Abstract
Toxic metals have received a paramount consideration to environmental chemists due to their toxic nature, they are toxic pollutants that may possibly cause a variety of illness and disorders when entering the living tissue.
Aim: This work investigates the adsorption capacity of biosorbent developed from Powder o Bambara Ground nut (PBG) and Soursop Seed (SSSP) powder. The adsorbents were useful in eliminating toxic metal ions; cadmium, chromium, cobalt, copper, iron, lead, manganese, lead, and thallium from aqueous solution. The study also examined the removal efficiency of PBG and SSSP.
Method and Materials: Constant mass of 0.2 gram of both PBG and SSSP were added in a volume of 50 mL of ultra-pure water, the samples were then analysed using Inductive Couple Plasma Optical Emission Spectrometer (ICP-OES).
Conclusion: The adsorption percentage for BGS reached 100% for Cd2+, Tl2+ and Pb2+, 94% for Cu2+, 81% for Zn2+, 68% for Co2+, 64% for Mn2+, 46% for Fe2+ and 07% for In2+, the results revealed that the metal ions were removed in the following order (Cd2+, Tl2+ and Pb2+)> Cu2+ > Zn 2+ > Co2+ > Mn2+ > Fe2+ > In2+ whereas for SSSP was 100% for Cd2+; Cu2+; Tl2+ and Pb2+; 83% for Zn 2+; 62% for Co2+; 49% for Fe2+; 21% for In2+; and 0% for Mn2+ with an order of (Cd2+= Cu2+ = Tl2+ = Pb2+)> Zn 2+ > Co2+ > Fe2+ > In2+ > Mn2+.
Based on the finding reported this study confirms that BGP are the perfect adsorbent for the recovery of selected metals.
Keywords: Adsorbent; Bambara Ground Nut; Soursop;Toxic Metals; Percent Adsorption
Introduction
Heavy metals are toxic pollutants that may possibly cause a variety of illness and disorders when they accumulate in the living tissues [1].
Toxic metals can be initiated from both natural and anthropogenic processes and end up in different environmental compartments [6].
Heavy metals generally present trace amounts in natural water but may pose danger and are very toxic even in low concentrations [7]. Metals like lead, cadmium, nickel, mercury, chromium, cobalt, zinc and selenium are highly toxic even in minor quantities [1].
Increasing quantity of heavy metals in our resources is currently an area of greater concern especially since many industries are discharging their metal containing effluents into water bodies without any necessary required treatment [1].
Toxic metals may enter human through food, water, air or absorbed through the skin when they encounter humans in agriculture, manufacturing of goods, pharmaceutical and through industrial operations [9].
Exposure of aquatic and terrestrial organisms to toxic metals can have devastating impacts to health of living creatures, toxic heavy metals have been associated with numerous diseases including cancer [2].
A number of methods have been employed to treat water contamination of heavy metals such as physio-chemical and biological techniques [3]. Physio-chemical methods such as chemical precipitation, chemical oxidation/reduction, filtration, ion exchange, electrochemical treatment have been commonly used for removal of toxic ions in waste water [7].
Biosorption has attained great concern as an alternative method that offers the use of biological materials for the same purpose of treatment of polluted water by eliminating trace metals. This study focuses on finding the solution to treatment of synthetic wastewater from toxic metal ions present in aqueous solution by using local available materials and cheap adsorbent developed from Bambara groundnut and sour soup seeds powder.
The use of natural adsorbents for removal of toxic metal ions from wastewater has been an effective technique with low cost and high adsorption efficiency [6].
Adsorbents are essential in the clean-up process because they are reusable in the physical adsorption process, cheaper and preferable for the environmental cleaning up thus, biosorption method could be the perfect alternative method to remove hazardous metal ions from the environment [5].
Developing low cost and eco-friendly technologies for the remuneration of soil and water dirtied with toxic elements is a global concern [8]. Many studies indicate that adsorbents from materials of biological origin can be very effective to eliminate toxic chemicals from aqueous solution because of its low cost, locally abundant and environmental friendly [4].
Due to the deterious effects of heavy metals around the world, there is a global environmental needs call for the application of adsorption process in pollution control. Thus, the present study focuses on using bambara groundnut powder as local available materials to develop low cost and eco-friendly adsorbents for removal and recovery of toxic heavy metals in aqueous solution.
Materials and Methods
Study Site
The materials used in this work were prepared in Zanzibar. However, the analysis of heavy metal was done at analytical chemistry laboratory in Brunei Darussalam (UBD).
Equipment and Apparatus
Inductively Coupled Plasma Optical Emission Spectrophotometer, digital weight balance, filter paper, spatula, 100 mL beaker, 100 mL conical flask, 1000 mL volumetric flask and funnel were used for the study.
Collection of Samples
The ICP multi-elements standard solution IV of analytical grade was used as control, 1 kg of bambara groundnuts bought from the local market at Mwanakwerekwe
Reagent and Chemicals
ICP multi-element standard solution and ultra-pure water
Preparation of Standard Reagent
1000 mL volumetric flask was cleaned, dried and labelled. A Stock standard solution containing 1000 ppm of cadmium (ii) ion, chromium (ii) ion, copper (ii) ion, cobalt (ii) lead (ii) ion iron (ii) ion, manganese (ii) ion, thallium (ii) ion and zinc (ii) ion by method of continuous dilution was diluted to 100 ppm. This was followed by a dilution to 10 ppm and then finally diluted to 2 ppm. 50 mL of 2 ppm of prepared standard solution was put into the flask labelled flask. They were all stored in a cool and dry place. 50 mL of Deionized water was used in the preparation of the above standard solution and then kept in another container labelled blank. The blank and the prepared standard solution of 2 ppm were analysed by Optical Inductive Coupled Plasma Spectrophotometer.
The Preparation of Multielement Standard Solution
The multi metal standard solution was prepared, containing 0.1922 ppm of cadmium, 0.1645 ppm of chromium, 0.1713 ppm of cobalt, 0.1908 ppm of copper, 0.1956 ppm of lead, xxxxg of iron, 0.2023 ppm of manganese xxxg of thallium and xxx g of zinc. 50 mL of multi metal standard reagent with 0.2 gram of powder of Bambara ground nut were transferred into conical flask, the mixture shaken by using rotary shaker at 200 rpm and kept overnight to allow maximum removals. The mixture of adsorbent and adsorbate was filtered by using a filter paper. The analyte obtained was analysed by Inductively Coupled Plasma spectrophotometer.The same conditions as applied in bambara ground nut powder were used for the soursop powder.
Removal of Heavy Metals Ions
Four conical flasks were cleaned, dried and labelled B1, B2, AB3 and B4, 0.2 gram of BGP was added into the flask libelled B1, B2, B3, and B4. 50 cm³ of synthetic wastewater were added into each labelled flask above containing 0.2 gram of powder of Bambara ground nut. The solutions in the flasks were homogenized and allowed to settle overnight. The mixtures were then filtered to become clear solutions. The analyte was put in 50mL sealed container labelled B1, B2, B3 and B4. The filtrate was stored in a cool and dry place.
Statistical Data Analysis
The experiment was carried out at room temperature, and the analysis was carried out using ICP – OES and repeated four times, the mean values from the data were taken for Statistical analysis. The removal efficiency of selected heavy metals in terms of percentage removals were calculated in excels software by typing formula:
Where Di represent initial concentration of heavy metal, Df stands for final concentration.
Other data analysis such as maximum % removal, minimum % removal, mean, standard deviation and variance were done by using IBM SPSS statistics 20 version.
Results Table 1 The removal efficiency of the heavy metal ions using BGP.
Parameter
Maximum adsorption
Minimum adsorption
Mean
Range
Standard deviation
Cd
100
100
100
0
0.00
Co
61.98
45.99
53.88
15.99
6.54
Cu
100
23.38
80.67
76.62
38.20
Fe
49.09
0.00
23.64
49.09
27,34
In
20.57
0.00
5.15
20.57
10.29
Mn
0.00
0.00
0.00
0
0.00
Pb
100
95.87
97.43
4.13
1.92
Tl
100
100
100
0.00
0.00
Zn
82.99
3.97
45.32
79.02
38.17
Table 1: Percentage adsorption of soursop.
Parameter
Maximum adsorption
Minimm
adsorptionMean
Range
Standard
deviationCd
100
100
100
0
0.00
Co
68
58
64
10
4.25
Cu
94
24
63
69
31.47
Fe
46
0
11.46
46
3.40
In
07
0
1.70
07
27.11
Mn
64
0
39
64
0.00
Pb
100
100
100
0
0.00
Tl
100
97
98.87
03
1.34
Zn
81
65
72.13
17
7.25
Table 2: Percentage adsorptions of bambara ground nut.
Results and Discussion
The results showed a high removal capacity of BGP for heavy metals except for indium which had poor adsorption level. This revealed that BGP is the best adsorbent for metals ion recovery from aqueous solution as shown in figure 3 below The results showed ahigh removal capacity of SSSP for heavy metals except for manganese which show no significant level of adsorption as well as iron which was adsorbed below 50%.

Figure 1: ICP multi-element standard solution IV.

Figure 2: Bambara ground nut.
Figure 3: Soursop fruit.
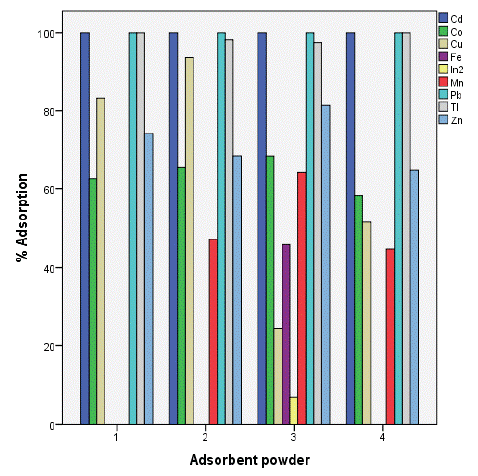
Figure 4: Percentage adsorption of selected metals.
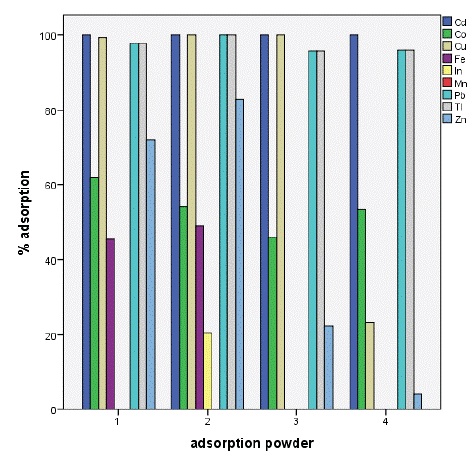
Figure 5: Percentage adsorption of selected metals.
Conclusion
To winded up, this study discovers an innovative cost-effective and ecofriendly biosorbent from locally available plant materials comprising bambara ground nut (BGP) and soursop seed (SSSP) as substitute to costly adsorbents used for the uptake of cadmium (II) ion, cobalt (II) ion, copper (II) ion, iron (II) ion, lead (II) ion, manganese (II) ion, indium (II)ion , thallium (II) ion and zinc (II) ion from the aqueous solution.
Conflicts of Interest
The author declares that there are no conflicts of interest.
With same conditions as applied on soursop the adsorption percentage of bambara ground nut is given as 100%, 94%, 81%, 68%, 64%, 46% and 07% for (Cd2+, Tl2+ and Pb2+), Cu2+, Zn 2+, Co2+, Mn2+, Fe2+ and In2+ respectively. The results revealed that heavy metals removal were successfully using BGP except for indium ions whose percentage adsorption was the lowest, the metal ions were removed in the following order (Cd2+, Tl2+ and Pb2+) > Cu2+ > Zn 2+ > Co2+ > Mn2+ > Fe2+ > In2+ (Table 1).
References
- Agarwal M, Singh K. Heavy metal removal from wastewater using various adsorbents: a review. Journal of Water Reuse and Desalination. 2017; 7: 387-419.
- Emenike PC, Omole DO, Ngene BU, Tenebe IT. Potentiality of agricultural adsorbent for the sequestering of metal ions from wastewater. Global Journal Environment Science Management. 2016; 2: 411-442.
- Jain CK, Malik DS, Yadav AK. Applicability of plant based biosorbents in the removal of heavy metals: a review. Environ Process. 2016; 3: 495–523.
- Robalds A. The use of biosorbents in the treatment of polluted waters. 2015.
- Sarada B, Prasad MK, Kumar KK, Murthy CVR. Cadmium removal by macro algae Caulerpafastigiata: Characterization, kinetic, isotherm and thermodynamic studies. Journal of Environmental Chemical Engineering. 2014; 2: 1533-1542.
- Sdiri A, Higashi T. Simultaneous removal of heavy metals from aqueous solution by natural limestones. Applied Water Science. 2013; 3: 29-39.
- Serrano-Gómez J, López-González H, Olguín MT, Bulbulian S. Carbonaceous material obtained from exhausted coffee by an aqueous solution combustion process and used for cobalt (II) and cadmium (II) sorption. Journal of environmental management. 2015; 156: 121-127.
- Swain G, Adhikari S, Mohanty P. Phytoremediation of Copper and Cadmium from Water Using Water Hyacinth,” Eichhornia Crassipes”. International Journal of Agricultural Science and Technology. 2014; 2: 1-7.
- Verma A, Kumar S, Kumar S. Biosorption of lead ions from the aqueous solution by Sargassumfilipendula: Equilibrium and kinetic studies. 2016; 4: 4587-4599.
