Abstract
Exopolysaccharides (EPS) are the polymeric substance obtained from diverse species of the genera Pseudomonas for a variety of purposes. Recently, large focus have been given on isolation and characterization of EPS from diverse microorganisms employing various strategies, but very less attention have been given on the theme of harnessing EPS from the master microbe Pseudomonas and exploiting its multifaceted roles. Hence, the main essence of this review article is to emphasize protocols opted for EPS production, extraction and optimization, detailing its architecture, composition, mechanisms and genetic regulation. In the process it will also discuss various approaches used nowadays for novel usage of EPS bioformulations derived from Pseudomonas for plant growth promotion, stress amelioration, bioremediation and disease management in an eco-friendly and sustainable manner.
Keywords: Bioformulations; Biofilm; Biocontrol; Exopolysacchrides; Metabolites; Pseudomonas
Introduction
Polysaccharides commonly known as glycans are abundant in nature. These polysaccharides can be isolated from various living organisms including algae, plants, animals, fungi, protozoa and bacteria [75]. Nature of polysaccharide varies in terms of physical properties, chemical nature and biological attributes. Some bacterial cells are enclosed by a slimy polysaccharide layer, commonly known as the glycocalyx. Capsular polysaccharides are produced by those organisms in which glycocalyx are attached to the bacterial cell surface by covalent bonds. When polymer is very loosely bounded to the cell surface, it creates slime, and then it is referred as Exopolysaccharides (EPSs) [47,66]. The concept of EPS was first introduced by Sutherland in 2001. They are high-molecular-weight compounds, made from carbohydrate, proteins, DNA and some non-carbohydrate components including acetate, phosphate, pyruvate, succinate etc depending on the bacterial strains [23,101]. EPSs are broadly classified as homo-EPS and hetero-EPS. Homo-EPS are the one made up of only one mono-saccharide unit and hetero-EPS constitutes diverse mono-saccharides unit within it [114]. In nature, EPS is present in the form of capsular material in bacterial cell, and even obtained in the structure of slimes. Bacterial cells have multifaceted reasons for releasing EPS, some of them includes [1] maintaining cell growth under stress environment, [2] providing attachment during biofilm construction, [3] nutrient pool or carbon storage, [4] plant growth promotion and [5] biological control.
Hence, the main aim of this chapter is to highlight how this metabolite EPS can serve as a game changer in future for attaining environmental sustainability. The chapter will also focus on the multi-dynamic roles of EPS with reference to the genera Pseudomonas. In the process it will also discuss various strategies; protocols opted for EPS extraction, detailing its architecture, composition and genetic regulation. Novel usage of EPS bioformulations derived from Pseudomonas for plant growth stimulation and disease management has been also included (Figure 1). This chapter will especially help those researchers who are exploring the areas of EPS in conjugation with the genera Pseudomonas for developing multifaceted bioformualtions in order to attain environmental sustainability.
Pseudomonas: Versatile Bacteria
The genus Pseudomonas includes the most ingenious and ecologically remarkable group of bacteria on the planet earth. Pseudomonas, belongs to the class gamma (γ) Proteobacteria. At present, there are more than ten genera of Pseudomonadales that includes, Burkholderia, Caulobacter, Ralstonia, Sphingomonas, Stenotrophomonas, and Xanthomonas [21]. The family Pseudomonadaceae, contain the foremost genera Pseudomonas, that are principally gram-negative, rod-shaped, straight and slightly curved shaped bacterial cells with polar flagella [21]. They are generally aerobes or facultative anaerobes, positive for catalase and oxidase test, negative for vogues proskauer, indole and methyl red tests. Advance methods for identifying the genus Pseudomonas have been done on the basis of 16S rRNA sequencing technique, with universal primers like, forward primer Ps-for (20-mer [5'-GGTCTGAGAGGATGATCAGT-3']) and reverse primer Ps-rev (18-mer [5'-TTAGCTCCACCTCGCGGC-3']) (Widmer 1998). Diverse members of the genera Pseudomonas, are established in majority of the natural environments including freshwater, marine, terrestrial, extreme habitats etc. Some of the Pseudomonas species can form close intimate relations with plants and animals [91]. Diverse Pseudomonas species including Pseudomonas alcaligenes, Pseudomonas chlororapsis, Pseudomonas dimunita, Pseudomonas elongata Pseudomonas fluorescense, Pseudomonas putida, Pseudomonas syringae etc are comprehensively used in the area of agricultural and environmental microbiology for its various applications.
A number of major Pseudomonas applications includes: plant growth elongation, biological control, biofilm formation, antimicrobial metabolite production, quorum sensing, plant microbe synergistic interaction, chemotactic interaction, uptake, and catabolism of various plant secretions [92]. There are many reviews and book chapters available on the subjected cited above [41], but the role of Pseudomonas in EPS production and exploiting its novel applications is reported rarely. Generally various species of Rhizobia are regarded as EPS producers and much work has been cited on this topic [105,106]. But, throwing light on the role of EPS with reference to Pseudomonas is a new thought that needs to be explored.
EPS Production in Pseudomonas
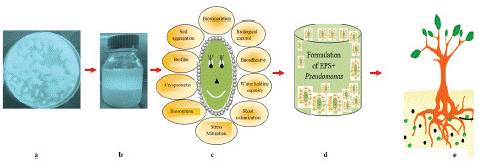
Figure 1: Plant growth stimulation and aggressive root colonization after applying EPS supplemented bioformulation.
a) Thick, slimy, or mucoid colonies of EPS producing bacteria on the growth medium; b) Extraction of EPS from bacteria, c) Diverse function performed by EPS; d) bioformulation developed from multifaceted EPS; e) Plant growth stimulation and aggressive root colonization after EPS amended bioformulation under stressed conditions.
Bacteria that produce EPS are usually characterized by thick, slimy, or mucoid colonies on the growth medium (Figure 1a). The concentration, amount and composition of EPS may vary within bacterial genera, species and strain [100]. Different Pseudomonas isolates obtained from the potato rhizosphere showed 1.75 to 2.24 mg/ml of EPS in the medium [100]. For producing large amount of EPS, bacteria desperately require energy from miscellaneous carbon sources. Hence, the type of carbon source utilized in the medium is an essential element that determines the quantity, composition and extent of EPS production. The EPS production is not solely dependent on carbon sources, but other factors including carbon/nitrogen ratio, genotype of microbe,phase of the microbial growth, salts used (NaCl, CaCl2), culture conditions (pH, temperature), and incubation time also impacts synthesis and production of EPS [95]. Composition of EPS varies in terms of carbohydrates, lipids, proteins, nucleic acids, humic substances etc depending on the strains. Different functional groups are embellished in the matrix of EPS such as amino group, amides, carboxylic group, hydroxyl group, phosphate etc that constitutes an essential component of EPS [22]. Bouchotroch et al. (2000) isolated 32 unusual Halomonas isolates from the salinized environment; authors also stated that all the diverse isolates showed variation in EPS productivity, chemical composition and physical properties, even under same environmental conditions.
In laboratory, EPS production is recorded maximum during the stationary phase [27,69], but there are few species of Pseudomonas, where the high EPS rate is observed during exponential phase. Bacterial genera including Alteromonas obtained from deep-sea hydrothermal vent showed high EPS production under nitrogen limited stationary phase [32]. The study was conducted by Nevot et al. (2006), where the worker isolated Pseudoalteromonas antarctica glacial marine sludge of South Shetland Islands. P. antarctica displayed, high EPS production during exponential phase. When Pseudomonas aeruginosa MTCC 1688 was inoculated in nutrient broth (pH 6) at 32oC for 4 days, maximum EPS production was monitored of about 26 mg/50 ml after 96 h of incubation [22]. Lower incubation temperature assist in manufacturing EPS by dipping P. aeruginosa growth and raising the accessibility of precursor for EPS synthesis. Production of EPS in case of P. aeruginosa was recorded significantly higher, when, there is an increase in incubation of bacterial cells under starved conditions. Under nutrient limitation, glucose is dominant in the EPS and glucosyl units are detected in the EPS matrix of P. aeruginosa cells [76]. Table 1elucidate diverse culture conditions, growth medium, required by the bacteria for enhancing EPS production in Pseudomonas sp.
EPS producing Pseudomonas
Media for EPS extraction
Dry weight of EPS
Mechanism of EPS for plant growth /biocontrol/bioremediation
References
Pseudomonassp.
Mineral salt medium at
0.2-mPa-
EPS creates a microenvironment around the bacterial cells, hold high amount of water, suggesting its moisture holding capacity and maintain bacterial survivability under high desiccation state.
[89]
Pseudomonas aeruginosa RB 28
Minimal medium
supplemented with 2% crude
oil2.7 g/l after 60 h incubation
High emulsification activity of the extracted EPS shows the presence of high glycolipid and rhamnolipid. These biosurfactants increased cell surface hydrophobicity that helped in biodegradation of crude oil.
[97]
Pseudomonas putida GAP-P45,
Trypticase soya broth
supplemented with 25%
polyethylene glycol4.06 mg/ mg protein under minimum water potential (-0.73 Mpa)
Strain displayed high drought tolerance by raising its cell number, increasing RAS/RT ratio, strengthening root colonization and soil aggregation.GAP-P45 produced biofilm like communities, where bacteria were connected together by an EPS matrix with micro colony formation under water stress condition and enhanced sunflower growth
[94]
P.aeruginosaPF23
Davis Minimal Medium
supplemented with 2000 mM NaCl1.323 g/l at 2000 mM NaCl
Saline tolerant EPS producing strain PF23 displayed multifaceted role of plant growth promotion, disease management and stress amelioration under non saline and saline conditions with sunflower crop
[103]
P.aeruginosa PF07
Davis minimal medium
supplemented with 0-1600 mM NaCl1.298 g/l EPS observed at 1600 mM NaCl
Bioformulation enhanced early seed germination, plant growth parameters, raising seed weight and ameliorating stress in saline affected regions by embracing RAS/RT, enhancing porosity, boosting nutrients uptake in sunflower crop
[104]
Pseudomonas
Mineral salt medium
-
EPS improved soil moisture contents, plant biomass, root and shoot length, and leaf area of maize Under drought stress, the inoculated plants showed increase in relative water content, protein, and sugar.
[79]
Pseudomonas sp. AK-1
Nutrient Broth
supplemented with NaCl5.3 g/l at 500 mM NaCl
Strain AK-1 was able to bind free Na+ from the soil, making Na+ unavailable to soybean roots under saline (200 mM NaCl) conditions.EPS binds with Na+ and reduces salinity stress in the soil and reduced electrical conductivity of soil from 1.1 to 0.9dS/m on soybean crop
[55]
Pseudomonas psd
Gluconate minimal medium
with and without
ZnSO4 supplementation.0.6 mg/mL of alginates after 5-days of incubation without Zn2+, In the presence of Zn2+, up to 3-fold increase was recorded in the amount of alginate produced
Zinc supplementation enhanced EPS production, siderophore production, phenazine-1-carboxylic acid synthesis, phenazine production displaying biofilm formation and aggressive root colonization that helped in the mungbean plant for growth stimulation
[108]
Pseudomonas fluorescens DR7+
P.fluorescens DR11+
Enterobacter hormaechei DR16+
Pseudomonas migulae DR35RCV-sucrose medium
supplemented with 40g/l sucroseP.fluorescens DR7 produced 11.63 mg/mg protein, DR16 displayed 5.44 mg/mg protein, DR35 showed 3.33 mg/mg protein (-1.03 MPa )
Multiple PGP traits (ACC, EPS, IAA, Phosphate solubilization) displayed by the organisms DR7, DR11, DR16, and DR35 increased the percentage of foxtail millet seeds germinating under drought stress conditions between -0.30 MPa and -1.03 MPa Such traits improved plant growth promoting activity, possibly by improving soil structure, soil texture and raising the number of root colonizing microbes around the root surface under drought stress conditions in Fox tail millet
[81]
P.fluorescence PF17 EPS+salicylic acid
Davis minimal medium
supplemented with 600 mM NaCl0.9 g/l at 500 mM Nacl
Combinational effect of EPS+SA helped in stress amelioration, plant stimulation and biocontrol,as stimulation of EPS enhanced SA production, augmenting seed germination thereby proving them to be biopriming agents on sunflower seeds
[105]
Pseudomonas sp. PFAB4
King’s medium B base
2.63 g/l
Thermotolerant EPS was mixed with AgNO3 in 1:1 ratio and this combination displayed effective killing against target bacterial and fungal pathogen
The non-porous, compact nature of the EPS
biopolymer is useful for several industrial applications; most
likely of which is bioplastic production.[10]
Pseudomonas entomophila PE3
Nutrient broth medium
supplemented with NaCl (0-8% NaCl)EPS production of 12.5g/l was observed at 2% NaCl
Enhanced EPS production during salinity stress (2% NaCl) resulted in increased antioxidant potential,hydroxyl scavenging activity, antioxidant activity and osmolyte accumulation. Excessive root colonization raised sunflower growth
[31]
P.aeruginosa MTCC 1688
Nutrient broth
26 mg/50 ml after 96h of incubation at pH 6 and 32°C temperature
EPS helped in sequestration of heavy metals.The extracted EPS has been applied for removal of Ni (II) and Cr (VI) ions from aqueous system by biosorption.
[22]
Table 1: Diverse mechanisms adopted by EPS producing Pseudomonas strains for plant growth stimulation, biocontrol and bioremediation using different medium and culture conditions.
Diverse Methods for Extracting Pseudomonas Exopolysaccharids
There are different methods and protocols employed for extracting and quantifying EPS form various species of Pseudomonas. Protocol for isolating EPS in P. fluorescens Biovar II, (ATCC 55421) was given by Hung et al. (2005). Total carbohydrates in the EPS of P. fluorescens Biovar II, was analyzed as per the modified protocol of Hung and Santschi (2001). Different sugars present in EPS consisted of arabinose, fucose, galactose, glucose, mannose, rhamnose, ribose, and xylose [49].
Titus et al. (1995) isolated EPS from marine bacterium P. alcaligenes using basal salt medium, EPS of diverse quantity was examined under different phases of growth medium. Slight variation in the concentration of C and N sources brought major change in amount and composition of EPS. Copious amount of EPS production was observed on active surfaces including copper, rather than on glass and titanium. EPS which promotes bacterial adhesion on immersed surfaces contained 19.8%, 16.8%, 3.5% and 0.7% of carbohydrates, proteins, uronic acids and sulphate respectively [107]. Amount of pyruvate was analyzed in traces and 52.5% of inorganic matter was also recorded in the EPS of P. alcaligenes.
Forde and Fitzgerald (1999) described the method for isolating EPS from P. aeruginosa ATCC 10145, in which amount of EPS production varied as per incubation time and nutrient availability. After 96 h and 120 h of incubation, the amount of EPS was recorded to be 5 μg/109 CFU and 18 μg/109 CFU respectively. Quantification of total EPS from P.aeruginosa ATCC 10145 was done by acid hydrolysis as per the protocol of Parkar et al. (2001). The quantified data was analyzed and it revealed different monomeric constituents within it including arabinose, fructose, glucose and galactose. The amount of these sugars showed variation in their values at 96 h and 120 h of incubation ranging from 0.17 to 6.60 % [76]. The mutational studies was conducted by Tewari and Arora (2014) on saline tolerant P.aeruginosa PF23, that displayed high EPS production under salinity stress conditions using using 5 bromouracil. It was observed that wild strain (PF23 EPS+) was EPS producer, whereas, mutant strain (PF23 EPS-) was defective in EPS production. Tewari and Arora (2014) conducted study on Davis minimal medium, and it was observed strain PF23 EPS+ showed high salinity tolerance upto 2000 mM NaCl concentration with 1.323 g/l of EPS production. With progressive increase in salinity from 100 to 2000 mM NaCl, there was significant enhancement in EPS production. Nearly, 66% increase in EPS was observed at 2000 mM NaCl concentration in comparison to non-saline conditions (0 mM salt). EPS defective mutant (PF23 EPS-) showed nearly, 87% reduction in EPS in comparison to wild strain (PF23 EPS+). Composition of EPS also showed variation in terms of raising salt concentration, for example at 0 mM NaCl, only glucose was present, whereas, progressive raise in salinity resulted in glucose, galactose and trehalose as its sugar components [103]. Sandhya et al. (2009) also reported the isolation and quantification of EPS from P. putida GAP-P45 by changing the amount of poly ethylene glycol in the trypticase soy broth medium. Authors reported high amount of EPS of about 4.06 mg mg-1 protein at water stress conditions (-0.73 Mpa), and this EPS producing strain was effectively used under drought stress for raising sunflower productivity. Characterization of EPS by TLC displayed presence of glucose, mannose, and rhamnose as its constituents.
Kasotia et al. (2016) performed EPS extraction using Pseudomonas sp AK-1 taking nutrient broth medium supplemented with 0 to 500 mM NaCl concentration. In absence of salt, strain AK-1 displayed 0.32 g/100 ml dry wt of EPS, that raised to 0.53g/100 ml at 500 mM NaCl. While performing experiments on two different species of Pseudomonas viz; P. aeruginosa G1 and P.putida G12, Celik et al. (2008) observed 3% and 2% xylose respectively as preferential carbon source for obtaining high EPS production of about 368 mg l-1 and 262 mg l-1 respectively. P. aeruginosa resistant to chromium was found to produce 863 mg l-1 of EPS [57]. Study was conducted by Upadhyay et al. (2017), in which workers have isolated EPS from Fluorescent Pseudomonas strain Psd, on Gluconate Minimal Medium (GMM) in two sets with and without ZnSO4.7H2O supplementation. EPS was extracted from strain Psd, using the protocol of Bitton and Freihofer (1977). High EPS production was obtained in zinc supplemented medium rather than other one. Freitas (2009) used glycerol rich products and pure glycerol as carbon source for the EPS production in P. oleovorans NRRL B-14682. Amount of EPS, obtained was approximately 11.82 g l-1 and 12.18 g l-1, when pure glycerol and glycerol rich products respectively, were used as carbon source, in the medium at 30°C and pH 6.8. P. oleovorans displayed heteropolysaccharide EPS, consisting of 37-80% galactose, 2-30% glucose, 0.5–25% mannose, 0.5-20% rhamnose and acyl group substituent’s like acetyl, succinyl and pyruvil. As per the findings of Dimitrijevic (2011), EPS extracted from extremophilic P. aeruginosa, documented alginate type EPS production of 36.5 mg l-1 in LB broth after supplementation of sunflower oil and tween 80. Several workers reported that mixing sunflower oil and detergents into the medium raised EPS production in Pseudomonas [62]. There is a proposed mechanism by which surfactants improve the EPS production. It may be due to the fact that surfactant molecules cooperate with the cell membrane in such a way that it would augment the polymerization process and thereby assist in the discharge of the polymer from the membrane [7]. Ali et al. (2020) documented elevated EPS from a psychotropic bacterium Pseudomonas sp. BGI-2 purified from the Batura glacier of Pakistan. EPS assembly was enhanced by changing the amount of different nutritional and physiological conditions. Glucose, galactose, glycerol, mannose and mannitol, were the preferable carbon sources that were used by this bacteria. Molasses (waste of sugar industry), was used as a major substrate for the bacterial growth and EPS production. Strain displayed high EPS production of 2.01g l–1, when following conditions were maintained in growth medium, viz: glucose (100g l–1), yeast extract (10g l–1), NaCl (10g l–1) and C/N ratio (10/1), pH 6 and temperature 15°C. Diverse sugar components present in EPS were glucose, galactose, and glucosamine that were detected using high-performance anion-exchange chromatography with pulsed amperometric detection technique.
Extracting EPS from Waste
It could be seen from various examples cited above that to obtain good quantity of EPS majorly good quality of carbon sources are required, that make the process of EPS extraction a bit expensive. Hence, it is utmost important to optimize the culture conditions for extracting high amount of EPS, using cheap nutrient sources or agrowaste materials. Different agro food industries are excreting hazardous waste materials that are toxic to the environment; hence, proper strategies should be opted for disposing agrowaste (Kanimozhi et al. 2018). Naturally these agro-wastes are allowed to decay in the fields. These waste are too polymeric and fibrous, to be digested by monogastric animals, so they are not suitable as animal food and fodder. Agro wastes are sparingly soluble in water containing upto 70% carbohydrates that can be effortlessly used by microbes as their energy source [112] (Sadh et al 2018). Agro waste material display dual role, firstly, they can be used as carbon source by the microbes to derive energy and secondly, they can be used to obtain EPS as by-products. As per the findings of Li P et al. (2016) Xanthomonas campestris can convert kitchen waste into xanthan gum. Similarly, agricultural waste could be converted to pullulan that is produced from Aureobasidium pullulans [96]. Kumar et al. (2022) observed that the Pseudomonas strain BGI-2 used crude glycerol (waste product of biodiesel industry) as a carbon source. To cut down the cost of EPS production, several workers examined the usage of citrus fruits, mango peel, milk whey, pineapple waste, cabbage, spoiled guava, beet molasses, olive mill wastewater, sweet lime, potato starch waste, date syrup, rotting tropical fruits and mixed fruit waste for EPS production [109,121]. Though, these substrates have been tried and tested to extract EPS from diverse bacterial genera, more focus should be given in using them with particular reference to the genera Pseudomonas for EPS extraction.
Genetic Regulation of EPS
Basic requirement for EPS synthesis is monosaccharide units that are obtained from catabolized sugars including nucleoside diphosphate sugars, Uridine Diphosphate (UDP)-N-acetylglucosamine and Guanosine Diphosphate (GDP)-mannuronic acid [9]. Plasmid or chromosomal DNA is the sites that target EPS gene synthesis in the bacterium. These genes are organized in the form of gene clusters. It has been observed that more than one gene cluster is present in a bacteria, that participates in EPS synthesis [59]. The gene cluster involved in EPS synthesis contain numerous functional genes, with desired properties such as gene assembly, determining chain length, gene exportation, gene polymerization, and gene regulation (De Vuyst et al. 1999). On the basis of detailed analysis of EPS biosynthetic and exportation mechanisms, three main pathways have been elucidated. First pathway is involved, in catalyzing both polymerization and exportation [115]. In the second pathway, ATP-binding cassette transporter plays an important role in exporting repeating biosynthesized unit. In the third pathway, Wzx-Wzy proteins are involved [117]. Wzy protein helps in polymerization and assist in elongating growing EPS chain whereas, final translocation is done by Wza [87,115]. Repeating unit is catalyzed by glycosyltransferase, and exported outside the cell across the inner membrane by Wzx protein [87,115]. Bacterial tyrosine kinases are coupled with EPS biosynthesis in bacteria [30]. Genes encoding bacterial tyrosine kinases are often associated with genes involved in EPS production. The kinases are known to regulate EPS production by phosphorylation and thereby, activating a biosynthetic enzyme in the pathway of EPS production [71]. Several workers reported that three major types of EPS are secreted by Pseudomonas sp. They are alg, Psl and Pel [36,43,67]. Alg for alginate synthesis, Psl constitutes galactose and mannose-rich polysaccharide that helps in the primary attachment, biofilm and micro colonies formation [67]. Pel, is a glucose rich cellulosic polymer necessary for pellicle development at air-liquid interface [39]. Divyashree et al. (2022) mentioned prevalence of top 5 gene for EPS synthesis including gene pslB (71.21%), gene pslA (57.57%), gene algD (43.93%), gene pelA (45.45%), and pelD (27.27%), respectively, in P. aeruginosa. Kamali et al. (2020) reported some interesting results in which 85% of P.aeruginosa isolates displayed algD, pslD, and pelF genes. The genes related with biofilm formation pslA (83.7%) and pelA (45.2%) respectively, were also reported by Ghadaksaz et al. (2015) in Pseudomonas. P. putida KT2440, contain four important genes, they are alg (alginate), bcs (cellulose), peb (biofilm stablization), pea (putida EPS A), important for forming biofilm, carbohydrate processing, polysaccharide synthesis and transport respectively. Pseudomonas entomophila L48 exhibited the presence of pea and alg locus and alginate biosynthesis loci [113].
Alginates are linear EPS consisting of Β-1,4-linked-D-mannuronic acid and a-L-glucuronic acid [44]. Alginate is a form of EPS secreted by various types of Pseudomonas sp. The three main gene clusters responsible for producing alginate are PA1381-1393, PA2231-2245, and PA3552-3558 [70]. In P. aeruginosa PAO1, gene cluster PA2231-2245 (pslA to -O) plays an imperative function in biofilm formation. 18-gene cluster are required for synthesizing EPS 273 in marine bacterium P. stutzeri, if any of the gene is deleted from this gene cluster it may affect EPS synthesis. Divyashree et al. (2022) observed 66 different isolates of P. aeruginosa, obtained from hospital waste.
Franklin et al. (1994) reported that in P.aeruginosa 13 proteins are directly linked with alginate synthesis (except AlgC), and encoded by the alg operon. 3 main enzymes are involved in synthesizing alginate precursors including guanosine 5'-diphospho-D-mannose pyrophosphorylase (Alg A), phosphomannomutase (Alg C) and GDP-mannose dehydrogenase (Alg D). These enzymes catalyze the conversion of fructose-6-phosphate to GDP-mannuronic acid via four steps: primarily, alginate is synthesized as a linear homopolymer from the GDP-mannuronic acid to polymannuronic acid by catalytic subunit alpha-1,3-glucosyltransferase (Alg8). Later, it interacts with Alg44 polymerase positioned at the cytoplasmic membrane, Thirdly, enzymes allow the movement of the alginate precursor across the inner membrane for polymerization, lastly, Alg44 also demonstrated the capability of binding the second messenger cyclic dimeric guanosine monophosphate (c-di-GMP) synthesized by MucR, a membrane-anchored protein, for synthesizing alginate [45,68].
The cellulose EPS has been described as a key component in the development of P. syringae pv. syringae UMAF0158 biofilm [46]. Strain UMAF0158 contains two additional genomic regions that putatively encode for alginate and a Psl-like polysaccharide. The Psl-like polysaccharide of UMAF0158 play an important role in virulence similar to that has been described for cellulose, also they are involved in surface colonization in case of P. aeruginosa. The significant role of Psl EPS in biofilm formation, plant colonization, plant microbe interaction and virulence, is well documented in case of the P. syringae. Since, the genomic region that encodes Psl is very well conserved. The strong relation is observed in between the production of Psl EPS and swarming motility. Direct relationship in between the two suggests the connection between the expression of psl gene and rhamnolipid genes (Heredia-Ponce et al. 2022).
EPS: A Game Changer for Environmental Sustainability
EPS are natural, non-toxic bioproducts with diverse biological activities. These natural biopolymers are serving as game changers for attaining environmental sustainability. As these polymers are pure, green and eco-friendly, so they can serve as a chemical free source for designing bioformulations including biopesticides, biofertilizers, biostablizers, bioadhesives, biosorptives and bioplastics (Figure 2). Various industries produces non-degradable plastics, that are extreme threat to the natural resources, mostly affecting the aquatic water bodies [77]. Usage of microbial EPS based bioplastics can serve as a next generation eco-friendly, and sustainable approach . Keshavarz and Roy (2010) reported the superiority of biological polymers in comparison to petro chemically derived polymers, in terms of biocompatibility, biodegradability, environmental and human compatibility. Recently the findings of Tewari and Sharma (2020) documented the role of microbial derived EPS as biostimulant, thereby serving as an alternative to replace the usage of hazardous agrochemicals, and safeguarding the survival and productivity of very important pulse crop Arhar in marginalized saline lands of India. Similar biostimulatory efficiency of Pseudomonas derived EPS was even proved by Fatimah and Arora (2021) and, its efficacy was tested on oilseed crop sunflower. Supplementation of pure bacterial EPS or in combination with Pseudomonas cells can provide a sustainable solution for bioremediation of salinized soil, augmenting plant productivity and disease management [103,104]. Authors also reported the usage of combinational bioformulation developed from Pseudomonas + EPS can work effectively as a potent biological control against Macrophomina phaseolina and a potent stress ameliorator protecting sunflower plants against salinity stress conditions [103-105]. Nutrient imbalance under salinity stress could be mitigated, by utilizing Pseudomonas EPS, as it promotes massive root colonization and assist in augmenting plant growth and development under osmotic stress conditions [104]. EPS producing Pseudomonas are the potent biofilm producers, and, helps in naturally creating slimy microbial aggregation rather than chemical films [43]. Nowadays, there is a massive interest in the usage of microbial derived EPS, at industrial level. EPS from psychrophilic bacteria Pseudoalteromonas sp KCTC 12867BP showed, excellent cryoprotective, membrane stabilizing and non cytotoxic nature (Roca et al. 2016). Hence, this EPS can be used as a substitute towards conventional cryoprotective agents that are cytotoxic and hazardous at high concentration to humans and animals. Certain bacterial species have been used commercially for exploiting various EPS such as alginate, xanthan, gellan, and dextran due to its gluing and bioadhesive nature. These microbial products could be used in future as a potent substitute over hazardous chemical adhesives [38]. Bioemulsifiers developed from Pseudomonas are more ecofriendly over synthetic emulsifiers (heptadecane) as they are biodegradable and non- carcinogens (Caruso et al. 2018). Another important feature of Pseudomonas EPS is its biosorption capacity that could be used to clean up the toxic heavy metal and salts from the environment. Microbe mediated biosorption is a green approach for sustainable future in comparison to chemical sorption [22]. A number of Pseudomonas species have been reported to sequester toxic contaminants including zinc, mercury, lead, chromium etc from waste water [22,108]. All these multifunctional attributes of Pseudomonas EPS, makes it a potent candidate for designing next generation bioformulations. Such EPS based biological products can serve as a game changer and set up a new horizon in attaining environmental sustainability in an eco-friendly manner.
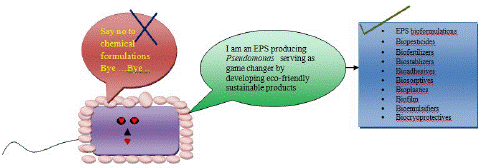
Figure 2: Pseudomonas EPS serving as game changer for attaining environmental sustainability.
Plant Growth Promoting Roles of Pseudomonas EPS
Structure, synthesis and genes responsible for EPS production are highly diverse. Besides this diversity, EPS also vary in terms of its functionality. Diverse functions have been carried out by EPS, like EPS matrix offer protection to bacterial cells against environmental stressors. It also covers bacterial cells and shields them from antimicrobial compound and toxic heavy metals. Water Holding Capacity (WHC) of EPS protects bacteria against excessive drought (Costa et al. 2018). Apart from this it also help in cell to cell communication, adhesion, aggregation, carbon storage, and a nutrient reservoir (Vardharajula and Ali 2015; Wang et al. 2015). In this section detailed diversity of EPS has been highlighted in terms of its functionality.
EPS offers tremendous protection to the microbial cells under stress conditions. Diverse abiotic conditions including drought, temperature, pH, and salinity can elicit the formation of EPS as a response to environmental stresses [110,119]. Roberson and Firstone (1992) observed that under drought stress conditions, EPS produced by Pseudomonas strain isolated from soil displayed high survivability in comparison to non-EPS producers. It was observed that under high desiccation state, EPS act as a protective covering, that help in trapping water and nutrients for bacterial survival, thereby allowing the bacteria to make metabolic as well as structural modifications [88]. Thus, it may act as a strategy for retaining water/nutrient and maintaining bacterial survival. Role of EPS in mitigating salinity stress has been elucidated by Tewari and Arora (2014) in P. aeruginosa PF23 under soil stress conditions while working on sunflower crop. The synthesis of EPS by salt tolerant isolates PF23 decreased Na+ uptake in plants by trapping and declining the amount of salt ions available (Upadhyay et al. 2011). EPS prevents nutrient imbalance under osmotic stress, and promote the growth of root colonizing Pseudomonas, thereby, benefitting the plant growth and development [104]. Kasotia et al. (2016) observed that EPS producing Pseudomonas sp. AK-1 was able to bind free Na+ from the soil, making Na+ unavailable to soybean roots and maintain normal plant growth up to 200 mM NaCl. Application of AK-1 EPS on soybean plant helped in minimizing soil electrical conductivity from 1.1 to 0.9 dS/m and protects bacteria from salinity stress. Seed pelleting with EPS of P. putida AKMP7 on wheat, under heat stress conditions enhanced the amount of cellular metabolites including proline, reduced membrane damage and the activity of several antioxidant enzymes such as super oxide dismutase, ascorbate peroxidase and catalase [44]. Treatment of plants with EPS-producing bacteria showed higher accumulation of proline, sugars, and free amino acids under water deficit stress conditions. Co- inoculation of PGPR with EPS producing bacteria is a promising measure to combat drought/salinity stress and, increasing global food security [78]. Importance of EPS has been also elucidated by Nunkaew et al. (2015) while performing experiments on rice crop using EPS producing bacteria Rhodopseudomonas palustris (strains TN114 and PP803).
Combinational effect of EPS producing P. fluorescens DR7+P.fluorescens DR11+Enterobacter hormaechei DR16+ P.migulae DR35 was observed on foxtail millet (Setaria italica), it enhanced seed germination and profuse plant growth under drought stress conditions (-0.30 MPa and -1.03 MPa). Multiple PGP traits displayed by the microbes including ACC deaminase activity+EPS+ IAA+ phosphate solubilizatio, could be one of the reason for plant growth stimulation. EPS producing P. fluorescens DR7 showed the highest level of ACC deaminase activity, DR7 could efficiently colonize the root adhering soil, increased soil moisture, and enhance the root adhering soil/root tissue ratio (RAS/RT). Bacteria having dual activity of EPS +ACC deaminase, may appears to improve the plant growth stimulation of desired crop plant, possibly by improving soil structure, soil texture and raising the number of root colonizing microbes around the root surface. Such EPS producing bioinoculants can be effective in long run for maintaining sustainable agricultural in drought effected arid habitat (Niu et al. 2018).
EPS producing strain of P. entomophila PE3 act as biostimulants for raising sunflower growth, yield and productivity under salinity stress. Talc based EPS bioformulation was prepared taking various concentration of purified EPS ranging from (0.1%, 1%, 2% and 5% EPS) and combination of EPS+PE3. Combinational set (EPS+PE3) and pure EPS (2%) set displayed significant enhancement in growth stimulation of sunflower crop in comparison to untreated sets receiving only bacterial treatment [31]. Potency of pure EPS bioformulation and combinational EPS bioformulation in growth promotion and biological control of pigeonpea crop was proven by Tewari and Sharma (2020) on Rhizobia. Naseem and Bano (2014) showed augmented growth and productivity of maize crop when combination of EPS+Pseudomonas was added in the saline soil. Tewari and Arora (2014) also developed talc based EPS bioformulation from saline tolerant P. aeruginosa PF07 and monitored its potency on sunflower crop under saline conditions in pot trails (125 mM NaCl concentration). Author’s suggested the benefits of employing EPS formulation in enhancing early seed germination, enhancing plant growth parameters, raising seed weight and ameliorating stress in saline affected regions. EPS bioformulation also enhanced soil texture by embracing RAS/RT, enhancing porosity, boosting nutrients uptake, and thereby considering it as a commercially important bioformulation for restoration of stressed sites. In 2018, same workers performed another experiment in which they have used the combination of two powerful metabolites EPS+Salicylic Acid (SA) for monitoring dual effect on sunflower seeds. The results highlighted that that combination of these two metabolites EPS+SA helped in enhancing plant growth of sunflower crop under saline conditions and combated the growth of dreadful phytopathogen M.phaseolina [103].
Certain Pseudomonas species produce high amount of EPS, that serves as potent cryoprotective agent, and help the microorganism against frost injuries. Mishra et al. (2011) documented, temperature ranging from 0-15°C display significant increase in EPS production in comparison to optimum conditions (20-30°C). Psychrotolerant Pseudomonas harvested from the north western Himalayan regions were able to produce higher amounts of EPS under low temperature conditions in comparison to ambient temperature (Mishra et al. 2011). The reason why EPS producing bacteria can help plants to thrive best under cold conditions may be due to the fact that the bacterial EPS act as an excellent chelator and, it chelates Na+ ions, restricting sodium uptake by roots, thereby, defending the plants against cold-mediated dehydration [74]. Ali et al. (2020) performed study on cold loving bacteria Pseudomonas sp. BGI-2, that displayed copious amount of EPS production, guarding bacterial cells against harsh environmental conditions including freeze thawing, chemical lysis or detergent treatment. It was amazing to observe that EPS extracted from strain BGI-2 safeguarded mesophilic Escherichia coli k12, suggesting its cryoprotective nature. BGI-2 also provided noteworthy stability to the RBC membranes by detergent treatment. Increased inhibition of lipid peroxidation by BGI-2 EPS, further authenticates its role in cell membrane protection. High colonization of EPS producing Pseudomonas on glacier ice was observed by [17]. Authors stated, the inhibitory effect of EPS from psychrophiles on lipid oxidation is very critical as oxidative stress increases at low temperature. Carrión (2015) isolated cryoprotective EPS from Antarctic Pseudomonas sp. ID1. Arcarons (2019) reported the cryoprotective role of EPS by monitoring its efficacy on adult cow oocytes. Application of 10 μg ml-1 EPS obtained from Pseudomonas sp. ID1 was added to vitrification and warming media, it protected bovine oocytes against cryodamage. EPS derived from Pseudoalteromonas sp. strain CY01 (KCTC 12867BP), is a novel strain isolated from the polar ice caps. It showed excellent ability to cryoprotect cells, and display no cytotoxicity. Thus, this EPS can be used as an alternative towards conventional cryoprotective agents that show cytotoxic attributes when used at high concentrations. This work paved new avenues for the usage of EPS as a cryoprotective and cell membrane stabilizing agents. With all these facts it could be speculated that, the usage of EPS as cryoprotective agent at industrial level will be a noteworthy. Currently, chemical based cryoprotective agents like dimethyl sulfoxide and methanol have taken a hold in the industries, but there usage will be toxic to cells at higher concentrations. Hence, microbial derived EPS can be used as a non-toxic, pure and biological approach towards the conventional method.
High-quality soil structure depends on its aggregation. Soil aggregation is important for agricultural sustainability [5]. Rearrangement of soil particles by flocculation, and cementation results in soil aggregation. Aggregated soil particles usually define the physical and mechanical properties of soil, including WHC, water upsurge and aeration which in turn affect chemical and biological processes [2,102]. Aggregates are important for the improving soil fertility, porosity and agronomic productivity as it influences plant germination and root growth [13]. The stability of aggregates depends on their internal cohesion, pore volume, and pore-wall hydrophobicity [20,64]. demonstrated that electrostatic interactions are important in between the EPS of P. putida X4 and mineral kaolinite, montmorillonite, and goethite. It was observed that Stenotrophomonas, Sphingobacterium, Bacillus, and Pseudomonas species could stabilize and increase aggregate strength. Interestingly, Bacillus and Pseudomonas are genus widely known to produce biofilms and EPS, which are involved in the stabilization of soil structure [93]. EPS obtained from P. aeruginosa, Erwinia, Ralstonia, and Azotobacter vinelandii helps in soil aggregation as it holds high adhesive properties. These adhesive natures help in gluing the bacteria to the soil particles and enhance root colonization. Mu’minah et al. (2015) reported high amount of EPS producing strains from the potato rhizosphere that were also potent IAA producers which in synergy assisted in plant growth development. There are several documented proofs that EPS producing Pseudomonas can enhance the aggregation of soil particles, this binding helps in maintaining soil moisture and nutrient entrapment. Within this process plants are benefitted and their productivity enhances. In addition to this gummy nature of EPS, it also provides unique traits including biocompatibility, gelling, stabilizing, and thickening capabilities, with enormous industrial applications. As per the reports of Roberson and Firestone (1992) EPS obtained from Pseudomonas can hold high amount of water, suggesting its moisture holding capacity. Addition of EPS in sandy soil, alters its moisture by allowing the EPS amended soil to hold more water than unamended soil. Endophytic bacterium strain PsJN (Pseudomonas sp. PsJN) was isolated from onion root that displayed root colonization on A. thaliana[40]. EPS are responsible for maintaining cohesive and adhesive interactions amongst microorganisms and biofilms respectively to the attaching surface. It influences the spatial association, allowing microbial interactions, and acting as cementing material between the cells [120]. These functions are imperative in establishing the biological activities of flocs and biofilms. EPS, provides mechanically support as it stabilizes the microbial cluster via various macromolecular interactions involving hydrogen bonds, dispersion forces and electrostatic interactions. These interactions leads to the formation of tridimensional gel like structure around the cells and help them in maintaining stable microbial consortia [33]. Exopolymers from Pseudomonas benefits the survival of other marine organisms by facilitating attachment and adherence to surfaces [48]. Chen and Stewart (2002) concluded that EPS is responsible for both adhesion and cohesion interactions and play a crucial role in maintaining structural integrity of P. aeruginosa biofilms. EPS act as a preconditioner, making the adhesion process more favorable. In some cases, EPS matrix also enables the marine bacteria to capture nutrients. In certain bacterial genera, exDNA is accountable for the cementing nature of EPS. exDNA is an autolytic secretion released by P. aeruginosa biofilms [72,116]. The exact function of exDNA has not been completely elucidated, but few studies demonstrated that may be related to the cohesion/ adhesion to surfaces and signaling [83].
Bioemulsifiers obtained from Pseudomonas have significant advantages over synthetic emulsifiers, as they are biodegradable and environmental friendly (Caruso et al. 2018). There are large number of Pseudomonas species that have been reported to form polymers with emulsifying property [24]. Microorganisms that produces bioemulsifiers can be categorized into three major groups: The first one, are those microbes that use only alkanes as carbon source. Second one, includes those organisms that uses only water soluble substrates as the carbon source and third one, involves those organisms which includes both alkanes and water soluble substrates as carbon source [112]. Pseudomonades are the main example included in the third category. There are certain species of fluorescent pseudomonads, where, the emulsification activity of bacterial EPS was almost found to be similar to that obtained from any hydrocarbon. The P. fluorescens EPS exhibited highest emulsification in comparison to controls including diesel and olive oil. Sifour et al. (2007) have reported high emulsification activity of EPS producing P. aeruginosa when sunflower oil, heptadecane and paraffins were taken as control. Abouseoud et al. (2007) reported the usage of diesel and kerosene oil as the substrate for enhancing the EPS production in P. fluorescens and it may further raise the emulsification activity. There is a direct correlation observed in between the emulsification activity and EPS production [112].
EPS producing strains play a significant role in biofilm formation and quorum sensing. Various studies have shown that Acylhomoserinelactone (AHL), (3OC6-HSL, 3OC8-HSL and C6-HSL) mediated quorum sensing is an important factor for biofilm enlargement, and in some species this is mediated through regulation of EPS production [25,61]. Genes required for EPS production are responsible for producing biofilm in bacterial cells. AHLs, are involved in the regulation of EPS production. P.aeruginosa has been regarded as model organism for biofilm formation. P.aeruginosa contain three types of diverse EPS synthesis viz alginate, Psl, and Pel. These EPS contribute to the formation of biofilm in this microorganism. Pel is a polymer of cellulose and highly rich in glucose. Increase in the amount of Pel production is related with the appearance of wrinkled colony and it is helpful in cell to cell communication that assists in the process of biofilm formation [43]. Polysaccharide containing Psl is highly rich in galactose and mannose. It is involved in initial adhesion and biofilm maturation during planktonic stage. In mature colonies Psl is linked with the caps of mushroom-like microcolonies. P. aeruginosa also produces alginates, a polyanionic EPS composed of uronic acids [39]. Excessive production of alginate results in forming large finger-like microcolonies. Alginate has been shown to contribute to decreased susceptibility of biofilms to antibiotic treatment and human antibacterial defense mechanisms [90].
Various industries releases huge amount of waste effluent containing heavy metal contamination that may cause serious threats to animals and other water bodies. Entrapment of this toxic heavy metal from such source using microbial assisted biosorption could be a clean and green approach for sustainable future. Detailed structure of EPS analyzed by electron microscopy shows many groves within it, in which multiple heavy metals can bind. It was observed that when P.aeruginosa is exposed to 10 mg l-1 of chromium dosage, there was significant increase in rhamnolipid concentration [85]. It indicates the involvement of rhamnolipids, that assist in chromium removal from the natural system like soil and water [85]. Taking into these considerations, EPS producing different species of Pseudomonas, have been used to sequester heavy metals such as cadmium, copper, chromium, nickel etc from aqueous solution [22]. EPS, obtained from P.aeruginosa MTCC 1688, has the potential to remove 26% Cr and 9% Ni respectively from the water waste system [22]. Fluorescent Pseudomonas strain Psd is a soil dweller, possessing zinc biosorption capacity. As per the findings of Upadhyay et al. (2017) it was documented that EPS play significant role in zinc biosorption. The reason for this was the production of alginates by Pseudomonas, that are involved in producing alg 8 gene. Alginate plays significant role in the biosorption of zinc. If alg8 gene was mutated, it may lead to the significant reduction in EPS production and compromised biofilm production. Authors also demonstrated that when Pseudomonas cells are exposed to zinc, it raises the concentration of alginate production, and further improved biosorption formation in psd strain.
Antagonistic role or biocontrol activity of EPS have been elucidated by various workers in details [10,103,106]. Role of EPS in antagonizing dreadful pathogen is new and very less literature is available on this subject. Aullybux et al. (2019) isolated EPS from bacteria residing in the marine water habitat, that displayed wide range of antibacterial activity against Acinetobacter, Bacillus, Campylobacter, Enterobacter, Enterococcus, Escherichia, Proteus, Salmonella, Streptococcus and Staphylococcus. Banerjee et al. (2020) isolated EPS producing novel thermotolerant and haloalkalophilic strain of Pseudomonas sp. PFAB4 from hot spring. Strain was able to produce 2.63 g l-1 dry weight of EPS on Kings B medium. EPS obtained from this strain was monitored for antagonistic activity against gram negative bacteria by agar cup assay. Equal ratio of AgNO3: EPS (1:1) demonstrated effective killing against gram negative bacteria E.coli. The EPS coated nanoparticles exhibited high antimicrobial property against target fungi and bacteria. Such green and eco-friendly measures could be used over conventional chemicals [10]. Tewari and Arora (2014) reported antifungal activity of P. aeruginosa PF23 EPS, against phytopathogen M. phaseolina that caused charcoal rot disease in sunflower crop under salinity stress conditions. Antimicrobial activity of EPS producing strains of P. aeruginosa B1 and B2 were monitored against gram positive and negative bacteria Onbasli and Aslim 2008).
Mixed Mechanisms Working With EPS
Multiple traits of EPS displayed by Pseudomonas are interwoven and knitted with each other. Pseudomonas strain psd is a soil bacteria present in the rhizosphere of mung bean plant. It has strong PGP and biocontrol potential. Psd is a potent EPS producer, displaying biofilm formation and aggressive root colonization. The stimulatory effect of Fluorescent Pseudomonas strain Psd on root growth has been studied by Sirohi et al. (2015). Biofilm producing Pseudomonas resulted in improved plant growth by facilitating dense bacterial population to produce various phytohormones, antibiotics, exoenzymes and secondary metabolites [60,98]. The secondary metabolites and antibiotics like Phenazine-1-Carboxylic Acid, (PCA) produced by Pseudomonas sp. contribute to the biocontrol properties. Supplementation of Zn2+ resulted in the stimulation of PCA production in P. fluorescens, which further strengthen the biocontrol potential of the strain (Slininger and Jackson 1992). Zn2+ absorption in plants is mediated by alginates in strain Psd that may also serve as important determinants in plant stimulation. Strain Psd showed an increase in the production of siderophores and phenazine in the presence of added Zn2+ in the medium, suggesting that Zn2+ modulates the production of these crucial secondary metabolites. The production of EPS is not only an advantage to the microbes but also to the soil environment in general. The adhesiveness is important for gluing soil particles together; high water locking capacity protects microorganisms and plants against drought, as well as permits the diffusions of nutrients in the environment. EPS production is also influenced by the interactions between plants and microorganisms, as it increases the availability of nutrients as a whole, promoting plant and microbial growth [23].
EPS can serve as a mechanical barrier between bacteria and plant defense compounds. EPS of Pseudomonas syringae pv. phaseolicola and S. meliloti protect the bacteria against Reactive Oxygen Species (ROS) produced by the plant host during infection, thereby decreasing oxidative stress [58,63]. Alginate, the EPS produced by P. aeruginosa, a human opportunistic pathogen, protects the bacteria against the inflammatory process of the host, by avoiding free radicals formation [90]. EPS can act as an antioxidant, but very less is known about the chemical mechanism of protection against ROS. Alginates produced by P. aeruginosa enhances bacterial survival in chlorinated water, and removal of the slime eliminates bacterial chlorine resistance (Grobe et al. 2001).
Conclusion
In the coming years, there has been a budding interest in the isolation and identification of new strains of Pseudomonas and exploiting them for harnessing EPS. Diverse species of Pseudomonas produce different exopolymers with exploitable properties. As the property of one polymer varies from other, it may provide opportunities to the researchers for further isolation, characterization and examination of many new EPS from novel strains. Till now isolation of Pseudomonas has been done from common environmental habitats including soil and water habitat, from which different Pseudomonas species and strains are isolated for routine laboratory experiments. In order to exploit novel EPS, more focus should be given on isolating new strains of Pseudomonas from atypical environment like extremophilic habitats (hydrothermal vents, hot sulphur springs) that are still untouched and unexploited. Deep-sea hydrothermal vents, marshy areas, hot deserts, salty lakes, ice frost glaciers etc may be recommended as a new source for isolating novel Pseudomonas species that could be purposefully used for isolating different exopolymers with exploitable properties. EPS from such thermopiles, alkalophiles and halophiles could be employed for remediating stressed sites, it may also serve as a game changer for designing pure green and ecofriendly emulsifiers, viscosifiers, stabilizers, gelling agents, plant growth promoters, root colonizers, pathogen controller, texture enhancers and bioformulation developers. More focus should be given in exploiting the strategies for isolating diverse EPS and, harnessing them at significantly higher rates by manipulating the current procedures using economical substrates. Hence, deeper insight is required, so as to underline the mechanisms, pathways and genetic regulation of Pseudomonas EPS. While studying novel EPS of biotechnological interest, working on various model organisms will help in investigating how different biomolecules present in the EPS are stabilized when subjected to extremities.
References
- Abouseoud M, Maachi R, Amrane A. Biosurfactant production from olive oil by Pseudomonas fluorescens. Comm Curr Res Educ Top Trends Appl Microbiol. 2007; 1: 340-7.
- Alami Y, Achouak W, Marol C, Heulin T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl Environ Microbiol. 2000; 66: 3393-8.
- Ali P, Shah AA, Hasan F, Hertkorn N, Gonsior M, Sajjad W, et al. A glacier bacterium produces high yield of cryoprotective exopolysaccharide. Front Microbiol. 2019; 10: 3096.
- Ali SZ, Sandhya V, Grover M, Linga VR, Bandi V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J Plant Inter. 2011; 6: 239-46.
- Amézketa E. Soil aggregate stability: a review. J Sustain Agric. 1999; 14: 83-151.
- Arcarons N, Vendrell-Flotats M, Yeste M, Mercade E, López-Béjar M, Mogas T. Cryoprotectant role of exopolysaccharide of Pseudomonas sp. ID1 in the vitrification of IVM cow oocytes. Reprod Fertil Dev. 2019; 31: 1507-19.
- Arockiasamy S, Banik RM. Optimization of gellan gum production by Sphingomonas paucimobilis ATCC 31461 with nonionic surfactants using central composite design. J Biosci Bioeng. 2008; 105: 204-10.
- Aullybux AA, Puchooa D, Bahorun T, Jeewon R. Phylogenetics and antibacterial properties of exopolysaccharides from marine bacteria isolated from Mauritius seawater. Ann Microbiol. 2019; 69: 957-72.
- Balíková K, Vojtková H, Duborská E, Kim H, Matúš P, Urík M. Role of exopolysaccharides of Pseudomonas in heavy metal removal and other remediation strategies. Polymers. 2022; 14: 4253.
- Banerjee S, Singh S, Pandey S, Bhandari MS, Pandey A, Giri K. Biocontrol potential of Pseudomonas azotoformans, Serratia marcescens and Trichoderma virens against Fusarium wilt of Dalbergia sissoo. Forest Pathol. 2020; 50: e12581.
- Bitton G, Freihofer V. Influence of extracellular polysaccharides on the toxicity of copper and cadmium toward Klebsiella aerogenes. Microb Ecol. 1977; 1: 119-25.
- Bouchotroch S, Quesada E, Izquierdo I, Rodriguez M, Béjar V. Bacterial exopolysaccharides produced by newly discovered bacteria belonging to the genus Halomonas, isolated from hypersaline habitats in Morocco. J Ind Microbiol Biotechnol. 2000; 24: 374-8.
- Bronick CJ, Lal R. Soil structure and management: a review. Geoderma. 2005; 124: 3-22.
- Byrd MS, Sadovskaya I, Vinogradov E, Lu H, Sprinkle AB, Richardson SH, et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol. 2009; 73: 622-38.
- Carrión O, Delgado L, Mercade E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. I. Carb Polym. 2015; 117: 1028-34.
- Celik GY, Aslim B, Beyatli Y. Characterization and production of the exopolysaccharide (EPS) from Pseudomonas aeruginosa G1 and Pseudomonas putida G12 strains. Carb Polym. 2008; 73: 178-82.
- Chattopadhyay MK, Raghu G, Sharma YV, Biju AR, Rajasekharan MV, Shivaji S. Increase in oxidative stress at low temperature in an Antarctic Bacterium. Curr Microbiol. 2011; 62: 544-6.
- Chen X, Stewart PS. Role of electrostatic interactions in cohesion of bacterial biofilms. Appl Microbiol Biotechnol. 2002; 59: 718-20.
- Wayman M, Jenkins AD, Kormendy AG. In: Ratledge C, Dawson P, Rattray J, editors. Biotechnology for the oils and fat industry. American Oil Chemists Communications’ Society, monograph no 11’ Society. Champaign. 1984; 129-14.
- Chenu C, Cosentino D. In: Ritz K, Young I, editors. Microbial regulation of soil structural dynamics. The architecture and biology of soils: life in inner space. Wallingford: CABI Publishing. 2011; 37-70.
- Chu TN, Tran BTH, Van Bui L, Hoang MTT. Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res Notes. 2019; 12: 11.
- Chug R, Mathur S, Kothari SL, Harish VS, Gour VS. Maximizing EPS production from Pseudomonas aeruginosa and its application in Cr and Ni sequestration. Biochem Biophys Rep. 2021; 26: 100972.
- Costa OYA, Raaijmakers JM, Kuramae EE. Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol. 2018; 9: 1636.
- Czaczyk K, Myszka K. Biosynthesis of Extracellular Polymeric Substances (EPS) and its role in microbial biofilm formation. Pol J Environ Stud. 2007; 16: 799-806.
- Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998; 280: 295-8.
- De Vuyst L, Zamfir M, Mozzi F, Adriany T, Marshall VM, Degeest B et al. Exopolysaccharide-producing Streptococcus thermophilus strains as functional starter cultures in the production of fermented milks. Int Dairy J. 2003; 13: 707-17.
- Decho AW. Microbial biofilms in intertidal systems: an overview. Contin Shelf Res. 2000; 20: 1257-73.
- Dimitrijevic A, Velickovic D, Rikalovic M, Avramovic N, Milosavic N, Jankov R, et al. Simultaneous production of exopolysaccharide and lipase from extremophylic Pseudomonas aeruginosa san-ai strain: A novel approach for lipase immobilization and purification. Carb Poly. 2011; 83: 1397-401.
- Divyashree M, Mani MK, Karunasagar I. Association of Exopolysaccharide genes in biofilm developing antibiotic-resistant Pseudomonas aeruginosa from hospital wastewater. J Water Health. 2022; 20: 176-84.
- Elsholz AK, Wacker SA, Losick R. Self-regulation of exopolysaccharide production in Bacillus subtilis by a tyrosine kinase. Genes Dev. 2014; 28: 1710-20.
- Fatima T, Arora NK. Pseudomonas entomophila PE3 and its exopolysaccharides as biostimulants for enhancing growth, yield and tolerance responses of sunflower under saline conditions. Microbiol Res. 2021; 244: 126671.
- Finore I, Di Donato P, Mastascusa V, Nicolaus B, Poli A. Fermentation technologies for the optimization of marine microbial exopolysaccharide production. Mar Drugs. 2014; 12: 3005-24.
- Flemming HC, Wingender J. Relevance of microbial extracellular polymeric substances (EPSs)-Part I: Structural and ecological aspects. Water Sci Technol. 2001; 43: 1-8.
- Forde A, Fitzgerald GF. Analysis of exopolysaccharide (EPS) production mediated by the bacteriphage adsorption blocking plasmid, pCI658, isolated from Lactococcus lactis ssp. cremoris HO2. Int Dairy J. 1999; 9: 465-72.
- Franklin MJ, Chitnis CE, Gacesa P, Sonesson A, White DC, Ohman DE. Pseudomonas aeruginosa AlgG is a polymer level alginate C5-mannuronan epimerase. J Bacteriol. 1994; 176: 1821-30.
- Franklin MJ, Nivens DE, Weadge JT, Howell PL. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and Psl. Front Microbiol. 2011; 2: 167.
- Freitas F, Alves VD, Carvalheira M, Costa N, Oliveira R, Reis MAM. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carb Poly. 2009; 78: 549-56.
- Freitas F, Alves VD, Reis MA. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011; 29: 388-98.
- Friedman L, Kolter R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol. 2004; 51: 675-90.
- Fu B, Yan Q. Exopolysaccharide is required for motility, stress tolerance, and plant colonization by the endophytic bacterium Paraburkholderia phytofirmans PsJN. Front Microbiol. 2023; 14: 1218653.
- García-Seco D, Bonilla A, Algar E, García-Villaraco A, Mañero JG, Ramos-Solano B. Enhanced blackberry production using Pseudomonas fluorescens as elicitor. Agron Sustain Dev. 2013; 33: 385-92.
- Ghadaksaz A, Fooladi AAI, Mahmoodzadeh Hosseini HM, Amin M. The prevalence of some Pseudomonas virulence genes related to biofilm formation and alginate production among clinical isolates. J Appl Biomed. 2015; 13: 61-8.
- Ghafoor A, Hay ID, Rehm BH. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl Environ Microbiol. 2011; 77: 5238-46.
- Gutsche J, Remminghorst U, Rehm BH. Biochemical analysis of alginate biosynthesis protein AlgX from Pseudomonas aeruginosa: purification of an AlgX-MucD (AlgY) protein complex. Biochimie. 2006; 88: 245-51.
- Hay ID, Remminghorst U, Rehm BH. MucR, a novel membrane-associated regulator of alginate biosynthesis in Pseudomonas aeruginosa. Appl Environ Microbiol. 2009; 75: 1110-20.
- Heredia-Ponce Z, Gutiérrez-Barranquero JA, Purtschert-Montenegro G, Eberl L, Cazorla FM, de Vicente A. Biological role of EPS from Pseudomonas syringae pv. syringae UMAF0158 extracellular matrix, focusing on a Psl-like polysaccharide. npj biofilms Microbio. 2020; 6: 37.
- Hidalgo-Cantabrana C, López P, Gueimonde M, de Los Reyes-Gavilán CG, Suárez A, Margolles A, et al. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob Proteins. 2012; 4: 227-37.
- Holmström C, Kjelleberg S. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Ecol. 1999; 30: 285-93.
- Hung CC, Santschi PH. Spectrophotometric determination of total uronic acids in seawater using cation-exchange separation and pre-concentration by lyophilization. Ann Chem Acta. 2001; 427: 111-7.
- Hung CC, Santschi PH, Gillow JB. Isolation and characterization of extracellular polysaccharides produced by Pseudomonas fluorescens biovar II. Carb Poly. 2005; 61: 141-7.
- Chenu C, Cosentino D. In: Ritz K, Young I, editors. Microbial regulation of soil structural dynamics. The architecture and biology of soils: life in inner space. Wallingford: CABI Publishing. 2011; 37-70.
- Jurasek L, Marchessault RH. Polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha cells: a computer simulation. Appl Microbiol Biotechnol. 2004; 64: 611-7.
- Kamali E, Jamali A, Ardebili A, Ezadi F, Mohebbi A. Evaluation of antimicrobial resistance, biofilm forming potential, and the presence of biofilm-related genes among clinical isolates of Pseudomonas aeruginosa. BMC Res Notes. 2020; 13: 27.
- Kanimozhi R, Prasath DA, Dhandapani R, Sigamani S. In vitro antioxidant and antibiofilm activities of Microcystis sp. against multidrug-resistant human pathogens. Ann Rom Soc Cell Biol. 2021; 25: 4419-30.
- Kasotia A, Varma A, Tuteja N, Choudhary DK. Amelioration of soybean plant from saline-induced condition by exopolysaccharide producing Pseudomonas-mediated expression of high affinity K+-transporter (HKT1) gene. Curr Sci. 2016; 111: 1961-7.
- Keshavarz T, Roy I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr Opin Microbiol. 2010; 13: 321-6.
- Kiliç NK, Dönmez G. Environmental conditions affecting exopolysaccharide production by Pseudomonas aeruginosa, Micrococcus sp., and Ochrobactrum sp. J Hazard Mater. 2008; 154: 1019-24.
- Király Z, El-Zahaby HM, Klement Z. Role of extracellular polysaccharide (EPS) slime of plant pathogenic bacteria in protecting cells to reactive oxygen species. J Phytopathol. 1997; 145: 59-68.
- Knoshaug EP, Ahlgren JA, Trempy JE. Exopolysaccharide expression in Lactococcus lactis subsp. cremoris Ropy352: evidence for novel gene organization. Appl Environ Microbiol. 2007; 73: 897-905.
- Koul S, Kalia VC. Multiplicity of quorum quenching enzymes: a potential mechanism to limit quorum sensing bacterial population. Indian J Microbiol. 2017; 57: 100-8.
- Koutsoudis MD, Tsaltas D, Minogue TD, von Bodman SB. Quorum-sensing regulation governs bacterial adhesion, biofilm development, and host colonization in Pantoea stewartii subspecies stewartii. Proc Natl Acad Sci USA. 2006; 103: 5983-8.
- Kumar A, Mukhia S, Kumar R. Production, characterisation, and application of exopolysaccharide extracted from a glacier bacterium Mucilaginibacter sp. ERMR7: 07. Process Biochem. 2022; 113: 27-36.
- Lehman AP, Long SR. Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J Bacteriol. 2013; 195: 5362-9.
- Lin Y, Ye G, Kuzyakov Y, Liu D, Fan J, Ding W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol Biochem. 2019; 134: 187-96.
- Li P, Li T, Zeng Y, Li X, Jiang X, Wang Y, et al. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr Polym. 2016; 151: 684-91.
- Lynch KM, Coffey A, Arendt EK. Exopolysaccharide producing lactic acid bacteria: their techno-functional role and potential application in gluten-free bread products. Food Res Int. 2018; 110: 52-61.
- Ma L, Conover M, Lu H, Parsek MR, Bayles K, Wozniak DJ. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLOS Pathog. 2009; 5: e1000354.
- Maharaj R, May TB, Wang SK, Chakrabarty AM. Sequence of the alg8 and alg44 genes involved in the synthesis of alginate by Pseudomonas aeruginosa. Gene. 1993; 136: 267-9.
- Manca MC, Lama L, Improta R, Esposito E, Gambacorta A, Nicolaus B. Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus. Appl Environ Microbiol. 1996; 62: 3265-9.
- Matsukawa M, Greenberg EP. Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J Bacteriol. 2004; 186: 4449-56.
- Mijakovic I, Grangeasse C, Turgay K. Exploring the diversity of protein modifications: special bacterial phosphorylation systems. FEMS Microbiol Rev. 2016; 40: 398-417.
- Minh Tran T, MacIntyre A, Khokhani D, Hawes M, Allen C. Extracellular DNases of Ralstonia solanacearum modulate biofilms and facilitate bacterial wilt virulence. Environ Microbiol. 2016; 18: 4103-17.
- Mishra MS, Singh RK, Chauhan S, Gupta P. Secretome of microbiota in extreme conditions. Microb Versatility Varied Environ Springer, Singapore. 2020: 85-99.
- Morcillo RJL, Manzanera M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites. 2021; 11: 337.
- Moscovici M. Present and future medical applications of microbial exopolysaccharides. Front Microbiol. 2015; 6: 1012.
- Myszka K, Czaczyk K. Characterization of adhesive exopolysaccharide (EPS) produced by Pseudomonas aeruginosa under starvation conditions. Curr Microbiol. 2009; 58: 541-6.
- Narisetty V, Prabhu AA, Al-Jaradah K, Gopaliya D, Hossain AH, Kumar Khare SK, et al. Microbial itaconic acid production from starchy food waste by newly isolated thermotolerant Aspergillus terreus strain. Bioresour Technol. 2021; 337: 125426.
- Naseem H, Ahsan M, Shahid MA, Khan N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J Basic Microbiol. 2018; 58: 1009-22.
- Naseem H, Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J Plant Inter. 2014; 9: 689-701.
- Nevot M, Deroncelé V, Messner P, Guinea J, Mercadé E. Characterization of outer membrane vesicles released by the psychrotolerant bacterium Pseudoalteromonas antarctica NF3. Environ Microbiol. 2006; 8: 1523-33.
- Niu X, Song L, Xiao Y, Ge W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front Microbiol. 2017; 8: 2580.
- Nunkaew T, Kantachote D, Nitoda T, Kanzaki H, Ritchie RJ. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym. 2015; 115: 334-41.
- Okshevsky M, Regina VR, Meyer RL. Extracellular DNA as a target for biofilm control. Curr Opin Biotechnol. 2015; 33: 73-80.
- Onbasli D, Aslim B. Determination of antimicrobial activity and production of some metabolites by Pseudomonas aeruginosa B1 and B2 in sugar beet molasses. Afr J Biotechnol. 2008; 7: 4614-9.
- Ozturk S, Kaya T, Aslim B, Tan S. Removal and reduction of chromium by Pseudomonas spp. and their correlation to rhamnolipid production. J Hazar Mat. 2012; 231: 64-9.
- Parkar SG, Flint SH, Palmer JS, Brooks JD. Factors influencing attachment of thermopilic bacilli to stainless steel. J Appl Microbiol. 2001; 11: 675-85.
- Reid AN, Whitfield C. Functional analysis of conserved gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition between Wza and Wzc for colanic acid biosynthesis. J Bacteriol. 2005; 187: 5470-81.
- Roberson EB, Chenu C, Firestone MK. Microstructural changes in bacterial exopolysaccharides during desiccation. Soil Biol Biochem. 1993; 25: 1299-301.
- Roberson EB, Firestone MK. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol. 1992; 58: 1284-91.
- Ryder C, Byrd M, Wozniak DJ. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr Opin Microbiol. 2007; 10: 644-8.
- Sah S, Krishnani S, Singh R. Pseudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr Res Microb Sci. 2021; 2: 100084.
- Saharan BS, Nehra V. Plant growth promoting rhizobacteria: A critical review. Life Sci Med Res. 2011; 21: 1-30.
- Sainju UM, Stevens WB, Caesar-TonThat T, Liebig MA, Wang J. Net global warming potential and greenhouse gas intensity influenced by irrigation, tillage, crop rotation, and nitrogen fertilization. J Environ Qual. 2014; 43: 777-88.
- Sandhya VZ, SK, Grover M, Reddy G, Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils. 2009; 46: 17-26.
- Sheng GP, Yu HQ, Li XY. Stability of sludge flocs under shear conditions: roles of extracellular polymeric substances (EPS). Biotechnol Bioeng. 2006; 93: 1095-102.
- Sheoran SK, Dubey KK, Tiwari DP, Singh BP. Directive production of pullulan by altering cheap source of carbons and nitrogen at 5l bioreactor level. Int Schol Res Not. 2012; 2012: 1-5.
- Sifour M, Al-Jilawi MH, Aziz GM. Emulsification properties of biosurfactant produced from Pseudomonas aeruginosa RB 28. Pak J Biol Sci. 2007; 10: 1331-5.
- Sirohi G, Upadhyay A, Srivastava PS, Srivastava S. PGPR mediated zinc biofertilization of soil and its impact on growth and productivity of wheat. J Soil Sci Plant Nutr. 2014; 15: 202-16.
- Slininger PJ, Dunlap CA, Schisler DA. Polysaccharide production benefits dry storage survival of the biocontrol agent Pseudomonas fluorescens S11: P: 12 effective against several maladies of stored potatoes. Biocontrol Sci Technol. 2010; 20: 227-44.
- Mu’minah H, Baharuddin, Subair H, Fahruddin. Isolation and Screening Bacterial exopolysaccharide (EPS) from potato rhizosphere in highland and the potential as a producer Indole Acetic Acid (IAA). Procedia Food Science. Proceedings of the food sci. 2015; 3: 74-81.
- Sutherland IW. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology (Reading). 2001; 147: 3-9.
- Tang J, Mo Y, Zhang J, Zhang R. Influence of biological aggregating agents associated with microbial population on soil aggregate stability. Appl Soil Ecol. 2011; 47: 153-9.
- Tewari S, Arora NK. Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr Microbiol. 2014; 69: 484-94.
- Tewari S, Arora NK. Fluorescent Pseudomonas sp. PF17 as an efficient plant growth regulator and biocontrol agent for sunflower crop under saline conditions. Symbiosis. 2016; 68: 99-108.
- Tewari S, Pooniya V, Sharma S. Next generation bioformulation prepared by amalgamating Bradyrhizobium, cell free culture supernatant, and exopolysaccharides enhances the indigenous rhizospheric rhizobial population, nodulation, and productivity of pigeon pea. Appl Soil Ecol. 2020; 147: 103363.
- Tewari S, Sharma S. Rhizobial exopolysaccharides as supplement for enhancing nodulation and growth attributes of Cajanus cajan under multi-stress conditions: A study from lab to field. Soil Till Res. 2020; 198: 104545.
- Titus S, Gasnkar N, Srivastava KB, Karande AA. Exopolymer production by a fouling marine bacterium Pseudomonas alcaligenes. Indian J Mar Sci. 1995; 24: 45-8.
- Upadhyay A, Kochar M, Rajam MV, Srivastava S. Players over the surface: unraveling the role of exopolysaccharides in zinc biosorption by fluorescent Pseudomonas strain psd. Front Microbiol. 2017; 8: 284.
- Vaishnav AM, Upadhyay KH, Tipre DR, Dave SR. Bio-prospecting of fruits waste for exopolysaccharide production by bacteria. Biotechnol Sustain Environ Springer Singapore. 2021: 353-71.
- Vardharajula S. SkZ A, Shiva KP, Vurukonda S, Shrivastava M. Curr Biotechnol. Plant growth promoting endophytes and their interaction with plants to alleviate abiotic stress. 2017; 6: 252-63.
- Vardharajula S, Zulfikar Ali S, Grover M, Reddy G, Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Inter. 2011; 6: 1-4.
- Vidhyalakshmi R, Valli Nachiyar C, Narendra Kumar G, Sunkar S, Badsha I. Production, characterization and emulsifying property of exopolysaccharide produced by marine isolate of Pseudomonas fluorescens. Biocatal Agric Biotechnol. 2018; 16: 320-5.
- Vodovar N, Vallenet D, Cruveiller S, Rouy Z, Barbe V, Acosta C, et al. Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nat Biotechnol. 2006; 24: 673-9.
- Wang B, Song Q, Zhao F, Zhang L, Han Y, Zhou Z. Isolation and characterization of dextran produced by Lactobacillus sakei L3 from Hubei sausage. Carbohydr Polym. 2019; 223: 115111.
- Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem. 2007; 282: 36777-81.
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002; 295: 1487.
- Whitfield C, Mainprize IL. TPR motifs: hallmarks of a new polysaccharide export scaffold. Structure. 2010; 18: 151-3.
- Widmer F, Seidler RJ, Gillevet PM, Watrud LS, Di Giovanni GD. A highly selective PCR protocol for detecting 16S rRNA genes of the genus Pseudomonas (sensu stricto) in environmental samples. Appl Environ Microbiol. 1998; 64: 2545-53.
- Wingender J, Neu TR, Flemming HC. What are bacterial extracellular polymeric substances? microbial extracellular polymeric substances. Berlin: Springer, Heidelberg. 1999; 1-19.
- Wolfaardt GM, Lawrence JR, Korber DR. Function of EPS. Microb Extracell Polym Subst Springer Berl, Heidelberg. 1999; 171-200.
- Yang BY, Tseng YH. Production of exopolysaccharide and levels of protease and pectinase activity in pathogenic and nonpathogenic strains of Xanthomonas campestris pv. campestris. Bot Bull Acad Sin. 1988; 29: 93-9.
- Zhou Y, Cui Y, Qu X. Exopolysaccharides of lactic acid bacteria: structure, bioactivity and associations: a review. Carbohydr Polym. 2019; 207: 317-32.
