- Dales RE, Zwanenburg H, Burnett R, Franklin CA. Respiratory health effects of home dampness and molds among Canadian children. American journal of epidemiology. 1991; 134: 196-203.
- Douwes J, Pearce N. Invited commentary: is indoor mold a risk factor for asthma?. Am J Epidemiol. 2003; 158: 203–206.
- deShazo RD, Chapin K, Swain RE. Fungal sinusitis. The New England journal of medicine. 1997; 337: 254-259.
- Jaakkola JJK, Jaakkola N, Ruotsalainen R. Home dampness and molds as determinants of respiratory symptoms and asthma in pre-school children. J Expo Anal Env Epidemiol. 1993; 3: 129–142.
- Husman T. Health effects of indoor-air microorganisms. Scandinavian journal of work, environment & health. 1996; 22: 5-13.
- Meadow JF, Altrichter AE, Kembel SW, Kline J, Mhuireach G, et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air. 2014; 24: 41–48.
- S´wiercz EZ. Analysis of the impact of the parameters of outside air on the condition of indoor air. Int J Env Sci Tech. 2017; l 14: 1583–1590.
- Frankel M, Bekö G, Timm M, Gustavsen S, Hansen EW, et al. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl Env Microbiol. 2012; 78: 8289– 8297.
- REN P, JANKUN TM, LEADERER BP. Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dusts of dwellings in one Northeast American county1. Journal of Exposure Analysis and Environmental Epidemiology. 1999; 9: 560-568.
- Atkinson RW, Strachan DP, Anderson HR, Hajat S, Emberlin J. Temporal associations between daily counts of fungal spores and asthma exacerbations. Occupational and Environmental Medicine. 2006; 63: 580-590.
- Irga PJ, Torpy FR. Indoor air pollutants in occupational buildings in a subtropical climate: comparison among ventilation types. Build & Env. 2016; 98: 190–199.
- Miller JD, Haisley PD, Reinhardt JH. Air sampling results in relation to extent of fungal colonization of building materials in some water-damaged buildings. Indoor air. 2000; 10: 146-151.
- Colbeck I, Nasir ZA, Ali Z. The state of indoor air quality in Pakistan—a review. Environmental Science and Pollution Research. 2010; 17: 1187-1196.
- NIOSH: Manual of Analytical Methods NMAM Edited by Kevin A, Paula FC. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. 2017.
- Valeriani F, Cianfanelli C, Gianfranceschi G, Stucki SR, Spica V.et al. Monitoring biodiversity in libraries: a pilot study and perspectives for indoor air quality. J Prev Med Hyg. 2017; 58: E238–E251.
- Taylor J, Shrubsole C, Davies M, Biddulph P, Das P, Hamilton I, et al. The modifying effect of the building envelope on population exposure to PM2.5 from outdoor sources. Indoor Air. 2014; 24: 639-651.
- Borrego S, Lavin P, Perdomo I, Saravia SGD, Guiamet P. Determination of Indoor Air Quality in Archives and Biodeterioration of the Documentary Heritage. ISRN Microbiology. 2012; 2012: 1-10.
- Vijayakumar R, Sandle T, Manoharan C. A review of fungal contamination in pharmaceutical products and phenotypic identification of contaminants by conventional methods. Eur J Parent Pharm Sci. 2012; 17: 4–19.
- Ellis D, Davis S, Alexiou H, Handke R, Bartley R. Robyn Bartley descriptions of medical fungi. Second edition, Mycology Unit, Women’s and Children’s Hospital, North Adelaide. Australia. 2007.
- Yassin MF, Almouqatea S. Assessment of airborne bacteria and fungi in an indoor and outdoor environment. Int J Env Sci Tech. 2010; 7: 535–544.
- Miletto M, Lindow SE. Relative and contextual contribution of different sources to the composition and abundance of indoor air bacteria in residences. Microbiome. 2015; 3.
- Gravesen S, Nielsen PA, Iversen R, Nielsen KF. Microfungal contamination of damp buildings--examples of risk constructions and risk materials. Environmental Health Perspectives. 1999; 107: 505-508.
- Khan AAH, Karuppayil SM. Fungal pollution of indoor environments and its management. Saudi J of Bio Sci. 2012; 19: 405–426.
- Nunez M, Hammer H. Microbial specialists in below-grade foundation walls in Scandinavia. Indoor air. 2014; 24: 543-551.
- Al Horr Y, Arif M, Kaushik A, Mazroei A, Katafygiotou M, Elsarrag E. Occupant productivity and office indoor environment quality: A review of the literature. Building and Env. 2016; 105: 369–389.
- Al Horr Y, Arif M, Katafygiotou M, Mazroei A, Kaushik A, Elsarrag E. Impact of indoor environmental quality on occupant well-being and comfort: A review of the literature. Int J Sustainable Built Env. 2016; 5: 1–11.
