Abstract
The coverage of land territorial areas with either natural forests and grasslands, or instead with cultured farmland or urbanized areas, plays a primary role in climate regulation of the planet. The contribution of forest hydrology and local temperature regulation to global climate change, however, is not simply an area surface-dependent measure, like some global surveys suggest. There is also an important vertical component to it, affecting the atmospheric conditions and turning the land coverage into a volumetric, beneficial effect on the climate. Wood fires, on the contrary, constitute an additional climate threat. Historically, a certain health risk resulting from monotonous wood coverage has been foretold almost two centuries ago, without an exact knowledge of how these man-made plantations would impact the future climate. A case study based on comparing the Alpujarra and Low Country forests is included to indicate the different approaches followed in divergent cultures.
In this paper, moreover, the notion of Woodland-Grassland Interface is introduced as a fractal model system. It not only describes the fractal geometry of the border, but also indicates the complex ecological interactions that constitute the biodiversity of an ecosystem. The same approach may be fruitful for modeling coral reefs and mangrove forests, all displaying some form of fractal network system. The appreciation of a border interface in all of these ecosystems is found crucial, both for mitigating climate adaptation and for improving biodiversity resilience.
Keywords: Woodland Cultivation in the Low Countries; Biodiversity resilience; Forest Hydrology; Forest temperature regulation; Woodland-Grassland Interface; Fractal Ecosystems and Sustainable development.
Introduction: (Modern) History of Woodland Cultivation
In 1868, some 150 years from the present, a famous pioneer of forestry and one of the founders of the Wageningen (Netherlands) School for Agricultural Sciences, the geologist Winand Carel Hugo Staring (1808 – 1877), son of the poet A.C.W. Staring, wrote a caustic paper on the ‘Cultivation of Pines’ [1]. W.C.H. Staring also developed the first geological map of the Netherlands. In the introduction of his work on the Cultivation of Pines, not lacking criticism of the common opinion and ruling government, he especially criticizes the hesitative arguments for not planting forests of Pinus sylvestris (the Scotch or Baltic pine) in the dunes along the Dutch coasts, similar to the coasts of the French Gascogne and in Jutland in Denmark. W.C.H. Staring hereby cites the ancient French engineer Nicolas Brémontier (1738-1809), an engineer that became famous by his dune and coastal fixation works under the reign of Napoléon I (Bonaparte) [2]. In another commentary of the epoch, written by the amateur-botanist F. W. van Eeden (1829 - 1901), a critical note appears on the diminished biodiversity of the rural environment, following the drainage of marshlands, the cultivation of heath grounds and planting of pine forests [3]. In fact, the planting of Scots pines in the coastal regions of North-Holland, started already in the late eighteenth century, to begin with in the region that presently is designated as Amsterdam’s Waterleidingduinen, a water winning region West of Amsterdam.
Interestingly, F.W. van Eeden was the father of the well-known novelist Frederik van Eeden (1860 – 1932), one of the founders of the late-romantic Eightiers movement in Dutch literature. It has been noted that F.W. van Eeden, in his two volume work Onkruid (1886), was the first to suggest the concept of ‘nature monuments’ as a means to safeguard parts of the Netherlands as natural reserves [4]. It would be too rash to call these time documents the source or the bifurcation points of the contemporary polarization between a romantic, holistic view on the biodiversity problem and the hailed, modern utilitarian views on land use and forestry practices in particular. But it was definitely an era when the awareness about our planet’s critical condition rose.
In contrast to the ‘modern’ traditions of forestry, that may seem outdated nowadays, in this paper we will especially focus on the borderline between grassland and woodland, following the sayings of another Dutch pioneer, Victor Westhoff (1916 – 2001), who stressed the importance of the border areas, where farmland and nature reserves meet [5]. Starting from a physical characterization of a number of important functions of forests in the subarctic and temperate regions of the planet, especially the functions of hydrology and temperature regulation, a closer look at the border region between grassland and forest is presented. Also their roles as carbon stores and refuges for many vertebrate and invertebrate animal species, are important to analyze the key factors and topological characteristics of the interface between woodland and grassland, as a buffer system to contemporary farmland.
This study therefore is an attempt to update the general concept of a fractal Global Ecosystem Approach, as presented before [6,7]. The fractal nature of the woodland-grassland interface appears as a collection of both abiotic or physicochemical and ecological characteristics, which are in a strong contrast with most surface-oriented management or cultivation practices (see ¶ 5. A Fractal versus Surface Approach for Ecosystem Development). Finally, the postmodern threats of the Anthropocene, such as the problems of Nitrogen-derived eutrophication, the bacteriological and/or mycological soil distortions and the problem of forest fire prevention are considered as the latest, but not the least threats to our environment (see ¶ 6. Conclusions and New Challenges of the Anthropocene).
A Comparative Case Study: the Alpujarras and the Dutch Woods
It was a moment of surprise and also of sheer happiness, when I heard the Golden Oriole’s (Oriolus oriolus) call in a small valley in the Alpujarran Corridor, south of the Sierra Nevada (Spain) [8]. It was a hot day late in April 2023, temperatures in Sevilla had topped 38 degrees Celsius, and the whole South of Spain was experiencing an extremely dry Spring, the warmest on record since the beginning of meteorological registration. The Oriole’s call made me very happy, because, when standing at the side of the road through that barren landscape, there, of all places, I wouldn’t have expected that bird, typical of the densely foliated Oak forests of the North. There it was, calling from the narrow valley below, that run as a slender blood vessel through the gigantic, dried mountain range.
But even more surprising was it to find a recent travel report of a British expedition to a valley nearby, the Mairena valley towards the city of Ugijar [9]. Herein, not only the Golden Oriole, but also other rare, colorful birds were mentioned, like the Woodchat Shrike (Lanius senator), the Blue Rock Thrush (Monticola solitarius), the Crested Lark (Galerida cristata), as well as Hoopoes (Upupa epops), Rollers (Coracias garrulus), Bee-Eaters (Merops apiaster) (Figure 1 a-d) and several Warblers and Tits, not to mention the Booted Eagle (Hieraaëtus pennatus) and Golden Eagle (Aquila chrysaëtos) on top of the food chain [9]. Beside the extensive list of Red list species and less rare bird species, as well as numerous other vertebrates and numerous invertebrates, especially butterflies and moths, also an extensive list of tree species was given [9]. The rich biodiversity of this so-called barren landscape was extra-ordinary and very unexpected according to my previous experiences. It is understood that also the special geological features, like the rock formations in the Alpujarran Corridor (Figure 2) [8,10], may have helped, for instance in creating a perfect habitat for nesting of e.g. Rock Sparrow (Petronia petronia) and Crag Martin (Ptyonoprogne rupestris) [9] (also own observations in the same area).

Figure 1: Colorful birds of Mediterranean and North-European temperate forest zones: A. Golden Oriole (Oriolus oriolus)(©2017, Imran Shah, Pakistan); B: Bee-Eater (Meriops apiaster); C: Hoopoe (Upupa epops); D: Pied Flycatcher (Ficedula hypoleuca) (©2023, Photographs Biological Publishing A&O, except Figure 1A).
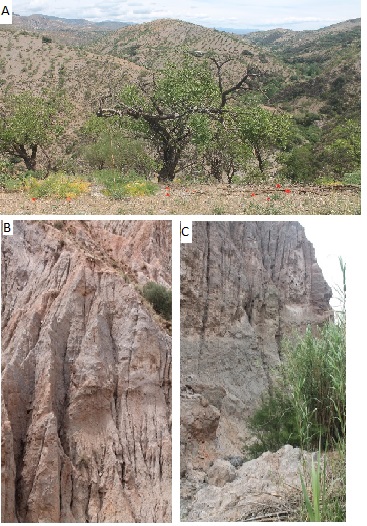
Figure 2: Panoramic View on Western Alpujarra (A), rock formations near Ugijar (B) showing nesting holes of Crag Martin (Ptyonoprogne rupestris) (©2023, Photographs Biological Publishing A&O).
Back in the Dutch Oak woods, far from the densely populated cities, rather at the Dutch-German border, I found several locations with many Golden Orioles (Oriolus oriolus) calling in the sunny months of May and June. However, not much of the other colorful rare birds and butterflies were seen, although quite some dragonflies and other bird species were also present. It made me think of the young waiter in a little village nearby Ugijar, who came from Columbia (South-America), deploring the fact that “not so many colorful birds were found here”. He meant in the Alpujarra region compared to the bird fauna of the Amazon forest. I was giving it a thought like “Oh boy, don’t come to the Netherlands, you would find even less colors there…”, but I didn’t want to spoil his dreams. After all, there must be good biological arguments available to explain the colors of bird feathers and also of butterflies and other insects in terms of evolutionary adaptation and selection mechanisms in relation to vegetation, temperatures and latitude. But the point was made, also for stressing the importance of the most threatened fauna and flora of the planet, that of the Amazon rain forest.
Despite the rich biodiversity of birds and other vertebrates and also invertebrates (see above), it has been noted that the diversity of tree species in Spanish woodland was rather low, at least in the natural reserves of Central Spain [11]: “Many dry, Mediterranean forests, such as the Alto Tajo Natural Park, exhibit a remarkably poor understory due, at least in part, to the combination of drought and shade coupled with a short growth period imposed by extreme temperatures” (p. 541). The dominant canopy (tree) species in Spain appear to be the Quercus ilex (Evergreen oak) and Pinus nigra (Black pine), Eurasian tree species that are known for high drought tolerance [11]. The understory in dry and shaded sites was found to consist predominantly of scattered individuals of Arctostaphylos uva-orsi (Bearberry) and Buxus sempervirens (Boxwood) [11]. For, indeed, in an extensive statistical analysis, Niinemets and Valladares [11] found a negative correlation between shade tolerance and drought tolerance as well as between drought tolerance and tolerance to flooding in all continents of the Northern Hemisphere (see below).
Specific to the Alpujarra region moreover, is the practice of diverting water from the rivers by an extensive network of irrigation channels (acequias), sometimes starting at high altitudes, to well-defined, highly permeable areas [12]. The objective of this centuries-old practice is to guarantee a supply of (drinking) water during the dry months as well as to improve the physicochemical characteristics of the water, and, as a bonus effect, the diversity of the local vegetation (see above) [12].
The difference with the Dutch landscape organization in fact is already very obvious when observed from an air plane high up in the sky. The clean, well-organized mosaic pattern of field patches almost like a checkerboard, sharply contrasts with the rough and desolate landscape of the Iberian highlands. But also when coming down to earth on the flat Dutch countryside, it was only deep in its forests, almost at the German borders, literally as far as possible away from the Dutch organized agricultural patchworks, that we heard the Golden Oriole singing again. Heard but seldom seen, the birds were hidden in the dark green impervious foliage.
The presence in mountainous regions of Southern Europe of bird species that became nearly extinct in North-Western Europe, mostly due to the extensive use of insecticides in the North-West [13], has been noted previously [5,14]. An interesting question, however, remains, namely that although the climate and hydrological conditions of these southern regions may seem far less optimal, the biodiversity of these mountainous regions is simply stunning. This paradoxical observation formed a novel impetus for the present modeling of the Woodland-Grassland Interface. But first, we have to look into the so-called abiotic, physicochemical characteristics of the woodland biotope.
Physical Characteristics: Forest Hydrology and Temperature Regulation
Forest Hydrology
It is well-known and it has been studied extensively, that trees may use a lot of water and play an important role in the hydrology of a landscape [15]. The processes that govern water use by trees are categorized in the following two main mechanisms: a) Transpiration: this process is effectuated by evaporation through the pores or stomata on the surface of the leaves; b) Interception: this is the process by which water, held on the surface of leaves, branches and trunk, after rainfall is directly evaporated back to the atmosphere [15]. The interception is often expressed as a proportion of annual precipitation (Table 1 for the Interception ratio of a number of tree species/groups). The combined processes of transpiration and interception, taken together with the direct evaporation from the soil surface, are called the Evapotranspiration of a forest [15]. The storage of water (together with carbon storage) due to the chemical processes of photosynthesis, length growth and leaf metabolism are often left out in hydrological analysis.
Forest Type/
Land Cover (15)Transpiration ratio (*)
Interception ratio (*)
Total Evapotranspiration (*)
Conifers
0.30 – 0.35
0.25 – 0.45
0.55 – 0.80
Broadleaves
0.30 – 0.39
0.10 – 0.25
0.40 – 0.64
Grassland
0.40 – 0.60
-----
0.40 – 0.60
Heather
0.20 – 0.42
0.16 – 0.19
0.36 – 0.61
Bracken
0.40 – 0.60
0.20
0.60 – 0.80
(*) Data on Transpiration, Interception and Transpiration are from Nisbet (2005), assuming an (annual) 1000 mm yr-1 rainfall [15].
Table 1: Compilation of hydrological data of some forest and tree species of the Northern Temperate climate zones: Transpiration and Interception ratios (top) and Tolerance to flooding (bottom).
Whereas the transpiration rates vary little between coniferous stands and deciduous or broadleaf forests, the interception ratios differ significantly (Table 1). When interception and transpiration are combined and assuming an annual rainfall of 1000 mm, “conifers are expected to use (sic) some 550-800 mm of water compared with 400-640 mm for broadleaves” [15]. In contrast to the interception ratio, trees are capable of controlling the use (or loss) of water via transpiration, because they may close their stomata in response to dry atmospheric or soil conditions. For the cold winter months, this is especially important for the non-deciduous conifer forests.
Also geology and soil conditions are important for a tree’s capacity to take up soil water, to enable the transpiration stream from the hair-like roots to the canopy leaves. When severe dryness occurs, like in the present summers with prolonged drought and elevated temperatures, tress may respond by early loss of foliage, dieback of twigs and even the death of branches and of the complete tree. It has been noted that some rock types, such as chalk, can continue the root water uptake by capillary action within the rock [15]. Obviously, this doesn’t work in clay soils that show a marked shrinkage during drought. On the other hand, some tree species are well adapted to survive in continuously high groundwater conditions, like some (but not all) Willow and Poplar species (Table 1).
The interception ratio of a forest depends significantly on the age and variations in canopy density of a forest, because of mixed-age and/or the mixture of tree species [15]. This follows from the impact of wind and the outer contact layer with the atmosphere causing air turbulence in the canopy zone [15].
It is interesting to note that in the past, a common view on forest hydrology was seen from the point of the ‘use’ of water by a forest, given a water supply that is never ‘short’, and not in the beneficial role of forests in containment of excessive rainfall following climate warming [19]. Halfway the previous century, a lot of data has been gathered following inundation studies, in order to calculate the damage to certain tree species following the engineering of water reservoirs in river valleys [17,18]. In recent years, it became clear that the deforestation of the largest rain forests of the planet has profoundly influenced water vapor circulation at a global scale, and, as a result, presumably has also contributed to the more extreme weather phenomena of recent decades [19].
Moreover, the importance of trees not only for captivating excess rain water, but also in attracting precipitation by directing water vapor streams that even may influence cloud formation, forms very intriguing new insights - although still speculative - of how forests react to climate change [20].
Temperature Regulation
Apart from the cooling effect of (water) evapotranspiration (see 3.1), obviously also other mechanisms play an important role in regulating the ambient air temperature in a forest. It may seem paradoxical too, that although the albedo, i.e. the fraction of sunlight reflected, is lower in deciduous and especially in coniferous forests (compared to grassland, or land covers like river sands) (Table 2), it is generally known that forests provide a much cooler ambient temperature, at least during hot summer days. The mechanism of this cooling effect, however, is far from being completely elucidated.
Surface
Albedo (#)
Surface
Albedo (#)
Snow
0.40 – 0.85
Green grass
0.26
Sea ice
0.36 – 0.50
High, dense grass
0.18 – 0.20
Water
0.03 – 0.40
Spring wheat
0.10 – 0.25
White sand
0.34 – 0.40
Canopy of Oak
0.18
River sand
0.43
Canopy of Pine
0.14
Desert loam
0.29 – 0.31
Canopy of Fir
0.10
(#) Albedo (A) defined as the fraction of reflected versus incident solar radiation.
Table 2: Albedo of natural surfaces. (Data adapted from Holman [1982] [21] and Eagleson [1970][1].
Radiation heat transfer to the environment in theory is governed by the absorption, scattering and reflection properties (dependent on the elevation of the sun) of the atmosphere and natural surfaces. The conventional approach in atmospheric problems, according to J.P. Holman (1982) [21], is to assume that the absorption and scattering processes are superimposed on each other and may be expressed in the form of Beer’s law over all wavelengths:
(Holman, ibidem, p. 388)
with aλ the monochromatic absorption coefficient and x the thickness of the layer absorbing the radiation. For a scattering process, one would replace aλ by a scattering coefficient kλ [21].
The interaction between absorption/reflection and scattering reminds us of the analysis of heat transfer through the mammalian fur [23]. Herein, a discussion is presented of the analysis of Kovarik (1964) [24], a methodology adopted by many others. In this study an integral model is proposed for the combination of the (inward) absorption/reflection and (outward) radiation fluxes through a cover zone. When combined with the interception and transpiration fluxes of the forest canopy (Figure 3 and [Allaerts, in prep.]), a truly integral model of forest thermoregulation could be built.
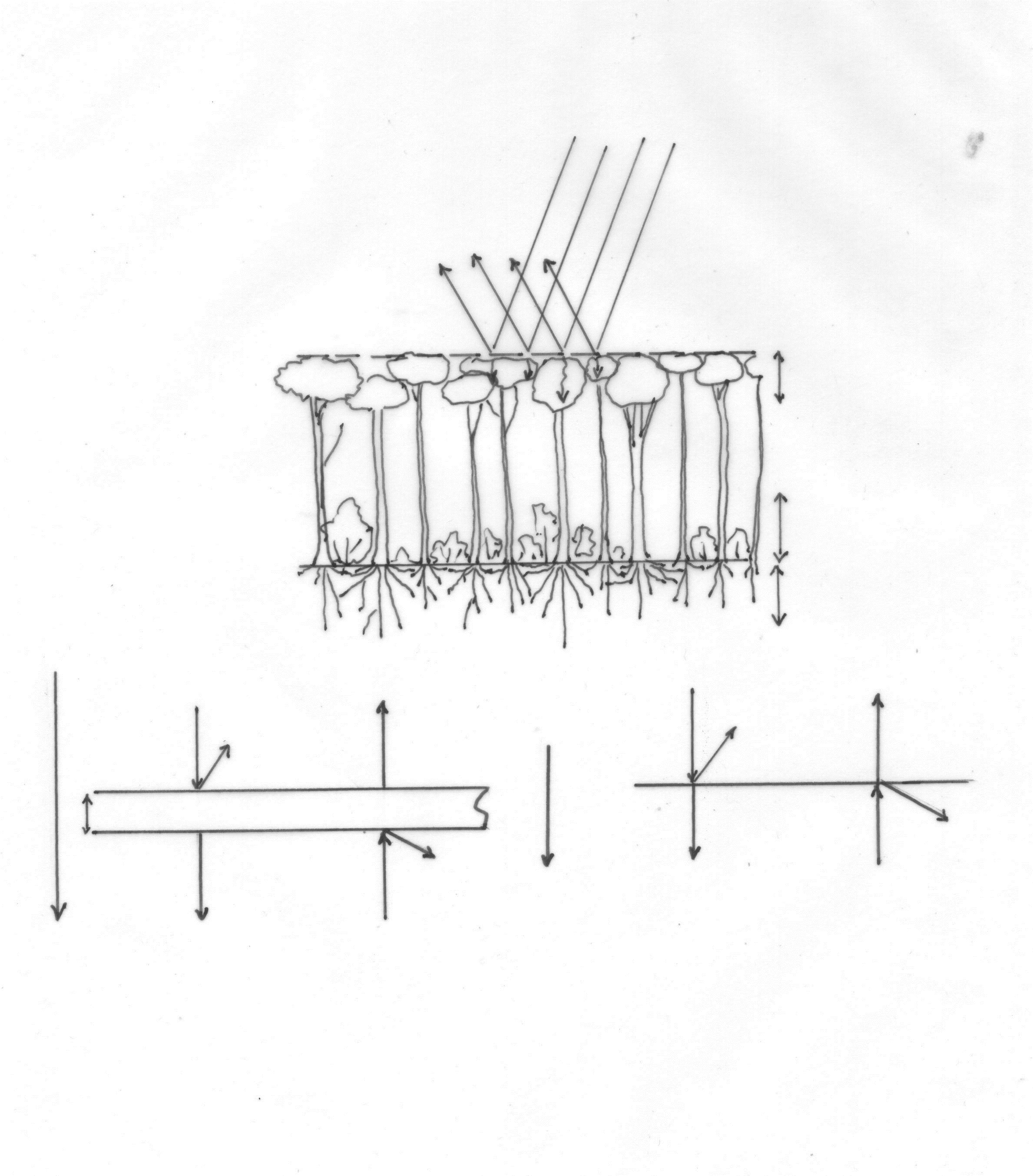
Figure 3: A. Scheme representing the main physical characteristics of the Hydrology and Temperature Regulation in a Temperate Forest, B-C. Mathematical representation of the Heat Flow through an irradiated protective cover according to Kovarik (1964) (adapted from Allaerts, 1984).
dY = - k Y dx – r Y dx + r Z dx, dZ = k Z dx – r Y dx + r Z dx
(Kovarik, 1964) (Figure 3B, C)
Using a system of coupled ordinary differential equations (see above), Kovarik [24] suggested that an increased absorption (in combination with constant or sometimes also an increased body temperature, especially in desert species) infers that the average depth where heat is accumulated becomes diminished. When the pigmentation of an integument (with sufficient insulation) is augmented, this would result in a diminished load (of the animal, or to a person or in a forest), what can be seen in desert mammals that show dark pigmented integuments.
A similar analysis may apply to the paradoxical finding that in forests of Oak, Pine or Fir, although the albedo of these tree canopies is lower than in grasslands (Table 2), more radiation is absorbed or captivated in forests especially during hot summer days. Nevertheless, a much cooler atmosphere is experienced, depending on the thickness of the insulating cover. Moreover, these findings reinforce the increased interception and evapotranspiration of forests (after rain), although more experimental data are necessary to model all the relevant parameters (Allaerts, in prep.). This is especially relevant for estimating the influence of increased surface coverage with the low scrubs of the Dwarf birch (Betula nana) in the Siberian tundra (following the rising temperature and melting permafrost) [25]. In the temperate zones obviously the trees grow taller, reaching their adult altitudes of tens of meters. Therefore, the combination of albedo or absorption/reflection and the vegetation structure (interception also depends on the age and mixture of a forest) becomes all the more important. For the interception of rain by other forms of cover (heath, bracken or simply brash), however, the thickness of the cover seems of less importance, since it has been noted that also a thick pile of brash can intercept as much as 15 % of annual rainfall, which is similar to the water lost from a broadleaved woodland canopy [26].
In the preceding paragraph, it appears that not only the type of vegetation matters, but also the thickness of the cover (to some degree) is relevant to the hydrological and thermoregulatory properties of woodland (as well as grassland) areas. From these observations a volumetric extrapolation of surface coverage may seem logical and useful for management purposes. In the next paragraph, however, it appears that from a biodiversity perspective, the woodland-grassland border should rather be seen as a fractal interface.
The Fractal Nature of the Woodland-Grassland Interface
Biodiversity of Woodland versus Grassland and Bracken
In addition to the sheltering functions of temperate Woodland and Grassland, both these biotopes harbor an abundance of prey animals, in particular Invertebrates (of the groups of Hexapoda, Arachnida, Acarina and Crustacea). Differences in insect biodiversity and abundance have been amply documented (see e.g. Ojija, et al. 2016) [27]. Several studies have revealed a higher diversity and abundance for grassland, particularly in the insect ordines of Hymenoptera, Coleoptera and Orthoptera. It was concluded that “grassland not only had the potential to support insect diversity”, but also “to act as refugia for some insects from woodland” [27].
Almost the same results, following the pioneering work of T.R.E. Southwood (1931 – 2005), were found in temperate climate regions, e.g. the British Isles, revealing a higher diversity in grasslands compared to woodland, and even more so when compared to bracken [28]. The lower insect diversity of bracken, although a favorite cover for sheltering mammals and birds, appears to result from the presence of toxic chemical compounds like cyanide, phenolic substances, thiamines and ecdysone (an insect hormone involved in molting) [29]. When an insect species (e.g in the group of Symphyta, Sawflies) becomes adapted to these toxic environments, e.g. by producing rhodanose, an enzyme that detoxifies cyanide, these species are no longer capable of evolving back to the ancestral status. A similar example is found in bacteria that produce thiamine-degrading enzymes, that may cause thiamine deficiency in animals and humans [30].
Although a quick examination of insect biodiversity in woodland and/or grassland is possible, for instance using Yule’s characteristic [31] (Table 3), this method of collecting and estimating animals obviously is not suited for estimating the abundance of larger, vertebrate animals like Birds and Mammals [32]. Woodland moreover offers specific niches for nesting, which obviously are not present in grasslands, or are inhabited by different species [5]. As a result, grassland and woodland differ strongly with regard to the larger vertebrate fauna [5].
Group
Species code
Ni
Ni2
S Ni2
Coleoptera
A
1
1
1
Lepidoptera
A
1
1
2
B
1
1
3
Heteroptera
A
2
4
7
B
8
64
71
C
5
25
96
D
1
1
97
E
1
1
98
Aphididae
A
50
2500
2598
Diptera, Nematocera
A
B1
11
1
2600
Diptera, Brachyptera
A
B
C
D1
4
2
11
16
4
12601
2622
Hymenoptera
A
B
C
D
E
F5
2
2
1
1
125
4
4
1
1
12647
2658
Neuroptera
A
1
1
2659
Thysanoptera
A
1
1
2660
Araneida
A
2
4
2664
Sample totals
S N = 96
N2 = 9216
Yule’s index
Y = N2/ S Ni (Ni-1) = 9216 : 2664 = 3.459 @ 3.5
Table 3: Example of a quick estimation of insect (and araneid) diversity using Yule’s characteristic (31) in woodland. (Insect collecting method using sweeping net) (Allaerts, unpubl. observations, Slapton Ley, Kingsbridge, Devon (UK), August 1979).
Yule’s characteristic was derived from linguistic analysis (regarding the frequency of nouns in a text by a certain author) [31], and, in fact was a modification of Fisher’s ‘Index of Diversity’ [33]. Yule’s characteristic actually is a simple, straight forward tool, very useful in the field, representing not only the number of individuals in a sample but also giving an estimate how ‘diverse’ the sampling is (Table 3). Nevertheless, Williams concluded that further investigation was needed, “both on the mathematical side and in testing against biological data” [31]. For larger animals, especially Birds and Mammals, but also for some of the larger Insects (like Odonata), it may be a naïve idea that something like ‘random sampling’ is possible. On the contrary, it takes a lot of time, an utmost patience and a whole lot of species-specific knowledge about the behavior (ethologically and ecologically) of a rare species to make an encounter or to establish the presence and the number of these extremely rare animals.
Therefore, in the next paragraph a different approach is followed, starting from a fractal approach of the nature of biodiversity [6].
Topological Features of the Woodland – Grassland Interface
To our opinion, the underlying failure of successfully estimating biodiversity as an a real abundance characteristic, is that most models presuppose a uniform areal distribution of animals (and also of plants) that runs into the hands of the observer by pure chance. An alternative way to look at biodiversity is to regard it as a chain of interacting species, that becomes increasingly difficult to unravel as the species become harder to get our hands on, because they are extremely rare species, or more difficult to discriminate from one another. The latter may for instance be the case with bacteria or viruses that follow a different mechanism of genetic information exchange between strains of variants and hence also exemplify a different concept of speciation [34].
When abandoning the areal connotation in biodiversity, and replacing it by the ecological network notion of interacting species, we also circumvent a possible bias like in the gate-keeping approach [7].
Herewith, the notion of the border zone between woodland and grassland could be instrumental. We call this zone the Woodland – Grassland Interface (Figure 4,5 & 6). It is not simply a demarcation line, but an area of multiple ecological interactions. The demarcation is an area, because it is not the line on the map, but the actual forest-grassland border that we envisage. It is a fractal area, because similar to the coast-line dimension, that by increasing the magnification shows a number of twists and contortions, also the border line between forest and grassland is irregular (at each scale) (Figure 6) [6,35]. But, not only the morphological irregularities constitute the fractal geometry, i.e. the shape of branches, leaves, the height of trees altogether, that form a complex border area between the two habitats. The fractal nature is also expressed in the ecological interactions at both sides of the border, i.e. between animals and their predators and parasites, ‘residing’ either in the forest or the grassland. Therefore, because of the mobility of animals between their foraging, resting and/or breeding areas, in reality, forest and grassland are interconnected, although we may draw them as separate areas on a map, divided by clear demarcation lines (see also legend to Figure 6). The border between forest and grassland thus literally form an interface.

Figure 4: A. Temperate coniferous forest with Hemlock (Pseudotsuga menziesi) and Scots Pine (Pinus sylvestris); B. Roe Deer (Capreolus capreolus) often appearing at the forest border; C. Group of female Red Deer (Cervus elaphus) withdrawn into the forest. Red Deer however are often found in open terrain, like in the Scottish Highlands and in the Central-European Alps above the timber-line as well as in marshlands in coastal areas (©2023, Photographs Biological Publishing A&O).

Figure 5: Grasslands are in fact a large number of vast ecological systems, and, in comparison with other biomes in the temperate climate zone, show the highest diversity of insect species. A. So-called Blue or Chalk (limy soil) grassland of Central Europe, with Field Scabious (Knautia arvensis) and Marbled White (Melanargia galathea). B. Four-spotted Chaser (Libellula quadrimaculata) and C. Male Scarlet Darter (Crocothemis erythraea) two dragon-flies of the Libellulidae in Wet grasslands, the first a quite common species and the second rather rare in the Netherlands (©2023, Photographs Biological Publishing A&O).
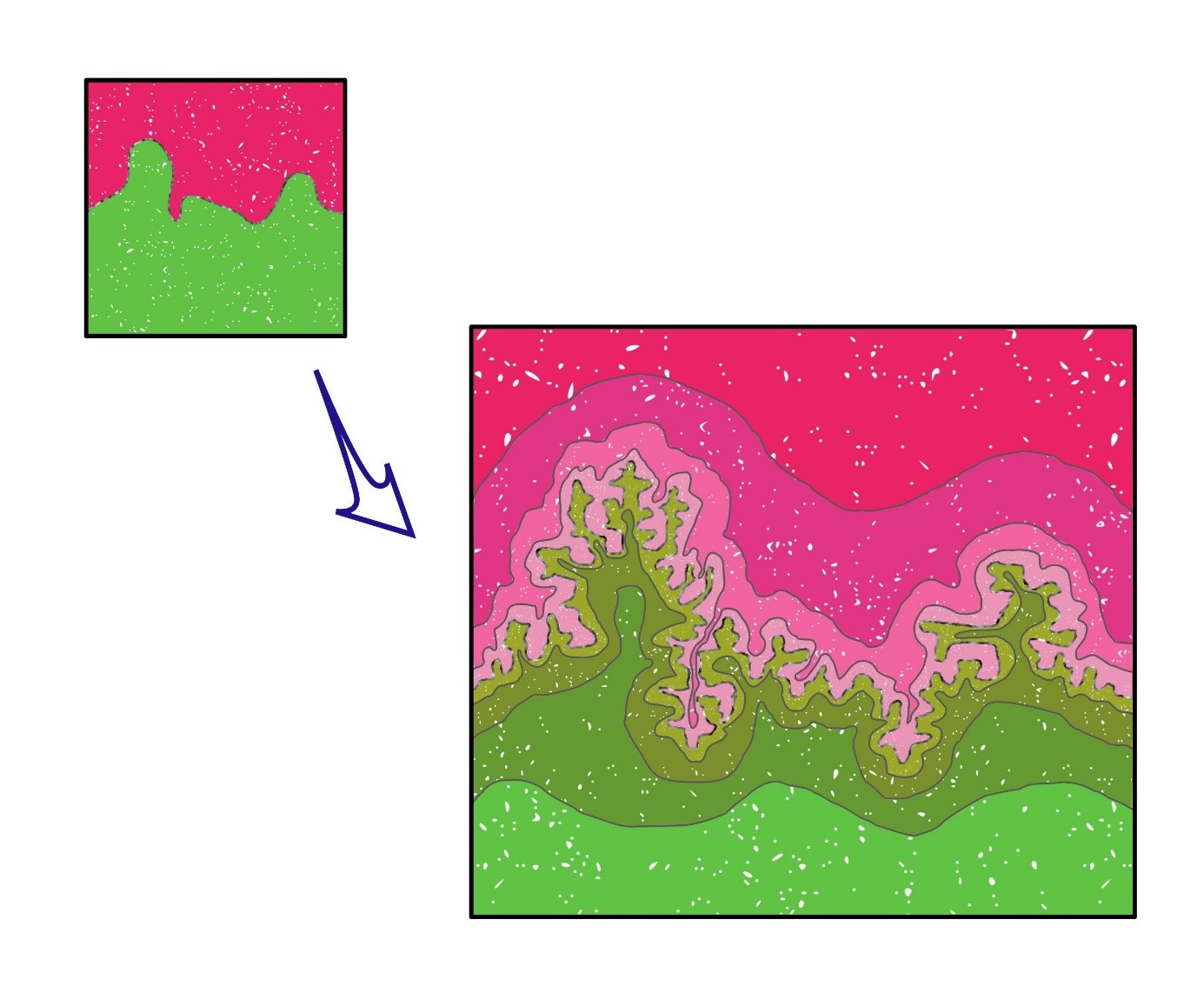
Figure 6: Symbolic representation of the fractal notion of the Woodland-Grassland Interface. The borderline (symbolized by grey-white line) that at each increasing magnification shows a further splintering of minuscule, fimbriated details, in reality is a fractal system that also turns the 2-dimensional area surface into a virtual 3D volumetric system. Obviously, this demarcation not exhaustively corresponds to the geometrical border between the forest trees and the surrounding environment, but reflects all life forms that inhabit at both sides of the border (represented by shades of either green or pink). Therefore, the Woodpecker (Figure 7A) making holes high up in the Oak tree and foraging for caterpillars, bees and other insects in the grassland below, as well as the Roe Deer finding shelter in the forest and coming out at dusk for eating the green herbs, all constitute the complex interconnected web called the ecosystem’s biodiversity. The next question, how the biodiversity of the system changes with flattening or smoothing the border line, not only is interesting from an ecological viewpoint. It also forms an interesting heuristic for defining the entropy change of the ecosystem in relation to biodiversity changes (see also main text, Allaerts [in prep.] and 6).
It is well known (among naturalists and hunters) that wild grazing mammals, like deer (Cervidae) mostly stay in the forest during the day, but, during the early morning and twilight hours, they leave the forest to find richer food in grasslands or pastures surrounding their shelter. Moreover, the larger Red deer (Cervus elaphus) withdraw deeper into the woods than the smaller Roe deer (Capreolus capreolus) (Figure 4, b-c), and this correlation between body size and daily movements is seen in many animal groups, including birds. Withdrawing deeper into the wood also means evading disclosure by predators, or human disturbance of the environment. According to the hypothesis of Janis and Carrano (1992) [36], called the JC hypothesis, there is a negative correlation between body size and litter size in terrestrial mammals, which however didn’t seem to exist in distinct archosaurs and also not in contemporary non-passerine birds [36,37]. As a result, large mammals on the average show little reproductive success (with some exceptions like the big herd-forming Red deer, C. elaphus, at least in certain environments). For, when a species has few, respectively many enemies or predators, a correspondingly small/large number of offspring is needed to improve their survival potential. On the other hand, species with large offspring and few predators, like in some exotic animals (but also in plants and in the Dutch populations of Red deer), therefore, may become a plague to the ecosystem.
The fractal nature of (ecological) biodiversity thus is related to the survival probability or the rate of reproductive success. If a number of generations of a species fail to procreate new offspring, or the population becomes too small, local extinction may become the outcome (if no new individuals are immigrating from surrounding territories) [7]. The resulting local biodiversity appears to display characteristics of a dynamic multidimensional network of interacting ‘trees’ (= the survival path, from conception to successful reproduction of a species at a local number of habitats), similar to the ‘Hyper-objects’ suggested by Timothy Morton (2013) [38].
When it comes to defining the fractal nature of a system, two questions seem of primary importance, namely (a): What is the self-similarity dimension?; and (b): What is the critical dimensionality, defined as the percolation probability or the critical probability below which a network disintegrates? [6,35]. The problem in earlier studies, however, appears to be the attempt to characterize a mean-field approximation of biodiversity [6], similar to the areal approach of defining an area by a number of characteristics (like water consumption, crop yield, number of grazers, etc.).
In the fractal approach of the woodland-grassland interface, a possible representation of the local interference with the (local) biodiversity may be represented as a ‘flattening’ or smoothing of the fractal interface, analogous to the flattening of the coast line of the British isles in the seminal work of B.B. Mandelbrot [35] (Figure 6). A simplified representation in the two-dimensional plane, follows from
L (e) = F e 1 – D (see Allaerts, 2020) [6]
with F, the number of fragments of a chosen length e and D the fractal dimension [6]. The total length L (e) is the add up or the total of line segments. When replacing length by a biologically relevant, derived parameter r (N), that occurs N times in a given environment, the following relation results:
log r (N) = log 1/ N 1-D= - (log N)/D (Mandelbrot, 1983) [35].
Or, when a positive measure (like the mass density) can be defined, within the ball B with radius r centered at x, for all x ∈ E, E being a regular set, then the fractal dimension equals the mass dimension [6]:
D = dim E = log μ [Br (x)] / log r (Marcelli, 2019) [39].
The former equation of Mandelbrot shows a striking analogy with the formula for the information state (H) or negentropy of a system:
H = - S pi log pi (Brillouin, 1953) [40].
Given the intuitive relationship between biodiversity and information, it would be conceivable to define biodiversity alterations in terms of changes of entropy [41]. However, just like in Williams’ conclusion on the statistical approach of biodiversity estimation (see 4.1 ) [31], more information is needed both on the mathematical side and regarding the ‘nature’ of the biological data. For instance, neither the homogeneity nor geometrical regularity of the local biodiversity, represented as a set of ecological interactions, can be ascertained (see also ¶ 5. A Fractal versus Surface Approach for Ecosystem Development). But at least one (trivial) solution may be obtained from comparing the equations above, namely that reducing the number of elements in the set (whether or not homogenously distributed) results in augmenting the entropy as well as reducing the biodiversity at a local scale [6].
A Fractal versus Surface Approach for Ecosystem Development
Not only the Woodland – Grassland Interface, also other ‘border’ regions are of primary importance for the conservation of our planet’s ecosystems. Examples that so far have received much public attention are the Great Coral Reefs (of the Pacific Ocean), the Mangrove Forests and many other Coastal Ecosystems. In fact, every ‘natural’ river bed could be regarded as a ‘border’ ecosystem, although a too high proportion of river beds worldwide is lacking a ‘natural’ border or riparian zone. As was mentioned previously [7], coral reef biodiversity refuted Hubbell’s UNTB. The ecological biodiversity in coral reefs strongly depends on the interaction between groups of coral species, such as algae, the Coelenterata (the builders of the stony coral structure) and fish species, but many interactions between them are species-specific. Each of the latter groups is important for keeping the ecosystem in a locally balanced equilibrium, preventing the spread of parasites or the growth of one group at the cost of another. What are the benefits and the costs of choosing a fractal approach instead of a surface (or volume) approach?
Fractal geometry has the disadvantage of making used of geometrically non-differentiable functions [6]. In order to enable a developmental description of ecosystem, including its injury and possible resilience, a time-integrative mathematical convolution technique could be useful.
Taking advantage of the physiological and anatomical analogs of the world’s respiratory system, the mammalian lungs, in analogy with the power-law description of the lung airway scaling, we may also write:
S (z, n) = z μ. F (z, n) (Sturm, 2017) [42]
(with μ representing the ‘power-law index’ [= fractional dimension], z the generation order of a bifurcating network system such as the mammalian lungs and F (z, n) a tailor-made function representing the harmonic periodicity) [42]. Similarly, a function describing a power-law rating of lung injury (Pinj) was inferred:
Pinj (z, n, θ ) = z θ - μ = z θ . F (z, n, θ) / z μ . F (z, n, θ) (Allaerts, 2020) [43]
Reflecting the proportion of the injured part of the lung compared to the healthy lung. A time-integrative approach is the following step, making use of a convolution equation involving a Laplace transformation. When a function is piecewise continuous in a finite interval, like the Heavyside function, then it is integrable over that interval [44]. This mathematical property can prove useful to explore the convergence of the Laplace transform:
L { P inj (…) } = ∫0 ∝ e -pt [ ∫0t Pinj (…) dt ] (Allaerts, 2019 and Allaerts, [in prep.]) [45].
Also here, more information is needed to enable mathematical model fitting to the biological data, in particular, for fitting the model in question to the relevant time scales of ecosystem destruction and recovery rates or the time-dependency resilience.
In case of the coral reef and mangrove forest regeneration, some experimental and observational data have been gathered, enabling an estimation of both the local growth rate of individual colonies of a population of Porites divaricate (LeSueur, 1821), a coral species occurring on Caribbean reefs [46], as well as the growth (and decay) rates of the reef supporting mangrove forest roots [47]. The ultra-fast, catastrophic decay rates resulting from a natural storm, or following human interference are also well-known, for instance resulting in 86 % loss of Florida’s mangrove coverage since the 1940s [47]. Directly, or in combination with other processes that follow climate warming, an almost countless number of species that depend on the mangrove habitat, from large mammals like Manatees, Dolphins and numerous birds, reptiles, fishes, etcetera, are under direct threat. It is one thing to estimate the (slow) linear growth rate of a single colony-forming coral species (of Coelenterata) [46], or to derive a volume growth rate of coral forming species in laboratory conditions or in the open ocean [48]. For a restoration of the full complexity of the mangrove and coral ecosystem, however, this is only the beginning of a slow recovery process, where we need to give way to Nature.
Returning to the Woodland – Grassland Interface (see above), a similar situation may be discerned. It is one thing to plant an array of new tree sprouts after deforestation, the recovery of a natural forest and/or grassland ecosystem is a much more elaborate process. In order to develop a balanced ecosystem with its natural inhabitants included, two examples of the resulting complexity of ecological web interactions are given below (Table 4-5 and Figure 7, a-c).
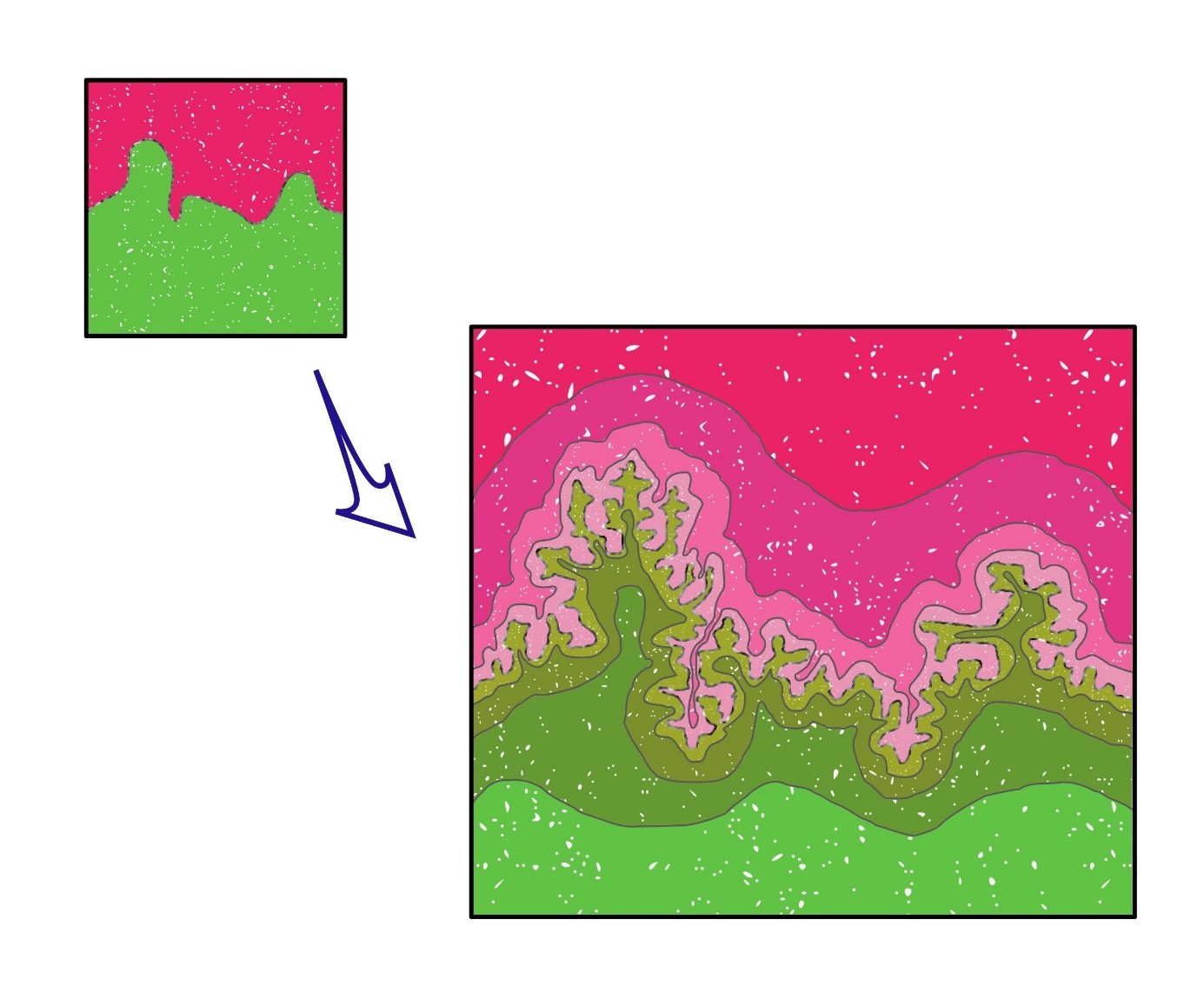
Figure 7: A. Schematic ecological web (detail) for the Red Mason Bee (Osmia bicornis) with 4 major predators and parasites (see Table 4 for estimated threat levels); B. One of the most iconic species of the Dutch Wet Grasslands, the Black-tailed Godwit (Limosa limosa) has become a Red List species (Status = Near threatened); C. General threat levels for the Black-tailed Godwit in The Netherlands (©2023, Photographs and Drawings by Biological Publishing A&O).
Key species (Protection status)
(Natural) Predators
Parasites and Parasitoids
General threats/dependencies or indirect effects of human interference
Red Mason Bee
(Osmia bicornis)
[= Osmia rufa]
(No direct threat or Least concern)Green Woodpecker (Picus viridis) (5)
European Beewolf (Philanthus triangulum) (2)Common Parasite Wasp (Cratichneumon sicarius ) (7)
Bee-fly or Bombyliid Fly (Anthrax anthrax) (8)Threatened by cold weather in early spring (3)
Asian or Yellow-legged Hornet (Vespa velutina) (4-5?)Large Scabious Mining Bee (Andrena hattorfiana) (genus of Sand Bees)
(Vulnerable)(see above)
Kleptoparasitic Cuckoo Bee, e.g. Nomada armata (2)
Sand Bee parasites like Stylops melittae (3)Feeds primarily on pollen of Knautia arvensis (secondly on Knautia dipsacifolia ) (4)
(* Polylectic species: feeds on (pollen of) many different plant species; oligolectic species: feeds on only a small number of plant species)
Table 4: Detail of Ecological web for two wild bee species (called key species), one rather common, polylectic species (*), the Red Mason Bee (Osmia bicornis) and one rather rare, vulnerable, oligolectic species (*) of the genus of Sand Bees (Andrena hattorfiana). Threats are scaled 1 to 10 (bold numbers) according to data by P. Van Gampelaere and others (personal communication, 2023). The estimated threat level for the Asian Hornet is uncertain, because unlike in the Honey Bee, the impact of this exotic species on both the common and rare wild bee species is largely unknown.
The rare occurrence of e.g. Andrena hattorfiana, also results from the scarcity of its food source, the pollen of the so-called Field Scabious (Knautia arvensis /K. dipsacifolia) flower (Figure 5A). This flowering herb of the Honeysuckle family (Caprifoliaceae), at present is a Red List species in NW-Europe (Belgium and The Netherlands). It became especially rare, primarily because of the disappearance of the traditional mowing practices using sickle and scythe, being replaced by the more industrialized machine mowing practices. Secondly, Knautia species prefer the non-fertilized, moderately limy soil grasslands (both in the class of the Sandy Dry Grasslands or Grey Dune Plant Communities, known as Koelerio-Corynephoretea) [49] and in the classis of Nutrient-poor Wet Grasslands (Molino-Arrhenatheratea) [50]. As a result, the anthropogenic influence has a major negative impact on these rare wild bee species. The same holds for a typical Wet Grassland bird species, the Black-tailed Godwit (Limosa limosa) (Table 5 and Figure 7, b-c).
Key species
(Protection status)(Natural) Predators
Competitors
General threat
Golden Oriole
(Oriolus oriolus)
(No concern)Several species of Birds of Prey (Accipitriformes; incl Eagles, Sparrow-hawks,..); Stork (Ciconiiformes)
Woodpeckers (Piciformes); Corvidae
Diminished area of Forest habitat
Black-tailed Godwit
(Limosa limosa)
(Near threatened – Red List species)Beech Marten (Martes foina); Red Fox (Vulpes vulpes); (Sea) Gulls, Birds of Prey.
Several sp. Geese (Anseriformes) and other charadriiform birds (Charadriiformes)
Loss of habitat owing to wetland drainage and agricultural intensification; notable decline in wintering areas in Morocco and Senegal (51).
Table 5: Detail of Ecological web for two bird species, one typical for forest habitat, the Golden Oriole (Oriolus oriolus) and a typical breeding species of Wet Grasslands, the Black-tailed Godwit (Limosa limosa). (Data on protection status of L. limosa obtained from Data Zone of BirdLife International) [1].
Conclusions and New Challenges of the Anthropocene
Historically, the importance of a significant proportion of land covered with forests has been recognized merely since the last two centenaries, at least for protecting the coastal dune areas (¶ 1. Introduction: (Modern) History of Woodland Cultivation). That was more than a century before climate warming became a matter of global concern. The opportunistic use and/or economic appreciation of woodland cultivation, however, hasn’t changed much in these 200 years, it appears. Woodland cultivation came to the forefront of climate awareness in recent years, also because of the increased risk of massive wildfires following periods of heatwaves and exceptional drought. It became especially harmful in the Portuguese summer fires of 2017, where in the vast, monocultural Eucalyptus forests, a tree species known for fueling the fires with exhaling highly inflammable, oleaginous substances, a record number of human casualties were counted [52]. In our short historical introduction, we mentioned that a certain criticism of the potential risks of monocultures already existed in the nineteenth century [3].
In an era where ecology is superseded by planimetry, and agriculture became a national, economical asset (or a burden), a plea for the restoration of the natural forestry and grassland management practices seems a desperate effort. But all the more, in this study we argue that the biodiversity paradigm requires the renewed appreciation of the notion of a border interface, albeit in Mangrove forests, coral reefs, in natural river beds and also in the grassland-woodland interface described in this paper. It is well known that grasslands may generate biotopes with the highest biodiversity on the globe, at least the natural steppes of the great plains in the Americas, Eurasia and Africa. Temperate forests on the other hand, from a managerial point of view, are often regarded as less interesting for the local biodiversity. Depending on the species, however, the size of these temperate forests matter, because the size of the habitat also defines the distance from a specific niche forming shelter to the border of its safety zone. Therefore, for those animals that seek shelter and especially the absence of human interference, which for most species on the planet constitutes the biggest threat, the size of forest habitats does matter. But the crucial parameter is the distance to the fractal border ‘line’, which as we have described, in the biome of the species involved in fact forms a space-filling, convoluted surface area (Figure 6). The physical characteristics of both the grassland and the forest habitat, like their role in hydrology and temperature regulation, reinforce the complicated role of forests and grasslands in protecting biodiversity as well as the direct effects of climate warming on the biosphere (similar to the pivotal role of mangrove forests, coral reefs and underwater ecosystems of seagrass meadows) [53].
The intensive, economically relevant use of terrestrial areas for woodland cultivation, or the modification of grasslands (of all kinds) for agricultural exploitation, seems a continuous threat for the survival of these ecosystems and for the biosphere as a whole. Not only the difficulties arising from combining agricultural intensification with the protection of natural reserves (see above) is menacing a large number of species. But more and newer threats are resurging from the drawing tables of human creativity. It has been found that agricultural activities of the past decennia, since a long time have spoiled the quality of the soil. In a negative way, this has affected their potential for growing new forests. One important reason for this deterioration is the distorted balance of bacterial infestation versus the mycological flora in the forest soil. Indeed, the mycorrhizal fungi are necessary for trees to effectively pick up nutrients from the soil [54]. However, the most significant threat looming from a corner of economic opportunism and some sort of scientific hubris, probably is the tendency or wish of certain stakeholders to replace the existing soil micro-organisms by genetically modified organisms, engineered in some highly specialized laboratories. For obvious reasons their names and studies will not be mentioned in a study devoted to the protection of global biodiversity, as long as possible and necessary to resist their greed and foolish ambitions.
References
- Staring WCH. Dennenteelt. In: van Eijk JA, Staring WCH, editors; 1868. Tijdschrift. De Volksvlijt. 1868.
- Nattes J-M. Nicolas Brémontier (1738-1809). Rev Hist. 1986; 32: 33-41.
- van Eeden FW. De Lochemse berg en zijne omgeving [in Dutch]. In: van Eeden FW, Onkruid, Wandelingen B, editors. Haarlem. 1886; 60.
- Brouwer FI. Leven en werken van E. Heimans en de opbloei der natuurstudie in Nederland in het begin van de twintigste eeuw [in Dutch]. 73; 1958 ([PhD thesis]. University of Amsterdam). Groningen: J.B. Wolters.
- Allaerts W. Further notes on the Estimation of biodiversity and the Influence of Land use and Climate Warming on European Heath bird Populations. Adv Earth Environ Sci. 2023b; 4: 11-22.
- Allaerts W. Estimating biodiversity and the fractal nature of ecosystems. Int J Bioinformatics Comp Biol. 2020; 5: 15-24.
- Allaerts W. The unified neutral theory of biodiversity and biogeography revisited. Adv Earth Environ Sci. 2023a; 4: 1-10.
- De Galdeano CS, Rodriguez-Fernandez J, Lopez-Garrido AC. A strike-slip fault corridor within the Alpujarra Mountains (Betic Cordilleras, Spain). Geol Rundsch. 1985; 74: 641-55.
- Gater S. Naturetrek. Tour report, 9-16 June. Spain: The Alpujarras & Alhambra; 2019.
- Santamaria-López Á, Abad I, Nieto F, Sanz de Galdeano C. Early mylonitization in the Nevado-Filábride complex (Betic cordillera) during the high-pressure episode: petrological, geochemical and thermobarometric data. Minerals. 2022; 13: 23.
- Niinemets ü, Valladares F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol Monogr. 2006; 76: 521-47.
- Pulido-Bosch A, Ben Sbih YB. Centuries of artificial recharge on the southern edge of the Sierra Nevada (Granada, Spain). Environ Geol. 1995; 26: 57-63.
- Geiger F, Bengtsson J, Berendse F, Weisser WW, Emmerson M, Morales MB, et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl Ecol. 2010; 11: 97-105.
- Allaerts W. Biodiversiteit, Insecten en achteruitgang Weidevogels. [Interview with Frank Berendse] [in Dutch]. bi-logical. 2017; 6: 21-3.
- Nisbet T. Water use by trees. Forestry Commission information note. 2005; 65.
- Whitlow TH, Harris RW. Flood tolerance in plants: a state-of-the-art review. Washington, DC: National Technical Information Service, United States Department of Commerce; 1979. Available from: https://hdl.handle.net/11681/4549.
- Hall TF, Penfound WT, Hess AD. Water level relationships of plants in the Tennessee valley with particular reference to malaria control. J Tenn Acad Sci. 1946; 21: 18-59.
- McKim HL, Gratta LW, Merry CJ. Inundation damage to vegetation at selected New England flood control reservoirs. Cold regions research and engineering laboratory, Hanover. NH: Army Engineer Division, New England. Waltham, MA. 1975.
- Gordon LJ, Steffen W, Jönsson BF, Folke C, Falkenmark M, Johannessen A. Human modification of global water vapor flows from the land surface. Proc Natl Acad Sci USA (PNAS). 2005; 102: 7612-7.
- Nobécourt E 2020. Trees, a Global Superpower / Le Génie des Arbres (French, TV Movie/documentary). IMDb Films (Source: https://m.imdb.com).
- Holman JP. Heat transfer. New York, London: McGraw-Hill; 1982.
- Eagleson PS. Dynamic hydrology. New York: McGraw-Hill; 1970.
- Allaerts W. Studie van Modellen voor Warmteoverdracht doorheen de Zoogdierenpels (in Dutch). (Dissertation, Mimeographed). Leuven, Belgium: Universiteit Leuven; 1984.
- Kovarik M. Flow of heat in an irradiated protective cover. Nature. 1964; 201: 1085-7.
- Allaerts W. Over smeltende gletsjers, ontdooiende permafrost en andere lastige bewijzen. [Interview with M. Heijmans and F. Berendse] [in Dutch]. bi-logical. 2010; 3: 24-7.
- Johnson RC. Effects of upland afforestation on water resources. The Balquhidder experiment 1981-1991. Institute of Hydrology Report No. 116. 2nd ed. Wallingford, UK: Institute of Hydrology; 1995.
- Ojija F, Sapeck E, Mnyalape T. Diversity analysis of insect fauna in grassland and woodland community at Mbeya University of Science and Technology, Tanzania. J Sci Eng Res (J.S.A.E.R.). 2016; 3: 187-97.
- Southwood TRE, Henderson PA. Ecological methods. 3rd ed. Oxford: Oxford University Press; 2000.
- Retnakaran A, Krell P, Feng Q, Arif B. Ecdysone agonists: mechanism and importance in controlling insect pests of agriculture and forestry. Arch Insect Biochem Physiol. 2003; 54: 187-99.
- Sannino D, Angert ER. Genomic insights into the thiamine-degrading enzyme Paenibacillus thiaminolyticus NRRL B-4156 and P. apiaries NRRL B-23460. Stand Genom Seq (SIGs). 2017; 12: 59.
- Williams CB. Yule’s ’characteristic’ and the ’index of diversity’. Nature. 1946; 157: 482.
- Begon M. Investigating Animal Abundance: capture-recapture for biologists. London: Edward Arnold Publishing Ltd; 1979.
- Fisher RA, Corbet AS, Williams CB. The relation between the number of species and the number of individuals in a random sample of an animal population. J Anim Ecol. 1943; 12: 42-58.
- Allaerts W. Deconstructing self-similarity and the four dissimilitudes of biodiversity. Philos Int J. 2021; 4.
- Mandelbrot BB. The fractal geometry of nature. San Francisco: W H Freeman and Company; 1983.
- Janis CM, Carrano M. Scaling of reproductive turnover in archosaurs and mammals: why are terrestrial mammals so rare? Ann Zool Fenn. 1992; 28: 201-16.
- Werner J, Griebeler EM. Reproductive biology and its impact on body size: comparative analysis of mammalian, avian and dinosaurian reproduction. PLOS ONE. 2011; 6: e28442.
- Morton T. Hyperobjects: philosophy and ecology after the end of the world. Minneapolis: University of Minnesota Press. 2013.
- Marcelli G. Structural properties of two-phases deterministic multifractals [Bachelor thesis]. Rome: “Sapienza” University of Rome; 2019.
- Brillouin L. The Negentropy principle of information. J Appl Phys. 1953; 24: 1152-63.
- Artstein S, Ball K, Barthe F, Naor A. Solution of Shannon’s problem on the monotonicity of entropy. J Am Math Soc. 2004; 17: 975-82.
- Sturm R. Chaotic lung airway scaling using Verhulst dynamics. Int J Bioinformatics Comp Biol. 2017; 2: 1-6.
- Allaerts W. Biophysical parameters affecting lung surfactant function, surface tension and the transition from aerosol to droplet exhalation (in relation to COVID-19 infection). J Phys Conf Ser. 2021; 1730: 012059.
- Williams J. Laplace transforms. Series: problem solvers. London: George Allen & Unwin Ltd; 1973.
- Allaerts W. Connectivity, continuity and distance norm in mathematical models for community ecology, epidemiology and multicellular pathway prediction. Int J Bioinformatics Comp Biol. 2019; 4: 1-10.
- Scavo Lord KS, Lesneski KC, Bengtsson ZA, Kuhn KM, Madin J, Cheung B, et al. Multi-year Viability of a Reef coral Population Living on Mangrove Roots suggests an important role for Mangroves in the Broader Habitat Mosaic of corals. Front Mar Sci. 2020; 7: 377.
- Botoman E 2022. Climate Change: Living in Mangrove Time. Long Now’s Website, August 3, 2022 (https://longnow.org/ideas/living-in-mangrove-time/).
- Ladd MC, Miller MW, Hunt JH, Sharp WC, Burkepile DE. Harnessing ecological processes to facilitate coral restoration. Front Ecol Environ. 2018; 16: 239-47.
- Laima B, Tjarve D. Grey dune plant communities (Koelerio-Corynephoretea) on the Baltic coast in Latvia. Tuexenia. 2009; 29: 409-35.
- Westhoff V, den Held AJ. Plantengemeenschappen in Nederland (in Dutch)(Natuurhistorische Bibliotheek, 16). Hoogwoud: North-Holland; 1969: Koninklijke Nederlandse Natuurhistorische Vereniging (KNNV).
- BirdLife International. Species factsheets: Limosa limosa. (Source; 2023 [cited 17-7-23]. Available from: http://datazone.birdlife.org/species/factsheet/black-tailed-godwit-limosa.
- Gill J. Portugal fights wildfires with new tactics as heat waves raise risk. Context news (Thomas Reuters Foundation), July 15, 2022 (Source: https://context.news/climate-risks/); 2022[accessed on 19-7-23].
- Unsworth RKF, Cullen-Unsworth LC. Seagrass meadows. Curr Biol. 2017; 27: R443-5.
- Hestrin R, Hammer EC, Mueller CW, Lehmann J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun Biol. 2019; 2: 233.
