Abstract
Pyrolysis is the thermochemical decomposition of organic materials at high temperatures in the absence of oxygen. The process usually takes place in a sealed vessel under high pressure. Pyrolysis involves the simultaneous change of chemical composition and physical phase and irreversible. Excessive pyrolysis which leaves most of the carbon as a residue is called carbonization. Pyrolysis can deal with a variety of organic materials including plastic waste, food waste, crop waste, used cooking oil, used engine oil, etc. Pyrolysis is used to transform a variety of household items and industrial residues into fuel by recovering them. During pyrolysis, the molecules of the object vibrate at high frequencies to the extent that the molecules break down. The rate of pyrolysis also increases with the increase of temperature. The temperature of household items ranges from 370°C to above 410°C. Industrial applications have temperatures above 430°C. Pyrolysis is the transformation of waste biomass into a liquid fuel form. The density of the product fuel is 0.89gram/millilitre. The fuel produced has a long carbon chain length C9-C24 which is determined by Gas Chromatography and Mass Spectrometer (GC/MS). The produced fuel functional group and energy fuel heat enthalpy value is shown in the Fourier Transform Infrared Spectroscopy (FTIR) analysis result. The fuel produced can also be used as an internal combustion engine. The feed stock can be used as feed for refineries or as power generation feed for power plants. The objective of this research is to address the problem of waste plastic by using economical technology pyrolysis for waste reduction and energy recovery. Along with these environmental problems can also be solved. With the help of economical technology, we can save the environment and the problem of inflation of petrol in our country can also be reduced to some extent.
Keywords: Plastic Waste; Pyrolysis; Thermal reactor; Polypropylene (PP) and Polyethylene (PE); GC/MS
Introduction
Plastics have light weight and can be simply formed Plastic pyrolysis involves heating and degradation of plastic polymers at temperatures between 350°C and 900°C in an oxygen deficient environment. The waste plastic pollution has created a major dilemma in the environmental conservation sector [1]. In general, waste plastic has the composition of 46% High and low Density of Polyethylene (HDPE and LDPE), 16% Polypropylene (PP), 16% Polystyrene (PS), 7% Polyvinyl Chloride (PVC), 5% Polyethylene Terephthalate (PET), 5% Acrylonitrile-Butadiene-Styrene (ABS), and 5 percent other polymers [2,7]. In general, the conversion of waste plastic into fuel requires feed stocks which are non-hazardous and combustible. In particular each type of waste plastic conversion method has its own suitable feedstock [4]. To investigate the effect of the experimental conditions on the amount of generated products in detail, a characterization of the pyrolysis liquid should be performed. In earlier works, characterization of such pyrolysis liquids was performed by using various spectroscopic techniques [8]. Economic considerations and difficulties with feedstock availability have inspired some authors to study pyrolysis of used oils and waste plastics material [5]. The plastics include polystyrene, poly (vinyl chloride), polypropylene, polystyrene terephthalate, acrylonitrile-butadiene-styrene [6]. India recycles about 60% of its plastics, compared to world’s average of 22%. Plastic waste contains the calorific value equal to fuel.
Thermal degradation of waste plastics into liquid fuel have been conducted. Thermal degradations are not only used for polymer but it is also used for aromatics and gas [3]. Plastics play an important role in day-today life, as in certain application they have an edge over conventional material. However, one has to accept that virtues and vices co-exist. Plastics are relatively cheaper and being easily available has brought about use and throwaway culture. Approximately 85 to 90% of plastic from our daily life can be recycled or use for the production of synthetic fuel. In order to decrease the volume of nondegradable plastic waste material and preserving valuable petroleum resources Pyrolysis is one of the best methods. It is also help full in environmental protection. Because of higher conversion rate of fuel from plastic waste pyrolysis process is in favored.
Thermal processing in complete absence of oxygen (at low temperature). It is the process of thermal decomposition of organic matter at high temperature (about 350 to 500°C) in an inert atmosphere or vacuum, producing a mixture of combustible Carbon monoxide, Methane, Hydrogen, Ethane gases, pyroligenous liquid, chemical and Charcoal. Thermal treatment involves conversion of waste in to gaseous, liquid and solid conversion products with concurrent or subsequence release of heat energy. To improve the quality of crude oil from waste plastic pyrolysis so many studies have been carried out by researchers. Objective of this study is to introduce a safe and economical technology for waste reduction and energy recovery to yield the efficiency of product. The experimental setup on laboratory scale for the pyrolysis is mostly batch reactor; which consist of ‘liquid phase contact’ and ‘vapor phase contact’.
Experimental Process Description
Raw Sample Preparation
Waste plastics were collected from domestic grocery store, door to door and restaurant. All waste plastics were coming with foreign material and foreign materials separated by manually. Waste plastics cleaned with detergent powder and water then dried in to laboratory floor with fan air. Waste plastics cut into small pieces by manually using scissor then transfer into grinder machine for grounded and size was 12-13 mm.
Grounded waste plastics was transfer into batch reactor chamber for liquefaction process. The process of conversion involves heating of the waste plastic to thermal heating of 370–450 °C of the plastic mixture, product fuel density is 0.89g/ml, distillation the plastic mixture without catalyst, condensing the liquid plastic mixture with distillate to recover the liquid hydrocarbon liquid fuel materials, no additional chemicals are used in the thermal degradation process. In the mini-scale conversion process, the weight of a single batch reactor of input plastics for the fuel production process ranges from 500 gm to 5 kg. The sample is pre analyzed using Gas Chromatography and FT-IR Spectrum PerkinElmer Spectrum Version 10.03.06.
Raw Sample Analysis
Figure 1 and Table 1 are analysis of the LDPE raw plastics using the GC/MS. This analysis shows that the raw sample contains mostly double bod compoundsPlastic bags, food container covers). The GC/MS analysis indicates that the raw LDPE plastic’shydrocarbon compound ranges from C9–C32withdifferent retention times. When heat is being applied during thermal degradation process the LDPE sample is broken down into shorter chain hydrocarbon compounds.
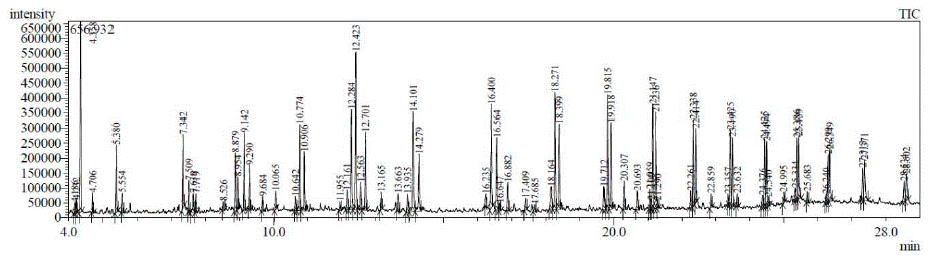
Figure 1: GC/MS chromatogram PP and PEmixture to produce fuel. Retention Time (Minutes)
Retention Time
Compund Name
Compound Formula
Retention Time
Compund Name
Compound Formula
4.186
2-Hexene, 4,4,5-trimethyl
C9h18
18.164
1-22-Docosanediol
C22H46O2
4.232
Cyclohexane, 1,3,5-trimethyl-
C9H16
18.271
1-Hexadecanol
C16H34O
4.358
2,4-Dimethyl-1-heptene
C9h18
18.399
Hexadecane
C16H34O
4.706
Cyclohexane, 1,3,5-trimethyl-
C9h18
19.712
9 -Octadecen- 1 -ol, (Z)-
C19H36O2
5.38
1-Undecene, 8-methyl
C12H24
19.815
1-Nonadecene
C19H38
5.554
Nonane
C9H20
19.918
Heptadecane
C17H36
7.342
1-Decene
C10H20
20.307
1-Decanol, 2-hexyl-
C16H34O
7.509
Decane
C10H22
20.693
1-Dodecanol, 2-hexyl-
C18H38O
7.639
Heptane, 3,3,5-trimethyl
C10H22
21.059
1,19-Eicosadiene
C20H38
7.719
Heptane, 3,3,5-trimethyl-
C10H22
21.1
Oxalic acid, 6-ethyloct-3-yl- prop
C15H28O4
8.526
Cyclohexane, hexyl-
C12H24
21.147
1-Nonadecene
C19H38
8.879
Isodecyl methacry late
C14H24O2
21.236
Eicosane
C20H42
8.954
2-Isopropyl-5-methyl-1-heptanol
C11H24O
21.29
Cyclotridecane
C13H26
9.142
Undecane
C11H24
22.261
9-Octadecen-1-ol, (Z)-
C18H36O
9.684
2-Undecanethiol, 2-methyl-,
C12H26S
22.414
Heneicosane
C21H44
10.065
(2,4,6-Trimethylcyclohexyl) meth
C12H21NSi
22.859
1-Decanol, 2-hexyl-
C16H34O
10.642
1,11-Dodecadiene
C12H22
23.357
1, 19-Eicosadiene
C20H38
10.774
1-Tridecene
C13H26
23.425
1-Nanodecene
C19H38
10.906
Dodecane
C12H26
23.49
Eicosane
C19H38
11.955
Cyclohexane, octadecyl-
C24H48
23.632
Cyclohexane, 1,2,3,5- Tetraisopro
C18H36O
12.161
Z-1,6-Tridecadiene
C13H24
24.376
1, 19-Eicosadiene
C20H38
12.284
1-Tridecene
C13H26
24.435
1-Nonadecene
C20H38
12.423
2-Isopropyl-5-methyl-1-heptanol
C11H24O
24.494
Eicosane
C19H38
12.563
11-Methyldodecanol
C13H28O
24.54
1, 10-Dicyanodecane
C12H20N2
12.701
2-Isopropyl-5-methyl-1-heptanol
C11H24O
24.995
Dotriacontyl pentafluoropropion
C35H65F5O2
13.165
1-Heptanol, 2,4-diethyl-
C9H20O
25.334
1, 19-Eicosadiene
C20H38
13.663
1,22-Docsanediol
C22H46O2
25.386
1-Nonadecene
C19H38
13.935
11-Hexadecen-1-ol, (Z)-
C16H32O
25.439
Dotriacontane
C32H66
14.101
1-Tetradecene
C14H28
25.683
Cyclohexane, 1,2,3,5- Tetraisopro
C18H36O
14.279
Tetradecene
C14H30
26.24
(Z)- 14-Tricosenyl
C24H46O2
16.235
(Z)-10-Pentadecene-1-ol
C15H30O
26.299
1-Heptacosanol
C27H56O
16.4
1-Pentadecene
C15H30
26.349
Dotriacontane
C32H66
16.564
Pentadecene
C15H18O2
27.313
1-Hexacosanol
C26H54O
16.647
Cyclopentadecane
C15H30
27.371
Tetracosane
C24H50
16.882
Pentadecaflurooctanoic acid, hexadecyl
C24H33F15O2
28.534
1-Hexacosanol
C26H54O
17.409
1-Dodecanol, 2-hexyl-
C18H38O
28.602
Tetracosane
C24H50
17.685
1-Octacosanol, 2,4,6,8-tetrameth
C32H66O
Table 1: GC/MS compound list with retention time of Conversion of Plastic Waste to Fuel.
Result and Discussion
GC/MS tests have been performed to investigate the composition of the produced fraction fuel. Varian GCMS/MS model 4000 with MS workstation. 1split/splitless injector and 2 PTV injectors, FID detector, MS/MS detector, Direct sampling system (Chromatoprobe) for mass of pure samples and Autosampler (Combipal) for liquid and HSS sampling Figure 2 shows chromatograms of producing 2nd fraction fuel illustrating the carbon chain length. GC/MS (Table 1) show higher peak intensity with retention-time major compound distribution throughout the range of hydrocarbon groups C9–C24, with retention time ranging from 4.0 min to 28.0 min.
FTIR analyses of Conversion of Plastic Waste to Fuel according to their wave number following functional groups are shown (Figure 2 and Table 2). The analysis spectrum is expressed in transmittance chart. The results declare that some functional groups are single bond and some are double bond cis and trans groups and non-conjugated groups are also present. In the spectrum field we noticed that higher wave number are emerged in the initial phase and middle index of the spectrum in higher wave number small and bulky both functional groups are available and in low wave number double bond and single bond functional groups are available such as methane group, trans and alkene group etc. Here after wave number 3076.08cm-1 functional group is free NH, wave number 2956.15 , functional group is C-CH3, wave number 1713.59 (cm-1), functional group is Non-Conjugated, wave number 1462.77 (cm-1), functional group is CH3 , wave number 1215.83 (cm-1 functional group is acetates, wave number 993.19 (cm-1),functional group is -CH=CH2, wave number 910.20 (cm-1),functional group is --CH=CH-(Trans), wave number 888.81(cm-1), functional group is -CH= CH2, and ultimately wave number 760.47(cm-1) functional group is -CH=CH-(Cis) etc. Energy values are calculated, using formula is E=hυ, where h=Planks Constant, h =6.626x10-34 J, υ=Frequency in Hertz (sec-1), Where υ=c/λ, c=Speed of light, where, c=299,792,458 m/s, W=1/λ, where λ is wave length and W is wave number in cm-1. Therefore, the equation E=hυ, can substitute by the following equation, E=hcW. According to their wave number several energy values are calculated such as for wave number 3076.08 (cm-1) calculated energy, E=6.11x10-22 J, wave number 2956.15 (cm-1) calculated energy, E=5.87x10-22 J, wave number 1713.59 (cm-1), calculated energy, E=3.40 x10-22 J, wave number 1462.77 (cm-1), calculated energy, E=3.90 x10-22 J, wave number 1215.83 (cm-1), calculated energy, E= 2.41x10-22 J, wave number 993.19 (cm-1), calculated energy, E=1.97 x10-22 J, wave number 910.20 (cm-1), calculated energy, E=1.80 x10-22 J, wave number (cm-1), calculated energy, E=1.76 x10-22 J and wave number 760.47 (cm-1), calculated energy, E=1.51 x10-22 J.
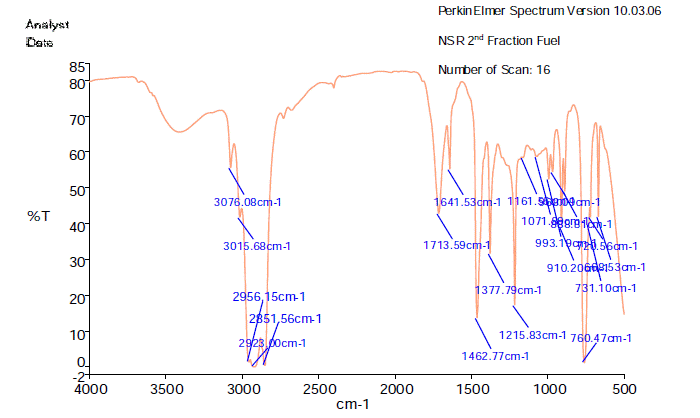
Figure 2: FTIR spectrum of conversion of plastic waste to fuel.
Band Peak Number
Wave number (CM-1)
Functional group
Band Peak Number
Wave number (cm-1)
Functional group
1
3076.08
Free NH
11
1071.89
2
2956.15
C-CH3
12
993.19
-CH=CH2
3
2923.00
13
968.09
4
2851.56
14
910.2
-CH=CH-(Trans)
5
1713.59
Non-Conjugated
15
888.81
-CH=CH2
6
1641.53
16
760.47
-CH=CH-(Cis)
7
1462.77
CH3
17
731.1
8
1377.79
18
72056
9
1215.83
Acetates
10
1161.55
19
668.53
Table 2: Conversion of plastic waste to fuel FTIR compound functional group list.
Conclusion
Waste plastics to fuel production process were used of PP and PE waste plastics without adding any kind of catalyst. Without catalyst fuel production yield percentage was almost 83% by wt. %. Waste plastics have different type of metal content because when plastic are made that time manufacturing company production procedure using different kind of additives for plastic shape. This metal content was helping when experimental heat applies on waste plastics to break down long chain to short chain hydrocarbon fuel production. Produced fuel has long chain and short chain hydrocarbon isomer and also alkene group compounds such as 2-Hexene, 4,4,5-trimethyl, 2,4-Dimethyl-1-heptene, C9 H18, 1-Undecene, 8-methyl-, C12 H24, 1-Tetradecene, C14H28 or CH3(CH2)11CH=CH2, 1-Pentadecene, C15 H30, 1-Hexadecanol, C16 H34 O, 1-Nonadecene, C19H38, Tetracosane, C24H50 and etc. because raw materials was mixed with Polystyrene (PS). By products of black solid residue percentage also less, the percentage residue is coming out as metal and some carbon and hydrogen because it was presented into pre-analysis trace metal present into the raw waste plastic. FTIR and GC/MS analysis also indicated that various kinds of hydrocarbon compounds are present in the obtained fuel including methyl group derivatives and alkene and a volatile organic compound etc. Various aliphatic compounds are also appeared in the GCMS analysis of fuel entities. Produce fuel could be use as internal combustion engines, feed for feed stock refinery or electricity generation feed for power plants. By using this technology can be solved PP and PE waste plastic problem as well as environmental problems and reduce some percentage of foreign oil dependency.
References
- Sarker M, Rashid MM, Molla M. Waste plastic conversion into chemical product like naphtha, Journal of Fundamentals of Renewable Energy and Applications. 2011; 1.
- Nagarjuna S, Bhosale SM. A Review: Energy recovery from plastic waste through pyrolysis, International Journal of Trend in Scientific Research and development. 2018; 3: 772-775.
- Kaustubh N, Devendra D. Conversion of selected waste Plastic in to Synthetic Fuel. International Journal of Engineering Sciences & Research Technology. 2014; 3.
- Gaurav, Madhukar, Arunkumar KN, Lingegowda NS. Conversion of LDPE plastic waste into liquid fuel by thermal degradation, Proceedings of IRF International Conference, Bangalore 23rd March-2014.
- Bidhya K, Cheng HN, Chandrashekaran SR, Sharma BK. Plastics to fuel: a review, Renewable and sustainable energy reviews. 2016; 54: 421- 428.
- Prajapati DM. Converting Plastic to Useful Energy Resources, International Journal of Trend in Scientific Research and Development. 2018; 2: 433-438.
- Rashid MM, Sarker M. Waste Polyethylene Terephthalate (PETE) And Polystyrene (PS) Into Fuel, International Journal of Scientific & Technology Research. 2013; 2.
- Sarker M, Rashid MM, Rahman MS. Randomly Mixture of Waste Plastics Conversion into Fuel by using Leftover Resi-due, World Research Journal of Environment and Waste Management. 2012; 1: 01-10.
