Abstract
Radioactive contamination of the Yenisei River bottom and (including the contamination with actinides) is the result of long-term operation of the Mining and Chemical Combine, which manufactures plutonium for weapons. The study considers the behavior of a submerged macrophyte Elodea Canadensis, one of the most widely spread species of aquatic plants in the River Yenisei. The values of the accumulation coefficients obtained for 242Pu, 13100 ± 2100 L·kg-1, were close to the concentration factor for 241Am–17100 ± 4300 L·kg-1, obtained for the Elodea shoots. Studies on the potential adaptation of one of the common aquatic macrophytes Elodea canadensis when immersed in a medium containing actinide. It was found that almost all the studied 241Am and 242Pu do not show a clear external effect on the solid fragments of the plant (cell membranes). Thus, it was shown that Elodea canadensis is tolerant of anthropogenic radio nuclides that differ in nature, physico-chemical properties, etc.
Keywords: River yenisei; Elodea Canadensis; Amerisium-241; Plutonium-242; Adaptation
Introduction
Aquatic plants are perennial (sometimes annual) plants whose necessary habitat is fresh (mostly), salty or briny water [16,17]. Radionuclides, as well as heavy metals from natural and anthropogenic sources when entering fresh water systems are primarily absorbed in the upper 10 cm of the bottom sediment layer [4,6,9,14]. The largest part of root systems of all the aquatic plants existing in a water body is usually located in this area. Consequently, surface bottom sediments are a potential supply of substances to be transported to the roots of aquatic macrophytes [6,9,14].
Aquatic macrophytes are able to absorb heavy metals. Substances are absorbed through the root system and leaves (submerged macrophytes) or, only through leaves (floating macrophytes). The accumulation of metals includes bioaccumulation (slow, irreversible process) and biosorption (which is fast and reversible). The active phase of the metal absorption occurs mainly in roots to be followed by their transfer to other parts of the plant, while the passive process of metal absorption is due to the direct contact of plants with the environment which results in the accumulation of metals mainly by the emergent part of the plant [7].
In general, the rate of absorption, accumulation and transport of a metal in a plant is dependent on the plant species and regulated by such environmental factors as: the nature of the metal, temperature, ??, oxidation-reduction potential and salinity.
Some plants are able to absorb extremely high concentrations (more than 1% of a metal in the dry matter) of pollutants from the environment with their subsequent accumulation in the roots, shoots and leaves. Such plants are referred to as hyper accumulators of metals [7,18,25].
Biological availability of radio nuclides from global particles is determined by the time of their formation, composition and dispersion. Leaching of radio nuclides is, as a rule, higher in fine particles than in coarse ones. Particles containing radio nuclides from the global fall out are extremely small (of up to about 1μm) and can almost completely be dissolved in water. The biological mobility of radio nuclides is commeasurable with their mobility in aqueous solutions [18].
Aquatic plants in freshwater, seas and estuaries accumulate a number of radio nuclides. Hyper accumulation results in a considerable number of radio nuclides being accumulated in the plant tissues, independent of the radionuclide concentration in bottom sediments [18]. Growth peculiarities for different plant species depend on the type of the water body. The flow rate and water transparency are the key factors. The Yenisei is one of the world’s largest rivers, over 3000 km long, flowing into the Kara Sea. It is a fast river with rapids, with the high flow rate being due to the large slope of the riverbed. In its estuary and gulf the flow rate radically decreases. Sometimes, under the influence of strong wind upsurge and, especially during tides the river flow can be reversed [1,21].
The Mining-and-Chemical Combine (MCC) in Zheleznogorsk is situated on the east bank of the Yenisei River, 60 km downstream of the city of Krasnoyarsk. The Combine produced weapons-grade plutonium in uranium-graphite reactors since the launch of the first reactor in 1958. At present, all the three nuclear reactors are out of operation (since 2010). The irradiated uranium is reprocessed at a radiochemical plant to separate uranium, plutonium, and fission products. Scientific survey reveals that the Yenisei River flood plain is contaminated with man-made radio nuclides, including plutonium isotopes, hundreds of kilometers downstream from the plutonium complex. The investigations of the sediment samples from the Yenisei River reveal high activity concentration of actinides (Pu isotopes, 241Am etc.), which are 100 times higher than their global fallout levels. Transuranic elements were detected not only in the soil and sediment but also in the biomass of aquatic plants. Plutonium isotopes were detected in the biomass of aquatic plants both near the discharge point of MCC and at a distance of 200 km downstream. The previously conducted laboratory experiments revealed a great capacity of aquatic plants and microalgae to accumulate transuranic elements. However, mechanisms of intensive accumulation of transuranic elements by living organisms still remain unknown.
The aim of the study was to investigate the properties, accumulation processes and peculiarities of interaction of the radio nuclides 241Am and 242Pu with the most widely spread species of submerged macrophytes – Elodea Canadensis.
Materials and Methods
To reveal the regularities and interaction mechanisms of the radio nuclides, a number of modeling experiments were carried out using the most common for the river Yenisei species of submerged macrophytes, Canadian pondweed Elodea Canadensis. This is a cosmopolitan species, which is widely used in toxicological experiments. Plant and water samples were collected from the Yenisei River upstream from the MCC discharge point. Plant samples were taken from the population growing in one of the river inlets. In our experiments we used 3.2-3.5 cm apical shoots. The plants were pre-washed with the river and tap water. The Yenisei River water was aseptically filtered through 0.2-μm-pore-size cellulose nitrate membranes (d=47cm, Shleicher & Shuell, Germany) to remove suspended particles and micro flora. The plants were kept in 0.5 L of water in 1.0 L cylindrical glass vessels at a temperature of 17-19°C. The vessels were illuminated by luminescent lamps during 12h a day and the side irradiance of a vessel was 4.5 klx. Fresh weight was determined for the plants blotted with water absorbing paper. Dry weight was determined for the dried plants. In some cases, the dry weight of the plants was calculated from the previously obtained calibration equations.
Accumulation and Peculiarities of 241Am Distribution in the Biomass of Elodea
To study the peculiarities of 241Am micro distribution in the structures of the submerged aquatic plant Elodea Canadensis Michx. (Canadian pondweed), young shoots with the length of 3 cm were chosen for the experiment, with the total weight of the dry biomass being 6.5 g. 241Amina 2? solution of HNO3 was twice introduced into the experimental system with the volume of 200 mL •The total radionuclide content in the experimental system was 1850 ± 31 Bq·L-1, or 370 Bq in 200 mL •After the experiment the plants were removed from the system, washed with distilled water and dried in air using filter paper.
Accumulation and Peculiarities of 242Pu Distribution in the Biomass of Elodea Canadensis
Series of experiments on the 242Pu accumulation were carried out with the introduction of Elodea 0.16 ± 0.02 g (or 2.5 ± 0.3 g fresh wt.). In the course of all the experiments the plants remained alive and at the end of the experiment (after 168 h) a certain increase in the wet weight was observed, up to 3.2 ± 0.3 g of fresh wt. in one vessel •To calculate the parameters of the 242Pu accumulation and release by Elodea, we used the averaged data for three experimental vessels with the Elodea shoots. The parameters of the radionuclide accumulation by the plants were calculated per unit of dry weight.
One of plutonium isotopes, 242Pu, was used in the experiments, its half-life period being 375000 years. 242Pu was added to the water as a solution of 242Pu in the presence of a 1 M HNO3 solution. The added solution of 242Pu was neutralized with a NaOH solution (0.1 M) up to pH 7.0. Then, the plants were placed into the water. The initial activity of 242Pu in the water was 4.0 ± 0.5 Bq per water sample (or 8 Bq·L-1). Each experiment on the accumulation of 242Pu by the Elodea shoots lasted 168 h. During the 242Pu accumulation experiments, at set intervals, aliquots of the water and plant shoots were analysed for the 242Pu concentration. To calculate the balance of the introduced amount of 242Pu, at the end of the experiments the outwash of HNO3 (8M) was obtained from the walls of the experimental vesseL •The results showed that 242Pu was hardly absorbed on the walls (below the detection level of the technique). The Concentration Factor (CF) of 242Pu was calculated as a ratio of the radionuclide concentration in the plant (Bq·kg-1 dry wt.) to the radionuclide concentration in the water (Bq·L-1). To calculate the parameters of the 242Pu accumulation by Elodea, we used the averaged data for three experimental vessels with the Elodea shoots.
Study of the Mobility of 242Pu and 241Am
To estimate the mobility of 242Pu and241Am in the plant, we used the method of sequential chemical fractionation [4,5,12]. 242Pu and 241Am of the exchangeable fraction was separated by exposing the plant biomass to the action of a CH3COONH4 solution (1M) for 24 hours. To separate 242Pu and 241Am of the absorbed fraction, the plant biomass was treated with a HCl solution (0.1M) for 20 min. 242Pu retained in the biomass was considered to be strongly bound to the plant components. 242Pu and 241Am bound by organic compounds and mineral residue of the plant biomass were separated by “wet combustion”, using H2O2 (30%) and a HNO3 solution (0.1 M).
241Am Determination
241Am concentration in the water and other liquids was measured using liquid scintillation spectrometry on a Tri-Carb-2800 spectrometer (Canberra Industries Co., Meriden, CT, USA). Immediately before the measurement, an aliquot of the liquid was mixed with Ultima Gold AB scintillation cocktail (Perkin Elmer, Waltham, MA, and USA) at a ratio of 8:12 (sample/cocktail) in a plastic viaL •The volume of the measured samples was 20mL •Each measurement lasted 300 to 420 min.
Olso, 241Am concentration in the liquid and solid samples was measured on a γ-spectrometer (Canberra, USA) coupled to an HPGe hyper-pure germanium detector, capable of measuring γ-spectra in the energy range from 30 to 3,000 keV. The γ-spectra were processed using the Canberra Genie PC software (Canberra, USA).
242Pu Determination
The 242Pu isotope from the samples under study was determined by the method of a-spectrometry after the radiochemical extraction in an anion exchange resin column AB-17-8 in the form of NO3-. The method is described in more detail in studies [10,11]. The chemical yield of plutonium was determined based on the yield of the 236Pu isotope introduced at the stage of the sample preparation. The chemical yield of 236Pu was as high as 80%. The activity of plutonium isotopes after the electrolytic deposition was measured with a Eurisys Measures a-spectrometer 7184 (France). The spectrometer was equipped with a low-background silicon semiconductor detector PLUS-300 with the area of 300mm² and resolution of 15keV. The detection limits for Pu isotopes (242Pu, 236Pu) were at the level of 0.0005Bq, with the measurement time being 200000 sec.
Results and Discussion
Aquatic plants living in a solution containing heavy elements are known to be able to accumulate them on their surface and/or absorb them into their tissues. Both the sorption by the surface and absorption can be either uniform or non-uniform.
Accumulation of 241Am an d242Pu by Elodea Canadensis
In the course of the experiments, it was revealed that most of the radio nuclides were absorbed by the biomass during the first 24 hours of the contact, i.e about 33-50% of the added activity, to be followed by further accumulation of radio nuclides by the biomass (Figure 1).
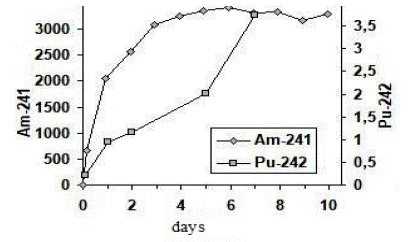
Figure 1: 241Am 242Pu accumulations in Elodea Canadensis biomass.
In our opinion, incomplete 242Pu depletion can be accounted for by a number of reasons. Firstly, 242Pu depletion from the aquatic phase depends on the capacity of the biomass tissues for binding the radionuclide and stability of the compounds being formed. In our experiments we used rather small volumes of Elodea (0.16-0.26 g. dry wt., or 2.5-2.6 g. fresh wt. per vessel) and, thus, the sorption capacity of the macrophyte was limited. Secondly, 242Pu depletion was limited by the properties of the aquatic phase (salt composition, content of the dissolved organic compounds).
In the case of the experiments with 241Am, the biomass used was sufficiently abundant as compared to the experiments with the introduced plutonium. Thus, the radionuclide had to be additionally introduced since upon the first introduction the whole amount of americium was absorbed by Elodea during 48 hours.
As is evident from the presented data, the maximum Concentration Factor (CF) was obtained for 242Pu which was equal to 13100 ± 2100 L·kg-1, with the specific activity of 242Pu amounting to 21 ± 2 Bq·g-1 dry wt. (Table 1). The values of the accumulation coefficients obtained for 242Pu, 13100 ± 2100 L·kg-1, were close to the concentration factor for 241Am–17100 ± 4300 L·kg-1, obtained for the Elodea shoots (Table 1). This is not in good agreement with the data provided by Eyman and Trabalka (1980) who reported that aquatic plants and microalgae accumulated americium more intensively than plutonium and the concentration factor of americium in aquatic plants could be an order of magnitude higher.
Dry mass, g
Fresh mass, gConcentration Factor,
L·kg-1initial
final
241Am
0.26±0.03
2.6±0.40.28±0.02
3.1±0.317100±4300
242Pu
0.16±0.02
2.4±0.30.18**
2.7±0.313100±2100
Table 1: The experimental data on the accumulation of 241Am and 242Pu by the plant samples.
As concerns the radionuclide forms which appear in the aqueous medium, there exists the following opinion. Due to the fact that plutonium in the aqueous medium can have several oxidation degrees, upon the introduction of 242Pu, colloids and (or) pseudo-colloids can be formed [8]. In this case, colloids and (or) pseudo-colloids are unevenly distributed in the liquid layer forming unstable complexes [2,8]. Besides, there exist thermodynamic and kinetic obstacles in the form of steric effects and coagulation rate of the complexes formed in the aqueous medium and thus, the radionuclide uptake by the plant surface can be more or less limited. This property is less typical for 241Am, since in the aqueous medium americium is present in the stable state with the oxidation degree (+3).
In order to confirm or refute this point of view, solutions of inorganic sodium salts in the form of citrates, ascorbates, sulfides, as well as sodium humate were added into the experimental systems. The concentration of the added salts was 0, 05M in the whole volume of the solution of the model systems.
(Figure 2) Diagrams of the radionuclide distribution among the plant fractions in the systems with the addition of salts of oxalic, ascorbic, hydrosulphuric and humic acids into the systems.
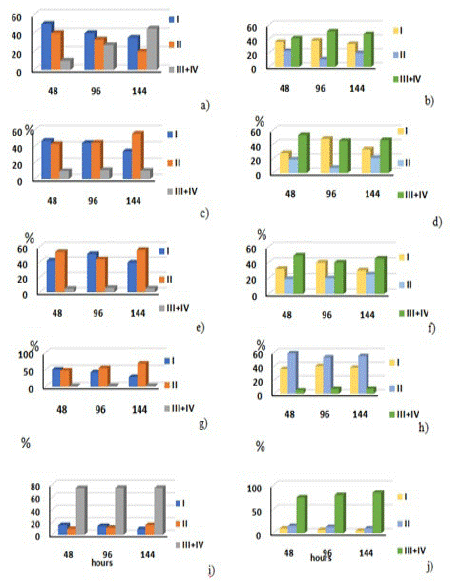
Figure 2: Dynamics of the distribution among the chemical fractions for 242Pu and 241Am accumulated by the Elodea biomass in the course of the experiment: I–exchange fraction; II–absorption fraction; III+IV–organic fraction + mineral residue upon the addition of salts of oxalic (c, d), ascorbic (e, f), hydrosulphuric (g, h) and humic acids (i, j) into the systems.
As can be seen from the obtained results, a larger content of the accumulated 241Am was found in the exchange and absorption fractions, i.e., on the plant surface. The surface of plants taken from the natural environment is known to be covered with sediment which consists both of mineral (carbonates, sulfates, particles of bottom sediment, etc.) and biological components, including the surfaces of the plant itself, i.e. epidermal tissue. In the medium where only the solution of 241Am is added, with time, there occurs the radionuclide redistribution into the biomass.
The redistribution between these fractions does change with time (Figure 2g). In the system with the added sulfide a rather predictable result was obtained. As most sulfides have low solubility, then in our case, all the introduced radionuclide remains on the plant surface and vessel walls. In the system with the addition of sodium humate the americium content in the plant biomass after as few as 48 hours from the beginning of the experiment reached the potential maximum and amounted to ~75% from the accumulated amount.
However, for 242Pu binding with the exchange and adsorption fractions is not dominant. This is likely to be caused by the fact that in the case of 242Pu, due to its physicochemical properties different from those of Am, there appear competing processes of sorption and oxidation-reduction. One can argue with more confidence that oxidation-reduction processes dominate in our experimental conditions. This results in tough binding of plutonium only by the biomass itself due to the reduction of Pu(V) to Pu(IV) on the surface of the cell membranes. In this case, plutonium is accumulated owing to bioreduction and, partially, due to biosorption. The exchange fraction in both cases plays the role of a diffusion layer, into which radio nuclides are transported from the layer of the experimental liquid due to the concentration gradient. The addition of salts of ascorbic and oxalic acids almost did not influence the radionuclide redistribution, with the variation being within the limits of experimental error. However, in the case of adding sulfide, similarly to the case of americium, the fraction of plutonium on the plant surface considerably increased, i.e., there occurred the formation of low-soluble compounds with ions S2- (Figure 2h).
As in the case with americium, when salts of humic acid were added into the experimental system the fraction of plutonium in the plant biomass considerably increased (85%), i.e. in both cases on can observe the process of hyper accumulation of the radio nuclides under study by the plant biomass; these radio nuclides exist in the aqueous medium bound to an organic ligand, in this case, to a ligand of humic acid. Similar results were obtained earlier with other macrophytes [13,14].
Adaptation of Macrophytes to the Content of Radio Nuclides in the Solution of 241Am and 242Pu
Aquatic biotic and abiotic components, between which various processes and exchanges occur, can be divided into three inanimate systems such as air, water and solid components, and living systems, namely flora, and fauna, including humans. All substances can be included in these systems in different concentrations. These concentrations depend on the chemical properties of substances and they are involved in the creation of a component matrix.
The organism and the environment are a unity which is constantly disturbed as a result of changes in the environment and in the organism itself. But almost all disturbances will sooner or later be removed as a result of the organism’s adaptability to the external environment surrounding it at a given moment and in a given space.
Adaptation (from Late Latin adaptation), as one of the fundamental properties of living systems, reflects, on the one hand, the stability of biological systems to environmental conditions, and on the other hand, this is the process of adaptation of living organisms to constantly changing environmental conditions. Adaptation is possible up to a certain level, which depends on the intensity and duration of the influence.
Adaptation of an aquatic plant organism includes beneficial changes at any level of the structure of a biological system (molecular, sub cellular, cellular, etc.) to help its survival under changing environmental conditions.
Possible disturbances of cell membranes can serve as an indicator of the resistance of an aquatic plant to stress.
The Main Method to Study this was Chosen to be FT-IR Spectroscopy
IR absorption spectra and diffuse reflectance spectra of the studied samples are similar to the IR spectra of any plant raw material, in particular, cellulose [24]. The spectroscopic study of such systems is based on the comparison of the spectral characteristics of the absorption bands of the functional groups of the control sample with the corresponding characteristics of these bands in the spectra of the treated samples. Let us consider the main absorption bands characteristic of the IR spectra of the samples under study.
(Figure 3) shows the IR absorption spectra and diffuse reflectance spectra obtained for the walls of the cell debris of plants of the control experiment (without the addition of metal) and experimental system with the addition of 241Am.
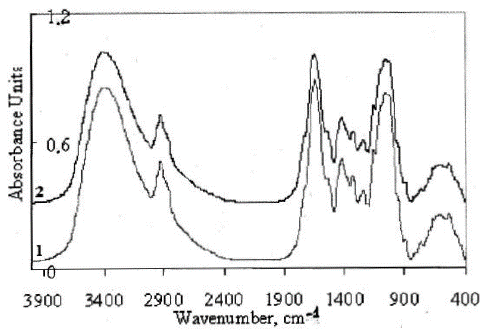
Figure 3: IR spectra of the Elodea samples: 1) control sample; 2) experimental sample.
The spectra presented show intense absorption bands with the maxima at ≈ 3400 and 1656 cm–1. An intense broad absorption band in the range of 3700–2200 cm–1 (the main maximum ≈ 3400 cm–1) is attributed to the stretching vibrations of hydroxyl groups linked by hydrogen bonds [3], and the absorption bands at 1656 and 620 cm–1, respectively, to in-plane bending vibrations of OH groups [3,22]. The absorption bands in the range of 3000–2800 and 1450–1370 cm–1 is due to the stretching and bending vibrations of aliphatic CH3 and CH2 groups, respectively [3,22]. The absorption band with the maximum at 1735 cm–1 is attributed to the stretching vibrations of carbonyl groups as in [3,22]. In addition, the analysis of the observed absorption bands in the region of 1000–1200 cm–1 in combination with the absorption at 1735 cm–1 allows us to make an assumption about the presence of keto-ester compounds in the structural components of the cell (cell walls) [3,19,22].
In the images presented in (Figure 3), the spectra exhibit intense absorption bands with the maxima at ≈ 3400 and 1656 cm–1. The intense broad absorption band in the range of 3700-2200 cm–1 (the main maximum ≈ 3400 cm–1) is attributed to the stretching vibrations of hydroxyl groups linked by hydrogen bonds [3,22], and that in the range of 1656 and 620 cm–1 to the in-plane bending vibrations of OH-groups [3,22].
The absorption bands in the range of 3000-2800 and 1450-1370 cm–1 are due to the stretching and bending vibrations of aliphatic CH3 and CH2 groups, respectively. The absorption band with the maximum at 1735 cm–1 is attributed to the stretching vibrations of carbonyl groups, as in [3,22].
The analysis of the observed absorption bands in the region of 1000-1200 cm-1 in combination with the absorption at 1735 cm-1 suggests the presence of keto-ester compounds in the cellulose composition [3,22].
(Figure 4) shows the IR absorption spectra of the samples treated with the solutions containing 242Pu with varying radionuclide content (curves 2 and 3, respectively) in comparison with the spectrum of the control sample (curve 1). The analysis of the presented curves evidences almost complete identity of the spectral patterns for all the samples. Apparently, changes are to be expected in the cellular fluid. Moreover, the identity of the spectral patterns also indicates that the plants were very carefully washed from the treatment solutions.
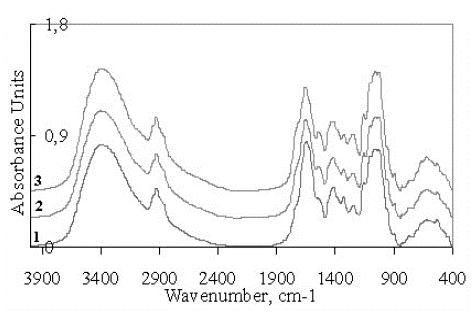
Figure 4: IR spectra of the cellulose samples: curve 1 – control; curve 2 - sample from the system with 242Pu (4 Bq·sample-1); curve 3 - sample from the system with 242Pu (10 Bq·sample-1).
Delayed Fluorescence as an Indicator of the Plant Viability
Delayed fluorescence is one of the indicators of the plant viability, and therefore, an indicator of their adaptation to stressful conditions.
(Figure 5) shows the time dependences of delayed fluorescence, obtained under the influence of radio nuclides on the Elodea shoots, with the content of radio nuclides in the solution being different.
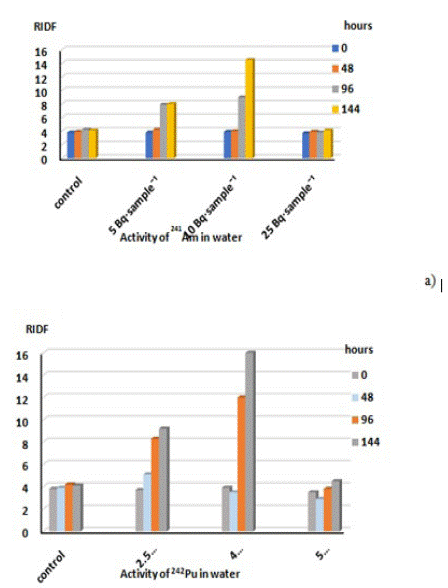
Figure 5: Dependences of Relative Index of Delayed Fluorescence (RIDF) from the content of radio nuclides in the solutions: a) 241Am, b) 242Pu.
The resulting dependence represents a regular change in the values of delayed fluorescence. With an increase in the content of radio nuclides in the solution, the inhibition of fluorescence is observed on the first day, followed by a stage of stagnation and a further very slow increase in fluorescence indices. The higher the content of radio nuclides, the more pronounced is the stage of inhibition. However, at the stage of increasing values of the fluorescence indices, at the maximum content of radio nuclides, ~25 Bq·sample-1 (americium) and ~ 5 Bq·sample-1 (plutonium), it was more intense than in other systems.
At the example of the studies performed, one can see that the structures of the cell membranes in the plants slightly change, despite the fact that the exposure level for Elodea was different, both in the physicochemical properties of radio nuclides and their content and in intensity (duration of exposure).
The analysis of the literature data indicates that when plants adapt to various unfavorable factors, there occur similar structural and metabolic changes if the unfavorable factor does not go beyond genetically inherent adaptive capabilities of the organism.
Substances dissolved in the aquatic environment are an important abiotic stress for living organisms developing in this environment. Like all other abiotic stresses, dissolved organic and inorganic compounds lead to biochemical and genetic changes [23].
A number of plant species, in particular, Elodea Canadensis, exhibit a rather high resistance to radio nuclides. Thus, Elodea retained its viability in the case of all the radionuclide content values considered. The toxic effect, which is recorded as a decrease in the fluorescent parameters 1 day after adding salts to the medium, decreases on the 5-7th day of cultivation, which is accompanied by an increase in the Relative Index of Delayed Fluorescence (RIDF).
The extent of accumulation of radio nuclides by plants depends on many external (concentration of an element in the environment and form of its existence, pH, Eh, seasonality and endogenous rhythms, light, temperature, salinity, currents, drains) and internal (species specificity, physiological state of an organism, development stage, age, genetic) factors.
Despite this, substances dissolved in a water body are not present in the form of simple inorganic substances since a significant amount of organic and inorganic substances is already present in the water of a surface water reservoir, which interact with substances entering the water. Therefore, either even more toxic forms or weak or non-toxic ones are formed.
Phytochelatins are identified in many aquatic plants and photosynthetic organisms, ranging from Elodea, gymnosperms to monocots and dicots. Phytochelatins (PCs) are synthesized from glutathione (in some cases, related compounds) by PC synthases (PCS), and play a role in the distribution and accumulation of Cd and other highly toxic metals like Ag, Hg, As [10,20].
All abiotic stresses can accumulate excessive Reactive Oxygen Species (ROS) at a certain stage of stress exposure leading to oxidative stress. However, ROS are not only toxic compounds, but sometimes act as important regulators in many biological processes in plants, namely, cell cycle, programmed cell death, hormone signaling, biotic and abiotic cell responses [15].
To deal with the heavy metal stress and associated oxidative stress, metallothionein, a well-known metal chelator and antioxidant, would possibly be a good option.
Thus, the studied external effects (factors affecting) on the aquatic plant did not go beyond the adaptive capabilities of Elodea Canadensis for almost all the studied model systems. Therefore, the submerged macrophyte Elodea Canadensis is tolerant to several abiogenic stress factors at once.
Conclusion
The processes of interaction of radio nuclides 241Am and 242Pu with the submerged macrophyte of Elodea depend on the physicochemical properties of the radio nuclides, state of the macrophyte (age, absence of damage to the leaf blades) and structure of the plant stem. In the case of a change in the salt composition of the aquatic environment (introduction of additional complex-forming ions), the distribution of the radio nuclides accumulated by the macrophyte biomass changes to some extent: in the presence of a sulfide ion, radio nuclides are firmly deposited on the plant surface (up to 85% of the total amount) and only their small amount is within the biomass; in the presence of humic acid salts, 241Am and 242Pu are largely accumulated by the plant biomass (up to 75%). However, in the case of all the investigated effects exerted on the submerged macrophyte Elodea, the studied aquatic plant was found to have very good adaptive abilities and it can effectively be used for phytoremediation of water bodies from such anthropogenic pollutants as transuranic radio nuclides 241Am and 242Pu.
References
- Antonenko AE. Some characteristics of the Yenisei. Krasnoyarsk: World of Wildlife. in Russ. 2012: 14-28.
- Beasley T, Cross F. A review of biokinetic and biological transport of transuranic radionuclides in the marine environment. In: Hanson W (ed.). Transuranic elements in the environment. Springfield, Virginia: U.S. Department of Commerce. 1980; 524-540.
- Bellamy LJ. The infrared spectra of complex molecules. Vol.1 (3rd ed.). Halsted Press: Wiley & Sons, Inc. New York. 1975: 433.
- Bolsunovskii AY, Zotina TA, Bondareva LG, Degermendzhi AG. Assessment of the Rate of Accumulation of the Transuranium Element Americium-241 by the Aquatic Plant Elodea canadensis Doklady Biological Sciences. 2004; 399: 467–469.
- Bolsunovsky AY. Artificial radionuclides in aquatic plants of the Yenisei River in the area affected by effluents of a Russia plutonium complex. Aquatic ecology. 2004; 38: 57–62.
- Bolsunovsky A, Bondareva L. Actinides and other radionuclides in sediments and submerged plants of the Yenisei River. Journal of Alloys and Compounds. 2007; 444-445: 495–499.
- Carbiener R, Tremolieres M, Mercier JL. Ortscheit A. Aquatic macrophyte communities as bioindicators of eutrophication in calcareous oligosaprobe stream waters (Upper Rhine plain, Alsace). Vegetatio. 1990; 86: 71–88.
- Choppin G, Morgernstern A. Distribution and movement of environmental plutonium. In: Kudo A (ed.). Plutonium in the Environment. edited. Proceeding of the second invited international symposium. Novenber 9-12, 1999, Osaka, Japan. Elsevier/Netherland, 2001: 91-105.
- Clarkson DT, Hanson JB. The mineral nutrition of plants. Annu Rev Plant Physiol. 1980; 31: 239–298.
- Cobbet CS. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol. 2000; 3: 211-216.
- Eyman L, Trabalka J. Patterns of transuranic uptake by aquatic organisms: consequences and implications. In: Hanson W (ed.). Transuranic elements in the environment. Springfield, Virginia: U.S. Department of Commerce. 1980; 612-624.
- Fukai R, Holm E, Ballestra S. Modified procedure for plutonium and americium analysis of environmental samples using solvent extraction. In: IAEA. Activities of the International Laboratory of Marine Radioactivity Rep. Ser. No. 187. Monaco: IAEA. 1976.
- Klark DL, Hekker ZC, Djarvinen GD, Ney MP. The chemistry of the actinide and transactinide elements. Ed. by Morss LR, Edelstein NM, Fuger J, Kaz JJ. Springer. 2010: 2: 530.
- Koranda JJ, Robison WL. Accumulation of radionuclides by plants as a monitor system. Environ. Health Perspect. 1978; 7: 165–179.
- Laloi Ch, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld J-Ph. The Arabidopsis Cytosolic Thioredoxin h5 Gene Induction by Oxidative Stress and Its W-Box-Mediated Response to Pathogen Elicitor. Plant Physiol. 2004; 134: 1006–1016.
- Lundgren RE, McMakin AH, Lundgren RE. Risk Communication: A Handbook for Communicating Environmental, Safety, and Health Risks. John Wiley & Sons. 2011: 384.
- Luoma SN. Bioavailability of trace metals to aquatic organisms. The Science of the Total Environment. 1983; 28: 1–22.
- Mason AZ, Jenkins P. Metal Speciation and Bioavailability in Aquatic International Union of Pure and Applied Chemistry series (IUPAC). Chapter 10: Metal Detoxification in Aquatic Organisms. J. Wiley Publishers. (Eds. A. Tessier and D.R. Turner). 1995; 479–609.
- Meek ME. Mode of Action Frameworks in Toxicity Testing and Chemical Risk Assessment / M.E Meek / PhD thesis, Institute for Risk Assessment Sciences (IRAS), Utrecht University, the Netherlands. 2009; 282.
- Rauser WE. Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin, and metallothioneins. Cell Biochem Biophys. 1999; 31: 19-48.
- Ryabovol SV. Flora of Krasnoyarsk (analysis, synanthropic changes, protection). Flora and vegetation of Siberia and the Far East. Krasnoyarsk: KSPU. in Russ. 2011; 424.
- Szymanski HA, Erickson RE. Infrared Band Handbook, Plenum Press. New York. 1970: 2.
- Toivonen Peter MA, Brummell David A. Postharvest Biology and Technology. 2008; 1–14.
- Vereecken H, Baetens J, Viaene P, et al. Ecological management of aquatic plants: Effects in lowland streams. Hydrobiology. 2006; 205-210.
- Wallentinus I. Introduced marine algae and vascular plants in European aquatic environments. In: E. Leppakoski, S.Gollasch and S. Olenin (eds), Invasive aquatic species of Europe – Distribution, impacts and management. – Dordrecht, the Netherlands: Kluwer Academic Publ. 2002; 27–52
