References
Microcystis aeruginosa is a toxin producing cyanobacterium responsible for dangerous Harmful Algal Blooms (HABs) in Lake Okeechobee Florida as well as worldwide. We investigated the potential utilization of organophosphates, as Dissolved Organophosphates (DOP), by this species to expand the knowledge of and eventually controls on nutrient sources and pollution. Axenic M. aeruginosa (PCC7806), which grew well on standard BG-11 media containing potassium dibasic hydrogen phosphate (K2PO4), was found to be unable to utilize certain organo-phosphates (D-Glucose-6-Phosphate {DG6P}, B-Glycerol-Phosphate {BGP}, Phytic Acid {PhA}). Non-axenic M. aeruginosa (UTEX LB2385) grew well on both standard BG-11 and BG-11 media in which the normal inorganic phosphate was substituted with DG6P or BGP but not with PhA. Heterotrophic bacteria in the non-axenic culture likely cleaved ortho-phosphate from the organophosphates while utilizing the organic portion as ‘food’. The addition of alkaline bovine phosphatase to the axenic cultures did not facilitate utilization of organophosphates. Letting the axenic cultures enter the lysis (death) phase did not allow activation of intrinsic phosphatase enzymes as added orgo-phosphates did not reactivate growth. Co-culturing M. aeruginosa with Anabaena flos-aquae, known to utilize phosphatase enzymes, did not provide phosphorus for M. aeruginosa. Collectively, these results reconfirm the concept of a synergistic microbiome (phycosphere, ‘interactome’) being required for the utilization of organophosphates as a phosphorus source by Microcystis aeruginosa.
Keywords: Harmful algal blooms (HABs); Microcystis aeruginosa; Organic phosphorous; Microbiome
Introduction
The following quote is from a US-EPA Funding Opportunity (Number EPA-G2017-STAR-A1) “The occurrence of HABs” {Harmful Algal Blooms} is increasingly common in inland freshwater ecosystems. --- Yet basic questions of HAB occurrence, extent, intensity, and timing are largely unanswered.” South Florida has been and still is experiencing nutrient (N,P) excesses in surface waters and sediments in Lake Okeechobee [1-5], coastal estuaries [6-9], and the Greater Everglades [10-14]. Sources include sewerage, notably septic systems (aka OSTDS, Onsite Sewerage Treatment and Disposal Systems) [15-18], agricultural operations [19-23], and a growing equestrian industry [24-27].
Drastic cyanobacterial blooms in Lake Okeechobee during the 1980s were due to the anatoxin producing diazotrophic (Nitrogen-fixing) species Anabaena flos-aquae and reductions in phosphorus loading in the 1990s appears to have help control that species [28,29]. However, as seen starting in 2005 and continuing to date, increasing dual nitrogen and phosphorus pollution now favors non-diazotrophic blooms of the toxic (microcystin) cyanobacterium Microcystis aeruginosa [30]. Cyanobacterial blooms dominate an ecosystem by blocking sunlight from photosynthetic organisms below. As a bloom senesces and dies, its organic matter is decomposed, removing available oxygen and leaving anoxic conditions leading to massive fish kills [31,32]. When the freshwater cyanobacterium M. aeruginosa begins to die, it releases large amounts of the hepatotoxic peptide microcystin [33] that can then leach into surrounding estuaries or marine waterways, expanding the detriment of the bloom. Microcystin-LR and its congeneric toxins are often responsible mammalian deaths such as dogs and cows [34-36]. In estuaries, this can pollute the water and decrease the success of many species that use these estuaries as safe havens for reproduction.
M. aeruginosa growth is facilitated by eutrophic conditions in lakes, which can be excessively fueled by pollution from various anthropogenic sources [37]. Nonpoint nitrogen and phosphorus pollution is a well-known worldwide problem [38,39,70].
It is known that the mucilaginous masses of cyanobacteria that cause these cyanoHABs are not homogeneous and it has been hypothesized that the “interactome” in these globs of bacteria, or a microbiome, are facilitating the metabolic reactions needed to fuel massive blooms [41]. Since then, the idea of an active synergistic phycosphere of these microbial colonies has expanded [42,43].
Regarding sources of phosphorus, specifically organo-phosphates, we previously found that sugarcane leaves, husks, stalks and roots contain beta-glycerol-phosphate, fructose-6-phosphate, glucose-6-phosphate as well as phosphate mono- and di-esters in addition to ortho-phosphate [21]. Therefore, the leaching of organo-phosphates into adjacent water bodies, such as Lake Okeechobee, can well be expected from sugarcane and other land plants as well [44]. One study has revealed that meadow or forest soils had between 79-92% or 13-37% organic phosphorous compounds, respectively [45]. Organo-phosphates are therefore phosphorus sources that need to be fully examined for their participation in the nutrient supplies creating harmful algal blooms.
Microcystis aeruginosa must compete with all other autotrophic and heterotrophic organisms, as well as inorganic precipitation reactions (e.g. Fe3+ + PO43- FePO4), for soluble reactive phosphorus (SRP, ortho-phosphate). Therefore, we undertook the current study to investigate the potential utilization of organic phosphorus by M. aeruginosa.
Materials and Methods
Stock culture conditions utilized light at 70 μmol phota m-2 s-1 in a 12hr light/dark cycle with BG-11 media [46,47] containing 2 mM NaNO3 and 0.23 mM K2PO4. The nitrate concentration was reduced from the standard 17.6 mM to 2 mM to better mimic [48,49] the Redfield Ratio of 16:1 N:P [50]. Tests on the utilization of organic-phosphates were performed by substituting the 0.23 mM K2PO4 with an equimolar amount of D-glucose-6-phosphate {DG6P: Sigma Aldrich #G7375}, b-glycerol-phosphate {BGP: SigmaAldrich #50020}, or phytic acid {PhA: SigmaAldrich #P8810}. Bovine alkaline phosphatase (SigmaAldrich # API-RO) was utilized for testing exogenous phosphatase activity on dissolved organophosphates. Glyphosate (SigmaAldrich #45521) was also tested as a phosphorous source in place of dipotassium phosphate. All samples were cultured in 125 mL PETG flasks (ThermoFisher # 50-233-5807) rotating at 130 rpm in a gyratory water bath shaker at 26°C. To ensure the axenic stocks and inoculates stayed axenic, all culture manipulations were performed in a Baker Sterile GARD-111 biosafety cabinet with sterile conditions. Media was sterile filtered or autoclaved, depending on the organic contents of the media. Extreme caution was taken when working with all stock and inoculates to ensure there was no contamination. Routine checks for contamination were performed using a fluorescent microscope and a light microscope, utilizing DAPI (ThermoFisher # EN62248) staining and the natural fluorescence of the cyanobacteria to evaluate contamination. All experimental trials were inoculated at a level of about 1x105 cells per mL and cultured in 50 mL of media. Growth was tracked over time utilizing cell counts (cells per mL) using a Thermo Fisher Invitrogen Countess II Cell Counter. The counter was checked for accuracy and precision. A standard curve was created with an r2 value of 0.996. All inoculates were grown into their stationary phase unless there was negative or no growth. Cell counts were performed every 3-4 days after an initial 4-day inoculation / lag phase period. Sterile procedures were ensured using the biosafety cabinet. It is estimated that a normal growth curve would take approximately 30-40 days, as cell counts will stop after reaching the end of their stationary phase (i.e., their lysis or death phase).
Axenic Microcystis aeruginosa stock (PCC7806) was from the Pasteur Culture Collection of Cyanobacteria (Institut Pasteur of Paris). The culture was incubated for at least 5 days to allow for adequate growth prior to being separated further into additional stock cultures. Stock solutions for these trials were kept under the same conditions as all other experimental trials.
The non-axenic strain of M. aeruginosa previously studied by our group. The UTEX LB2385 strain is another widely used and studied culture of M. aeruginosa. This strain was obtained through the University of Texas at Austin’s Algal culturing center (UTEX). This species was grown in the same conditions given above and stocks were allowed to incubate and routinely refreshed.
A culture of Anabaena sp., UTEX 2576, was acquired from the University of Texas at Austin’s algae culturing center (UTEX) and used as a potential source of phosphatase activity. This culture strain was also grown in the same conditions as the M. aeruginosa strains of PCC7806 and UTEX LB 2385. The same gyratory water bath shaker with the same RPM and water temperature, as well as the 125 mL PETG flasks, were used. The Anabaena sp. stocks were also grown in BG-11 medium. Anabaena sp. cells could not be counted with the cell counter as they are filamentous. Therefore, manual microscopic cell counts were utilized to assess growth.
Results and Discussion
Growth of Axenic and Non-Axenic M. Aeruginosa on BG-11 Media
Both the axenic (Figure 1a) and non-axenic (Figure 1b) M. aeruginosa cultures grew well on normal BG-11 in which phosphorus is provided as a form of ortho-phosphate (PO43- as K2HPO4). Each of the three separate trial data sets are plotted as the mean of three runs, equaling nine trials for both the axenic (1a) and non-axenic (1b) cultures. Cell counts and growth was stopped when the cultures were more than 10 days int the stationary phase.
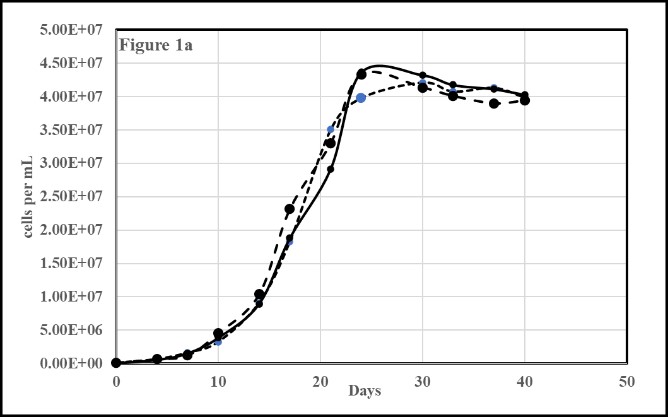
Figure 1: Growth of (1a) axenic PCC-7806 and (1b) non-axenic UTEX-LB2385 Microcystis aeruginosa in standard BG-11 media.
Zero (0.00E+00) on the Y-axis in these and following plots indicates the inoculation stage of ~ 1E+05 cell / mL.
Growth Trials of Axenic and Non-Axenic M. Aeruginosa with Organophosphate Substituted BG-11 Media
Next, we substituted the standard Dissolved Inorganic Phosphate (DIP), dipotassium phosphate, with various Dissolved Organic Phosphate (DOP) species. These are coded within the legend for Figure 2. As always, each set of trial data plotted is the mean of triplicate cultures. Each trial also included a reference run (black trendline) with standard BG-11 for both the axenic and non-axenic cultures.
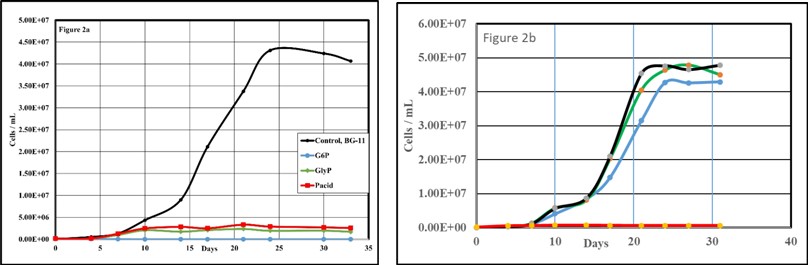
Figure 2: Axenic (2a) and non-axenic (2b) cultures of M. aeruginosa grown on BG-11 (black) and BG-11 phosphate substituted with D-glucose-6-phosphate (blue), β-Glycerol-phosphate (green) or phytic acid (red).
The axenic M. aeruginosa cultures were unable to grow on any of the three DOP compounds provided as a potential phosphorus source. This indicates a lack of ‘active’ phosphatase enzymes in this species, or at least this clade. The very slight increase in cell density for the phytic acid and b-glycerol-6-phosphate trials on the axenic culture is attributed to the use of ‘storage’ phosphorus reserves [51-53]. The non-axenic culture grew equally well on D-glucose-6-phosphate and b-glycerol-phosphate but was unable to utilize the hexaphosphate compound phytic acid. The activities if coincident heterotrophic bacteria in the microbiome [42,43,54] forming the “interactome” [41] of this culture are responsible for the cleavage of phosphate from the organophosphate species.
Aside from synergistic heterotrophic bacterial activities releasing phosphate from organophosphate compounds for use by M. aeruginosa, it is reported that high Ultraviolet (UV) radiation can alter phosphatase activities and high Dissolved Organic Matter (DOM) can act as an antioxidant decreasing that effect [55]. This point should be remembered when dealing with the native ‘interactomes’ in waters such as Lake Okeechobee which is high in DOM. That is, the phosphatase activities of the coincident heterotrophic bacteria would likely be little affected in high DOM containing waters.
Growth Trials of Stationary-lysis Phase Axenic M. Aeruginosa on Organophosphate Substituted BG-11 Media
Phosphate stress in M. aeruginosa may induce phosphatase activities [55,56]. We grew the axenic culture (PCC7806) on BG-11 with limited (10% normal; 0.023 mM [23 mM] K2PO4) phosphate (Figure 3a). We then took the stressed M. aeruginosa in the beginning of the death or lysis stage (~62) days and inoculated it into BG-11 media that had the DIP (K2PO4) replaced with B-Glycerol-6-Phosphate (BGP) or glucose-6-phosphate (G6P). The three runs with the BGP substituted BG-11 is shown in Figure 3b. A very small increase (appx. doubling) in cell density occurred within the first week, likely due to legacy (storage) P within the inoculum. After that cell death was very fast, again indicating that axenic M. aeruginosa cannot obtain phosphate from the cleavage of organophosphates without a synergistic microbiome. An identical trial (not shown) was performed with G6P substituted BG-11 and the results were essentially identical.
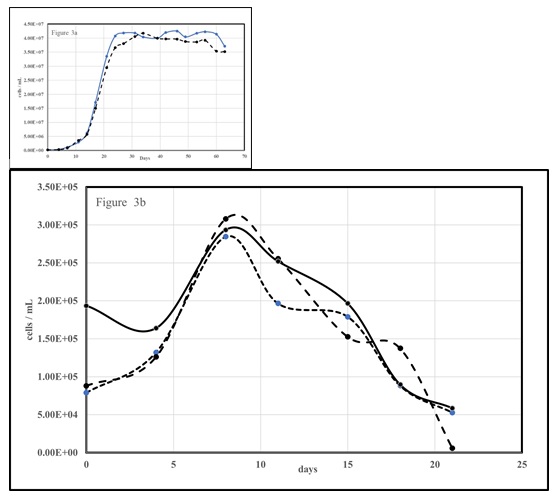
Figure 3: (3a) Axenic M. aeruginosa grown with 10% P for induction of P stress (3b) P-stressed cells incubated with B-Glycerol-6-Phosphate substituted (BGP) BG-11.
Growth Trials of Axenic M. Aeruginosa in Organophosphate Substituted BG-11 Media in the Presence of Alkaline Phosphatase
It is noted here that certain subspecies of Microcystis (e.g. have been shown to possess alkaline phosphatase enzymes [58] and/or activity [59,61]. However, as we have shown (Figure 2 above), M. aeruginosa (PCC-7806) lacks Alkaline Phosphatase Activity (APA). This and the following section probe potential extracellular APA.
Bovine alkaline phosphatase was added as18, 36 or 73 mL of 1500 U stock to BG-11 media that had the DIP (K2PO4) in BG-11 substituted with D-glucose-6-phosphate, b-Glycerol-phosphate or phytic acid. The results of these culture trials are shown in figure 4. It is noted here that all trendlines are present in this figure but overlap to the point that they, especially the blue and green trendlines, are not apparent. The presence of extracellular alkaline phosphatase without an organism’s participation did not result if phosphate cleavage from these three organophosphates. Again, it is the activity of the microbiome [42,43], also called interactome [41], that is required to provide DIP from DOP for use by M. aeruginosa.
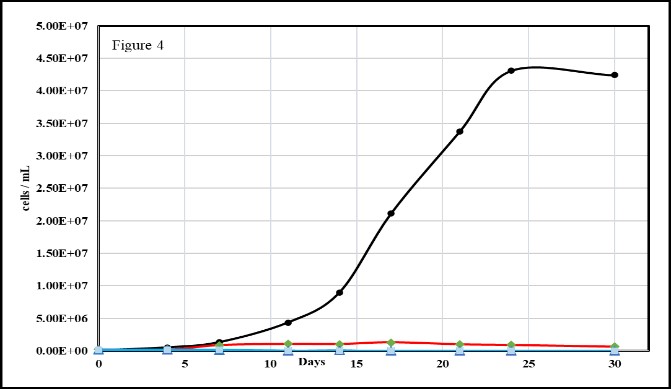
Figure 4: Axenic Microcystis aeruginosa grown on standard BG-11(reference, black trendline) and BG-11 media with dipotassium phosphate being substituted with an equimolar amount of b-glycerol-6-phosphate (green trendline), glucose-6-phosphate (blue trendline), or phytic acid (red trendline).
Growth Trials of Axenic M. Aeruginosa Co-Cultured with Anabaena Flos-aquae
Microcystis aeruginosa and Anabaena flos-aquae often coexist in Lake Okeechobee [62] and A. flos-aquae is known to utilize phosphatase enzymes [58,60]. We co-cultured these two species to determine if axenic M. aeruginosa could obtain DIP from the activities of A. flos-aquae.
The data in Figure 5 reveals that, even though A. flos-aquae can obtain its own inorganic P from the DOP compounds b-glycerol-6-phosphate and glucose-6-phosphate, as seen by microscopic evaluation of its growth in the co-culture, it does not supply DIP for uptake by M. aeruginosa.
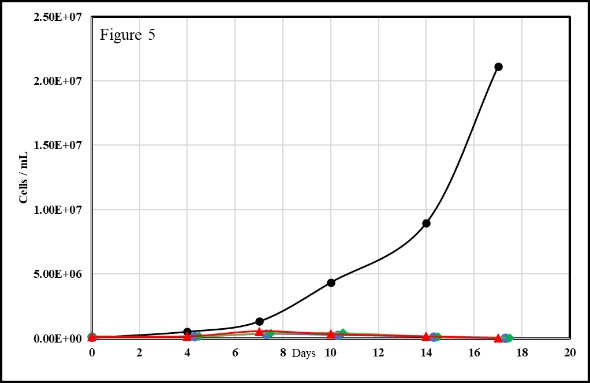
Figure 5: Axenic Microcystis aeruginosa co-cultured with Anabaena flos-aquae on standard BG-11 (reference, black trendline) and BG-11 media with dipotassium phosphate being substituted with an equimolar amount of b-glycerol-6-phosphate (green trendline), glucose-6-phosphate (blue trendline), or phytic acid (red trendline).
Growth Trials of Axenic M. Aeruginosa Substituting Glyphosate for Inorganic Phosphorus
Glyphosate (N-phosphonomethyl-glycine), the active ingredient in Roundup® is a herbicide in widespread use [63] and degrades rapidly in the environment [63,64]. Its high use in agriculture [63], notably around Lake Okeechobee [65], and reports that it can serve as a phosphorus source for certain phytoplankton [66,67] prompted us to test it with axenic M. aeruginosa. Figure 6 contains the results of three separate trials using glyphosate as the sole P source. Immediately apparent is that glyphosate did not aid growth but rather led to the rapid death of all M. aeruginosa cells. Glyphosate in natural Lake Erie waters was shown lead to a decrease in M. aeruginosa abundance [68]. However, it is also known that glyphosate degradation by bacteria, fungi and light can lead to increases in dissolved inorganic phosphorus which aids the growth of phytoplankton in P depleted environments [68-70].
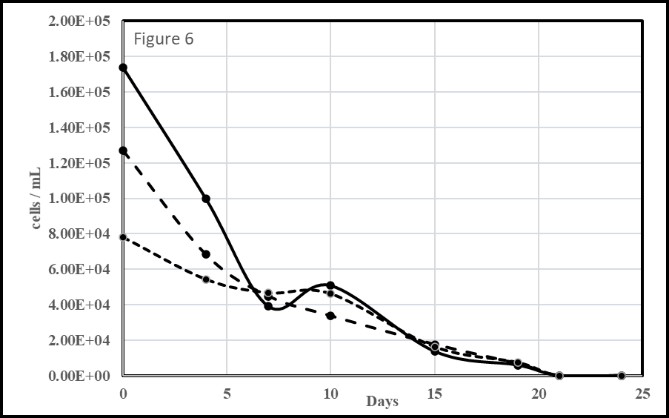
Figure 6: Microcystis aeruginosa growth trials of in BG-11 media having inorganic phosphate (K2PO4) substituted with an equimolar amount (0.23mM) of glyphosate. Trendlines for means of triplicate runs of three different starting cell concentrations.
Conclusions
Axenic Microcystis aeruginosa (PCC7806), grew well on standard BG-11 media containing potassium dibasic hydrogen phosphate (K2PO4), and was found to be unable to utilize certain organo-phosphates (D-Glucose-6-Phosphate (DG6P), B-Glycerol-Phosphate {BGP}, Phytic Acid {PhA}). M. aeruginosa was found to not be able to use glyphosate directly as a phosphorus source. Non-axenic M. aeruginosa grew well on both standard BG-11 media and BG-11 media with DG6P and BGP as the sole phosphorus source but was unable to utilize phytic acid. It is apparent that heterotrophic bacteria in the non-axenic cultures were responsible for cleaving phosphate from the organophosphates. It is likely that the heterotrophic bacteria utilize organophosphates for phosphate as well as organic matter as food. Therefore, once the phosphate requirements are met for the heterotrophs, additional phosphate cleaved from the organophosphates is released into the media for use by other organisms such as M. aeruginosa. This synergistic action whereby the heterotrophs release phosphate for use by M. aeruginosa substantiates the concept of a synergistic microbiome or “interactome” [41].
References
- Fisher MM, Reddy KRR, James RT. Long-term changes in the sediment chemistry of a large shallow subtropical lake. Lake Reserv Mng. 2001; 17: 217-32.
- Havens KE. Secondary nitrogen limitation in a subtropical lake impacted by non-point source agricultural pollution. Environ Pollut. 1995; 89: 241-6.
- Havens KE, East T. In situ responses of Lake Okeechobee (Florida, USA) phytoplankton to nitrogen, phosphorus, and Everglades agricultural area canal water. Lake Reser Mng. 1997; 13: 26-37.
- Havens KE, Thomas JR. The phosphorus mass balance of Lake Okeechobee, Florida: implications for eutrophication management. Lake Reser Mng. 2005; 21: 139-48.
- Pollman CD, James RT. A simple model of internal loading of phosphorus in Lake Okeechobee. Lake Reser Mng. 2011; 27: 15-27.
- Duersch BG, Louda JW. Phosphorus speciation using P-31 nuclear magnetic resonance spectroscopy in order to trace phosphorus sources and movement in the C51 basin and northern Everglades National Meeting, Aug 20-24 [poster]. Washington, DC: American Chemical Society; 2017. p. 254th:(Abstract #2729989).
- Liu Z, Choudhury SH, Xia M, Holt J, Wallen CM, Yuk S, et al. Water quality assessment of coastal Caloosahatchee River watershed, Florida. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2009; 44: 972-84.
- Pant HK, Reddy KR. Phosphorus sorption characteristics of estuarine sediments under different redox conditions. J Environ Qual. 2001; 30: 1474-80.
- Phlips EJ, Badylak S, Grosskopf T. Factors affecting the abundance of phytoplankton in a restricted subtropical lagoon, the Indian River Lagoon, Florida, USA. Estuar Coast Shelf Sci. 2002; 55: 385-402.
- Bruland GL, Osborne TZ, Reddy KR, Grunwald S, Newman S, DeBusk WF. Recent changes in soil total phosphorus in the Everglades: water Conservation Area 3. Environ Monit Assess. 2007; 129: 379-95.
- Childers DL, Doren RF, Jones R, Noe GB, Rugge M, Scinto LJ. Decadal change in vegetation and soil phosphorus patterns across the Everglades landscape. J Environ Qual. 2003; 32: 344-62.
- Corrales J, Naja GM, Bhat MG, Miralles-Wilhelm F. Modeling a phosphorus credit trading program in an agricultural watershed. J Environ Manage. 2014; 143: 162-72.
- Louda JW, Grant C, Browne J, Hagerthey SE. Pigment-based chemotaxonomy and its application to Everglades periphyton. In: Entry JA, Jayachandrahan K, Gottlieb AD, Ogram A, editors. Microbiology of the Everglades ecosystem. Science Publishers; 2015; 287-347.
- Reddy KR, Newman S, Osborne TZ, White JR, Fitz HC. Phosphorus cycling in the Greater Everglades Ecosystem: legacy phosphorus implications for management and restoration. Crit Rev Environ Sci Technol. 2011; 41: 149-86.
- Badruzzaman M, Pinzon J, Oppenheimer J, Jacangelo JG. Sources of nutrients impacting surface waters in Florida: a review. J Environ Manage. 2012; 109: 80-92.
- FDOH. Onsite Sewerage Treatment and Disposal Systems installed in Florida. Florida Department of Health; 2012. Available from: http://www.floridahealth.gov/environmental-health/onsitesewerage/newinstallations.pdf.
- Lapointe BE, Herren LW, Paule AL. Septic systems contribute to nutrient pollution and harmful algal blooms in the St. Lucie estuary, Southeast Florida, USA. Harmful Algae. 2017; 70: 1-22.
- Meeroff DE, Bloetscher F, Long SC, Bocca T. The use of multiple tracers to evaluate the impact of sewered and non-sewered development on coastal water quality in a rural area of Florida. Water Environ Res. 2014; 86: 445-56.
- Boggess WG, Johns G, Meline C. Economic impacts of water quality programs in the Lake Okeechobee watershed of Florida. J Dairy Sci. 1997; 80: 2682-91.
- Duersch BG, Bhadha JH, Root TL, Louda JW. The role of rice (Oryza sativa L.) in sequestering phosphorus compounds and trace elements: speciation and dynamics. Sci Total Environ. 2020; 725: 138366.
- Duersch B, Ricca J, Louda JW. Bioavailability of organic phosphorus compounds with respect to the growth of Microcystis aeruginosa. Fla Scient. 2021; 84: 282-302.
- Entry JA, Gottlieb A. The impact of stormwater treatment areas and agricultural best management practices on water quality in the Everglades Protection Area. Environ Monit Assess. 2014; 186: 1023-37.
- Stuck JD, Izuno FT, Campbell KL, Bottcher AB, Rice RW. Farm-level studies of particulate phosphorus transport in the Everglades Agricultural Area. Trans ASAE. 2001; 44: 1105-16.
- Cintron C, Louda JW. Extractability of phosphorous from horse manure and implications for the pollution of the surficial waters and aquifers of southern Florida. 71st. Annual Meeting of the Florida Academy of Science. St. Petersburg, FL. 2007: Abstr ENV.
- Louda JW, Duersch B, Querioz V, Cintron C. Equestrian waste streams as a source of surface-water phosphorus pollution Annual Meeting. Orlando, FL. 2019. American Chemical Society. Environmental Division. p. 257th. # ENVR-20726.
- Louda JW, Duersch BG, Osetek JT, Cintron C, Chaljub L, Queiroz V. Phosphorus non-point pollution from equestrian wastes and the need for recycling. Environ. and Pollut. 2021; 10: 21.
- Osetek J, Louda JW. Water quality analyses of canals in the agricultural / residential community of Loxahatchee Groves, Florida. 68th. Annual Meeting of the Florida Academy of Sciences. Orlando, Fl. 2004: Abstract ENV-10.
- Recheigl JE. Phosphorus-Natural versus Pollution Levels: Lake Okeechobee Case Study. Florida Beef Cattle Short Course. University of Florida – Institute of Food and Agricultural Sciences; 2017.
- Rechcigl JE, Boucher AB. Fate of phosphorus on bahiagrass (Paspalum notatum) pastures. Ecol Eng. 1995; 5: 247-59.
- Rosen BH, Davis TW, Gobler CJ, Kramer BJ, Loftin KA. ’Cyanobacteria of the 2016 Lake Okeechobee and Okeechobee Waterway Harmful Algal Bloom.’ USGS numbered Series 2017-1054. Cyanobacteria of the 2016 Lake Okeechobee and Okeechobee Waterway Harmful Algal Bloom. Vol. Open File Rep. 2017–1054.
- Lehman PW, Teh SJ, Boyer GL, Nobriga ML, Bass E, Hogle C. Initial impacts of Microcystis aeruginosa blooms on the aquatic food web in the San Francisco estuary. Hydrobiologia. 2010; 637: 229-48.
- Tian R, Chen J, Sun X, Li D, Liu C, Weng H. Algae explosive growth mechanism enabling weather-like forecast of harmful algal blooms. Sci Rep. 2018; 8: 9923.
- Hooser SB, Beasley VR, Lovell RA, Carmichael WW, Haschek WM. Toxicity of microcystin LR, a cyclic heptapeptide hepatotoxin from Microcystis aeruginosa, to rats and mice. Vet Pathol. 1989; 26: 246-52.
- Clark JM, Schaeffer BA, Darling JA, Urquhart EA, Johnston JM, Ignatius AR, et al. Satellite monitoring of cyanobacterial harmful algal bloom frequency in recreational waters and drinking water sources. Ecol Indicat. 2017; 80: 84-95.
- Herren CM, Webert KC, Drake MD, Jake Vander Zanden MJ, Einarsson Á, Ives AR et al. Positive feedback between chironomids and algae creates net mutualism between benthic primary consumers and producers. Ecology. 2017; 98: 447-55.
- Pippin C. ’Fish Kill Prompts Concern from Locals about Lake O Discharges.’ WPEC; Mar 22, 2016. Available from: http://cbs12.com/news/local/fish-kill-prompts-concern-from-locals-about-lake-o-discharges.
- O’Neil JM, Davis TW, Burford MA, Gobler CJ. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae. 2012; 14: 313-34.
- Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appls. 1998; 8: 559-68.
- Lankoski J, Ollikainen M. Counterfactual approach for assessing Agri-environmental policy: the case of the Finnish water protection policy. RAEStud. 2013; 94: 165-93.
- Xia Y, Zhang M, Tsang DCW, Geng N, Lu D, Zhu L, et al. Recent advances in control technologies for non-point source pollution with nitrogen and phosphorous from agricultural runoff: current practices and future prospects. Appl Biol Chem. 2020; 63.
- Cook KV, Li C, Cai H, Krumholz LR, Hambright KD, Paerl HW, et al. The global Microcystis interactome. Limnol Oceanogr. 2020; 65: S194-207.
- Lü P, Li HL, Xu Y, Zheng XX, Huang ZH, Wang C, et al. Effects of nutrients on the growth of Microcystis aeruginosa and bacteria in the phycosphere. Huan Jing Ke Xue. 2022; 43: 4502-10.
- Li J, Wang Z, Cao X, Wang Z, Zheng Z. Effect of orthophosphate and bioavailability of dissolved organic phosphorous compounds to typically harmful cyanobacterium Microcystis aeruginosa. Mar Pollut Bull. 2015; 92: 52-8.
- McDowell RW, Worth W, Carrick S. Evidence for the leaching of dissolved organic phosphorus to depth. Sci Total Environ. 2021; 755: 142392.
- Heron T, Strawn DG, Dobre M, Cade-Menun BJ, Deval C, Brooks ES, et al. Soil phosphorus speciation and availability in meadows and forests in Alpine lake watersheds with different parent materials. Front For Glob Change. 2021; 3: 604200.
- Allen MM, Stainer RY. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1968; 32: 270-7.
- UTEX BG-11 Recipe. Available from: http://web.biosci.utexas.edu/utex/Media%20PDF/bg-11-medium.pdf.
- Thurber K. What is the Redfield ratio and why should I Care? ATI North America; 2019.
- Sharp JH. Marine and aquatic communities, stress from eutrophication. Levin SA editor. In: Encyclopedia of biodiversity. New York: Elsevier. 2001; 1-11.
- Redfield AC. On the proportions of organic derivatives in sea water and their relation to the composition of plankton. In: James Johnstone memorial Volume, University Press of Liverpool. 1934; 176-92.
- Kromkamp J. Formation and functional significance of storage products in cyanobacteria. N Z J Mar Fresh Res. 1987; 21: 457-65.
- Galvanese EF, Chuang A, Hamilton DP. Phosphorus storage and utilization strategies of two bloom-forming freshwater cyanobacteria. Proc R Soc Lond A. 2023; B.290: 2023120420231204.
- Xiao M, Burford MA, Prentice MJ, Galvanese EF, Chuang A, Hamilton DP. Phosphorus storage and utilization strategies of two bloom-forming freshwater cyanobacteria. Proc R Soc Lond B.2902023120420231204. 2023; 290: 20231204.
- Zhang M, Lu T, Paerl HW, Chen Y, Zhang Z, Zhou Z, et al. Feedback regulation between aquatic microorganisms and the bloom-forming cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol. 2019; 85: e01362-19.
- Ghaffar S, Stevenson RJ, Khan Z. Effect of phosphorus stress on Microcystis aeruginosa growth and phosphorus uptake. PLOS ONE. 2017; 12: e0174349.
- Hong S, Pan Q, Chen S, Zu Y, Xu C, Li J. Identification and characterization of alkaline phosphatase gene phoX in Microcystis aeruginosa PCC7806. 3 Biotech. 2021; 11: 218.
- Zhao L, Lin LZ, Zeng Y, Teng WK, Chen MY, Brand JJ. et al. The facilitating role of phycospheric heterotrophic bacteria in cyanobacterial phosphonate availability and Microcystis bloom maintenance. Microbiome. 2023; 11: 142.
- Lin W, Zhao D, Luo J. Distribution of alkaline phosphatase genes in cyanobacteria and the role of alkaline phosphatase on the acquisition of phosphorus from dissolved organic phosphorus for cyanobacterial growth. J Appl Phycol. 2018; 30: 839-50.
- Zhang T, Lu X, Yu R, Qin M, Wei C, Hong S. Response of extracellular and intracellular alkaline phosphatase in Microcystis aeruginosa to organic phosphorus. Environ Sci Pollut Res Int. 2020; 27: 42304-12.
- Pandey M, Tiwari DN. A correlation matrix of alkaline phosphatase and sporulation in diazotrophic cyanobacteria and its thermo-tolerant mutant. Pol J Microbiol. 2004; 53: 257-65.
- Shi X, Qian S, Kong F, Zhangm YY. Differences in growth and alkaline phosphatase activity between Microcystis aeruginosa and Chlorella pyrenoidosa in response to different organic phosphorus. J Limnol. 2011; 70: 21-5.
- Havens KE, East T. In situ responses of Lake Okeechobee (Florida, USA) phytoplankton to nitrogen, phosphorus, and Everglades agricultural area canal water. Lake Reservoir Mng. 1997; 13: 26-37.
- Herbert M-P, Fugere V, Gonzalez A. The overlooked impact of rising glyphosate use on phosphorus loading in agricultural watersheds. Front Ecol Environ. 2018; 17: 48-66.
- Hove-Jensen B, Zechel DL, Jochimsen B. Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol Mol Biol Rev. 2014; 78: 176-97.
- De María M, Silva-Sanchez C, Kroll KJ, Walsh MT, Nouri MZ, Hunter ME, et al. Chronic exposure to glyphosate in Florida manatee. Environ Int. 2021; 152: 106493.
- Wang C, Lin X, Li L, Lin L, Lin S. Glyphosate shapes a dinoflagellate-associated bacterial community while supporting algal growth as sole phosphorus source. Front Microbiol. 2017; 8: 2530.
- Wang C, Sun X, Wang J, Tang JM, Gu Y, Lin S. Physiological and metabolic effects of glyphosate as the sole P source on a cosmopolitan phytoplankter and biogeochemical implications. Sci Total Environ. 2022; 832: 155094.
- Saxton MA, Morrow EA, Bourbonniere RA, Wilhelm SW. Glyphosate influence on phytoplankton community structure in Lake Erie. J Gr Lakes Res. 2011; 37: 683-90.
- Drzyzga D, Lipok J. Glyphosate dose modulates the uptake of inorganic phosphate by freshwater cyanobacteria. J Appl Phycol. 2018; 30: 299-309.
- An J, Jiang Y, Cao H, Yi C, Li S, Qu M, et al. Photodegradation of glyphosate in water and stimulation of by-products on algae growth. Ecotoxicol Environ Saf. 2023; 263: 115211.
