Abstract
Molybdenum (Mo) is an essential micronutrient for most living organisms but also an emerging contaminant in the environment. A narrow range exists between critical requirements for plants (0.5 mg kg-1) and threshold toxicity of molybdenum (10 mg kg-1) to ruminants, considered as “molybdenosis”. Adsorption is one of the most important chemical processes in soils that affect the mobility of metals/contaminants in soil. To evaluate the sorption behaviour of molybdenum in soils, twenty bulk surface soil samples (0–15 cm) with diverse physical and chemical properties were collected from different parts of India. A laboratory experiment was conducted to study the sorption behaviour of Mo in soil under two temperature (20 and 300C) conditions. Five graded Mo concentrations (1, 2, 5, 10, 20, and 50 mg L-1) were prepared using (NH4)6Mo7O24 in a 0.005 M Ca(NO3)2 background solution. Maximum adsorption of Mo was observed in the soils where pH ranges from 4-5, while in alkaline soils (pH > 8) negative adsorption phenomena were found. Freundlich isotherm fitted better than Langmuir isotherm in a wide range of soil’s pH. A highly significant negative correlation was observed between soil pH and adsorption parameters, while a significant positive correlation was found between SOC and adsorption parameters. The thermodynamic parameters i.e., free energy (ΔGo), enthalpy (ΔHo), and entropy (ΔSo) were determined using sorption data in two different temperature conditions. It was observed that molybdenum sorption in soil is a spontaneous endothermic reaction. This study highlighted the role sorption mechanism in the evaluation of mobility and availability of molybdenum in different soil chemical environments.
Keywords: Adsorption; Molybdenum; Sorption isotherm; Thermodynamic parameters
Introduction
Molybdenum is an essential trace element for plants and animals for normal growth and metabolism. In plant systems, molybdenum acts as a cofactor for more than 50 enzymes; among them, five are the most important: nitrogenase, nitrate reductase, sulfite oxidase, xanthine dehydrogenase, and aldehyde oxidase [19]. Molybdenum has an indispensable role in biological nitrogen fixation, nitrogen metabolism, pollen viability, sulphur metabolism, plant hormone biosynthesis, catabolism of purine compounds, and drought resistance [18]. However, there is a narrow span exists between the nutritional deficiency of the molybdenum for the crop (0.5 mg kg-1) and the potential toxicity of molybdenum to ruminants (10 mg kg-1) [22]. Plants don’t show any toxicity symptoms when an elevated amount of Mo is present in the plant tissues. However, the toxicity of molybdenum becomes pronounced in the ruminant bodies that are grazed into those crops. The toxicity of Mo is induced by the Cu deficiency, an excess level of Mo interferes with sulfide oxidase, causing sulfide levels in the animal to increase, thus decreasing the Cu availability to the animal [7,24], a situation called "molybdenosis.". There is a safe ratio (2:1) of Cu and Mo reported in a study [21], which suggested that with lower ratios of molybdenum, Cu deficiency in ruminants is prominent, especially in cattle. Molybdenum is a geogenic element that depends primarily on the formation of rocks and the parent materials. The average concentration of molybdenum in the earth's crust is 1.1 mg kg-1, with a higher value up to 7 mg kg-1 [17]. However, a very high level of molybdenum concentration was also reported in anoxic basin sediments including the Black Sea, Cariaco Trench, Framvaren Fjord, and Saanich Inlet where molybdenum concentration ranges within 20 to 160 mg kg-1 [14]. The modern advancement in technology and rapid industrilization has resulted in a tremendous amount of effluent discharge in the water bodies. In Tyrol, Austria, molybdenum pollution has been caused by the discharge of industrial effluent in the pasture area, reaching a level of approximately 200 mg kg-1 [12]. The occurrence of molybdenum in soil mostly spiked due the anthropogenic activities, i.e., mining and industrial activities related to alloys, catalysts, ceramics, lubricants, and pigments. The recent use of waste-water irrigation as an alternative to freshwater in agricultural activities also made a path for the element to enter the soil system [9].
The mobility of any contaminant or nutrients depends on the sorption-desorption mechanism of soils. Molybdenum is mostly prevalent in MoO4-2 form under an oxidized environment [10]. Several studies reported the role of pH, clay, organic matter, and sesquioxides content of the soil in the adsorption of molybdate ions in the soil chemical environment [11,33]. Studies have found that the Molybdenum adsorption by iron or aluminum oxides increased with increasing solution pH from 2 to 4, exhibited a peak near pH 4 to 5, and decreased with increasing solution pH above 5 [8]. Marks et al. [20] showed that organic matter played an important role in controlling Mo mobility in forest soils.
The adsorption of Mo in soils can be explained by the theory of specific adsorption, in which covalent bonds are formed to some degree between soil constituents and Mo ions. Another theory used to explain the strong adsorption of Mo to oxides is ligand exchange or anion penetration [3]. Scientists also reported the inner-sphere as well as outer-sphere complexation of molybdate ions by oxides and clay minerals [32]. These results infer the roles of soil constituents in the molybdate mobility and availability in soil. To predict the toxicity of molybdenum in a soil environment it is imperative to understand the role of soil physicochemical factors on the adsorption of molybdenum. Sorption isotherms provide useful information about the soil retention capacity and the strength by which the sorbate is held onto the soil. For proper evaluation of the environmental threat posed by the molybdenum, understanding of the sorption characteristics is essential.
The distribution of metal/contaminant between soil solid and solution phases has often been described empirically, either by Freundlich, Langmuir, or other modified adsorption isotherms. Theoretically, maximum monolayer sorption, empirical adsorption constants, and other important adsorption parameters derived from sorption isotherms help in predicting the behaviour of any contaminant in the soil chemical environments [5].
The thermodynamic approach in the sorption process can provide deeper insight to predict the final state of the metal in the soil solution equilibrium [4]. Evaluation of the free energy change corresponding to the transfer of elements from bulk solution into the appropriate site of the double layer or clay mineral lattice helps in comprehending the complex sorption process. Understanding the change in enthalpy and entropy helps in determining the free energy change and disorders that occur during the sorption process [1]. Using this background knowledge, the current study aims to identify the thermodynamic parameters and molybdenum sorption behaviours that will help us better understand how molybdenum behaves in the chemical environment of soil.
Materials and Methods
Soil Sampling
In the present study, twenty bulk surface soil samples (0–15 cm) with diverse physical and chemical properties were collected from different parts of the country. The soil samples were collected from the area of New Delhi (4), Bihar (2), Haryana (3), West Bengal (5), Arunachal Pradesh (1), Assam (2) and Punjab (3). A brief descriptive information about studied soils were discussed in supplementary Table S1. The numbers within the parenthesis represents the number of sampling points from that particular state in a country. The soil samples were collected from the arable lands during fallow periods excluding the border regions (sampling locations are depicted in Figure 1).
Figure 1: Soil Sampling Locations from seven different states of India (only for illustration purposes).
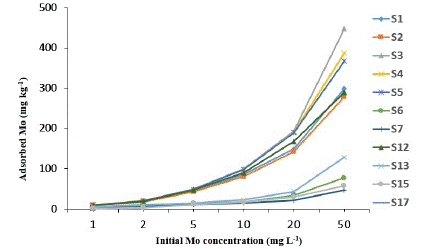
Figure 2: Comparison of Mo adsorption in different soil based on soil’s pH.
S3, S4, S5: pH 4-5; S1, S2: pH 5-6; S6, S7, S12, S13, S17: pH 6-7; S15: pH 7-8.
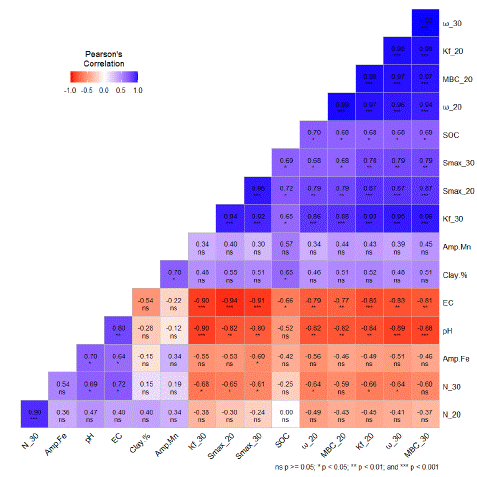
Figure 3: Correlation coefficients between Initial soil characteristics and Langmuir, Freundlich adsorption parameters of sorption for molybdenum.
pH=Initial soil pH, EC = Electrical Conductivity (μS cm-1) SOC = Soil organic carbon (%), Amp Fe= Amorphous Fe (mg kg-1), Amp Mn = Amorphous Mn (mg kg-1), Clay % = Clay (%) in soils, N_30 = Freundlich’s N constant at 30°C, N_20 = Freundlich’s N constant at 20°C, MBC_30 = Maximum Buffering Capacity at 30°C, MBC_20 = Maximum Buffering Capacity at 20°C, w_30 = Langmuir constant (ω) at 30°C, w_20 = Langmuir constant (ω) at 20°C, Kf_30 = Freundlich affinity/partitioning coefficient(Kf) at 30°C, Kf_20 = = Freundlich affinity/partitioning coefficient(Kf) at 20°C, SMAX_30 = Langmuir sorption maxima (Smax) at 30°C, SMAX_20 = Langmuir sorption maxima (Smax) at 20°C.
Soil Analysis
The soil samples were allowed to air dry at room temperature before processing. The samples were well mixed to achieve homogeneity before passing through a 2 mm sieve. The homogenized samples were used for the determination of initial soil properties and sorption experiments. The mechanical composition of soil was determined by hydrometer method [13]. The soil pH was determined in 1:2 (soil:water) suspension using the combined electrode of a digital pH meter. The electrical conductivity (EC) was determined in the supernatant liquid of the same extracts with the help of an EC meter (Eutech Instruments; model: pc510). The Soil Organic Carbon (SOC) content in soil was determined by the wet digestion method [28]. The NH4-Oxalate extraction (pH 3.3) was performed for amorphous Fe and Mn in soil which were analyzed using AAS (Atomic Absorption Spectroscopy) [13]. The total Mo of the soil was analyzed by digesting the soil with the di-acid mixture (HNO3:HClO4::3:1) followed by cooling and making the final volume with 5 % HNO3 for better compatibility under ICP-MS [2].
Molybdenum Sorption and Desorption Experiments
A laboratory experiment was conducted to study the sorption behaviour of Mo in soil under two different temperature (20 and 30 C) conditions. Five graded Mo concentrations (1, 2, 5, 10, 20, and 50 mg L-1) were prepared using of (NH4)6Mo7O24 in a 0.005 M Ca(NO3)2 background solution without adjusting the pH to maintain a constant ionic strength. About 3 g of air-dry soil in triplicate was suspended in 30-ml solution in Teflon centrifuge tube. The mixtures was shaken for 24 h at 150 rpm on an environmental shaker and subsequently centrifuged for 15 min to get a clear supernatant. After that, the supernatant was filtered through Whatman No 42 filter papers before analysing in ICP-MS.
Desorption of Mo was made from the adsorbed soils where highest concentration of Mo (50 mg L-1) was added and this was carried out through sequential dilution to encourage Mo desorption. Desorption of Mo was initiated from adsorbed soil suspension by replacing the 20 ml aliquot with 20 ml of 0.005M Ca(NO3)2 background solution. Five steps of desorption were conducted after every 24 h, 48 h, 72 h, 96 h and 120 h. After shaking, the samples were centrifuged at 5000 rpm for 15 min, and filtered through Whatman no. 42 filter paper, and equilibrium concentration of Mo was measured using ICP-MS. The amount of Mo desorbed from the soils were calculated on the basis of change in concentration of solution (before and after desorption).
Standard metal solutions of Merck KGaA, 64271 Darmstadt (Germany) were used to calibrate the ICP-MS. Standard solutions were also tested after each batch of 20 samples to check the stability of the reading. All the soil samples were analyzed in triplicate.
Data Analysis
Adsorbed Mo: Using the following formula, the amount of Mo adsorbed was calculated as the difference between the Mo added and that which remained in the equilibrium solution.
X/m = (Ci-Ce) X V/G
X/m = Amount of Mo adsorbed (mg kg-1 soil)
V = volume of solution (ml)
G = Weight of soil (g)
Ci = Mo concentration in added solution (mg L-1)
Ce = Mo concentration in equilibrium solution (mg L-1)
2.4.2 Adsorption equations/isotherms
2.4.2.1 Langmuir’s adsorption isotherm equation
X = ωSmaxC/(1+ ωC)
C/X = C/Smax + 1/ωSmax (Linear form)
X = (Total) Amount of Mo retained by the soil (mg kg-1)
C = Equilibrium Mo concentration (mg L-1)
Smax = Adsorption maximum (mg kg-1)
ω = Langmuir coefficient related to binding strength (L mg -1)
Freundlich’s adsorption isotherm equation
X = Kf CN
Log X = N Log C + Log Kf (Linear form)
X = (Total) Amount of Mo retained by the soil (mg kg-1)
C = Equilibrium Mo concentration (mg L-1)
Kf= Partitioning coefficient/ affinity constant (L kg -1)
N = Dimensionless reaction order (1/n)
Maximum buffering capacity: Maximum buffering capacity (MBC) was calculated as a product of adsorption maxima (Smax) and Langmuir constant (ω), an affinity term related to bonding energy (Holford and Mattingly, 1976).
Thermodynamic parameters for adsorption: The thermodynamic parameters i.e., equilibrium constant Ko, free energy ΔGo, enthalpy Ho and entropy ΔSo were determined using sorption data at two different temperatures
The standard free energy (ΔGo) was calculated as follows:
ΔGo = -RT ln Ko
R= Universal Gas Constant; (0.001987 kcal K-1 mole-1)
T=Temparature, kelvin
Ko = Equilibrium Constant (Freundlich’s equation Kf was used here)
The standard enthalpy (ΔHo) was obtained from integrated form of the Vant Hoff equation:
ln Ko2 / Ko1 = - ΔHo/R (1/T2 - 1/T1 )
Ko1 and Ko2 are the Freundlich Kf value at temperature 293 and 303 K
The standard entropy (ΔSo) was calculated as
ΔSo = (ΔHo - ΔGo) / T
Desorption index: A desorption index was used to describe the desorption characteristics (DI) and it is the ratio of Freundlich slope for adsorption and desorption.
DI = (n des)/(n ads)
Statistical Analysis
In order to estimate the Pearson’s correlation matrix for different soil physicochemical properties with the soil adsorption parameters the R (4.3.2) software had been used.
Results and Discussions
Initial Soil Properties
The soil samples collected from different parts of the country showed a diverse nature in their physicochemical properties (Table 1). The mean pH of the studied soil samples ranged between 4.61 and 8.96 (Table 1). The lowest (4.61) and highest (8.96) mean pH values were observed in S4 (Jorhat, Assam) and S11 (Fategarh sahib, Punjab), respectively. The mean EC (μs cm-1) of the studied soil samples ranged between 75.8 (S3, S5) and 342 (S11). The mean clay percentage ranged from 6 to 46%, showing various types of soil texture. The investigated soil was sandy loam to clay loam in nature. The mean organic carbon of the soil ranged from 0.32 to 1.68% (Table 1). The highest (1.68%) and lowest (0.32%) mean soil organic carbon were recorded in S4 (Jorhat, Assam) and S10 (Mahendragarh, Haryana), respectively. Mean amorphous Fe and Mn ranged from 2964 to 9865 mg kg-1 and 0.7 to 11.5 mg kg-1, respectively. The total Mo level of the investigated soils ranged from 0.57 to 1.65 mg kg-1 (Table 1). The maximum (1.65) and minimum (0.57) mean total Mo were observed in S3 (Longding, Arunachal Pradesh) and S16 (Madanpur, Delhi), respectively.
Location
pH
EC (μS/cm)
Clay (%)
Texture
SOC (%)
Amorphous Fe (ppm)
Amorphous Mn (ppm)
Total Mo (mg kg-1)
S1
5.39±0.04
103.2±1.95
31±1.52
Clay loam
1.15±0.05
5411±211
4±0.21
0.97±0.015
S2
5.82±0.07
117.6±3.75
16±1
Sandy Loam
0.85±0.025
7492±172
1.13±0.40
0.58±0.033
S3
4.71±0.07
75.8±5.36
46±1.53
Clay loam
1.31±0.03
8496±381
10.53±0.95
1.65±0.05
S4
4.61±0.03
105.4±5.10
20±1
Sandy Loam
1.68±0.15
5095±461.5
4.73±0.35
1.21±0.025
S5
4.77±0.06
75.8±5.35
34±2.08
Clay loam
1.17±0.05
2964±136.5
0.83±0.35
0.71±0.02
S6
6.71±0.09
186.76±5.4
6±1
Loamy Sand
0.66±0.04
7989±451.4
1.9±0.4
0.82±0.047
S7
6.89±0.08
176.8±5.20
11±1.52
Sandy Loam
0.57±0.03
9495±868
0.7±0.36
0.95±0.073
S8
8.12±0.03
327.1±5.20
16±1.15
Loamy sand
0.48±0.02
8883±276.8
2.89±0.28
1.30±0.054
S9
8.38±0.04
221.5±4.85
6±0.57
Loamy sand
0.38±0.02
8365±292.9
1.4±0.6
0.94±0.067
S10
8.87±0.03
318.4±5.56
11±1.52
Silt Loam
0.32±0.02
8910±118.7
2.6±0.46
0.75±0.055
S11
8.96±0.040
342.4±3.44
15±1
Sandy Loam
0.40±0.03
7427±268.30
2.26±0.321
0.780±0.035
S12
6.04±0.2
121.33±2.46
23±1.53
Loam
1.12±0.1
7788±378
4.07±0.45
1.65±0.08
S13
6.51±0.03
166.76±2.98
20±1.52
Loam
0.94±0.08
9865±361.95
6.4±0.75
1.431±0.155
S14
7.93±0.07
193.3±6.83
24±2
Loam
0.72±0.04
8426±415.8
3.93±0.65
0.614±0.110
S15
7.63±0.08
169.5±3.78
44±1.52
Sandy Clay
1.34±0.07
9103±394.94
7.16±0.351
0.908±0.092
S16
7.96±0.07
201.6±7.31
6±1.52
Sandy Loam
0.516±0.03
6462±279.94
3.33±0.21
0.570±0.144
S17
6.63±0.07
214.93±4.55
17±2
Silt Loam
0.62±0.045
7786±410
2.45±0.53
0.974±0.088
S18
8.36±0.075
299.7±3.80
18±1.52
Sandy loam
0.45±0.045
5180±573.7
0.73±0.35
0.970±0.054
S19
8.30±0.055
286.3±4.45
16±1.52
Sandy Loam
0.56±0.051
3535±344
1.5±0.62
0.837±0.040
S20
7.82±0.042
191.2±3.05
26±1
Sandy clay loam
0.69±0.05
8969±542
3.13±0.40
0.869±0.060
Table 1: Initial physicochemical properties of the investigated soils.
Sorption of Molybdenum in Soils
Adsorbed Mo: The adsorption characteristics of Mo were shown in Figure 1 based on the variation of soil pH levels. It was observed that the soils that are acidic, ranges 4-5, showed maximum Mo adsorption. After pH 5, Mo adsorption showed a decreasing trend, and negative adsorption was recorded after pH 8. Hence, only 11 soils out of 20 were plotted for adsorption characteristics. This adsorption behaviour of Mo suggests that the higher the soil pH, the lower the adsorption of molybdenum will occur that gradually increase the availability of molybdenum in the soil solution. Similar findings also presented by Goldberg et al. [8], they concluded that molybdenum adsorption on both Al and Fe oxides exhibited a maximum value at a low pH of about 4 to 5. Above pH 5 adsorption decreased rapidly, while little adsorption occurred above pH 8.
Adsorption isotherm: Adsorption data of eleven soils were fitted to the Freundlich and Langmuir equations. The mean values of equation parameters were represented in Table 2. Langmuir and Freundlich equations are the two major types of isotherms used to describe the Mo adsorption process. The Langmuir equation is based on the kinetic theory of gaseous adsorption onto solids, but is often used to model the adsorption of ions from solution [6]. Langmuir adsorption isotherm is based on a hypothesis that the energy of adsorption on a uniform surface is independent of surface coverage [25]. In our present study the Langmuir adsorption isotherms were fitted well in the case of S1, S2, S3, S4, S5, S12 and S17 in two different temperature conditions (Table 2). In those soils the R2 was found more than 0.85. However, S6, S7, S13, and S15 soils were not fitted well to Langmuir equations. The adsorption parameters in Langmuir equations have been increased due to an increase in temperature because of an increasing number of adsorption sites. The sorption maxima (Smax) in the Langmuir equation ranged from 78.7 (S17) to 500 mg kg-1 (S3) and 161 (S17) to 523 mg kg-1(S1) under 20 and 30 °C, respectively. The Langmuir constant (ω) that is related to the bonding energy in the Langmuir equation ranged from 0.01 (S15) to 1.66 L mg-1 (S3) and 0.02 (S15) to 2.50 L mg-1 (S3) under 20 and 30 °C, respectively. The MBC (Maximum Buffering Capacity) derived from the Langmuir equation ranged from 2.16 (S15) to 833 (S3) and 4.19 (S15) to 1250 (S3) under 20 and 30 °C, respectively.
Soils
Langmuir
Freundlich
Smax
(mg Kg-1)ω
(L mg -1)MBC
R2
N
Kf
R2
20°C
30°C
20°C
30°C
20°C
30°C
20°C
30°C
20°C
30°C
20°C
30°C
20°C
30°C
S1
370
526
0.23
0.68
86.2
357
0.86
0.82
0.65
0.60
58
153
0.99
0.99
S2
345
417
0.18
0.77
61.0
323
0.97
0.98
0.65
0.57
43.9
130
0.84
0.99
S3
500
500
1.66
2.50
833
1250
0.98
0.98
0.59
0.55
219
263
0.91
0.96
S4
400
454
1.56
2.00
625
909
0.99
0.99
0.49
0.51
153
196
0.94
0.96
S5
385
417
1.36
1.50
526
625
0.99
0.99
0.51
0.51
137
156
0.94
0.96
S6
100
164
0.05
0.08
4.91
12.6
0.51
0.80
0.54
0.60
8.09
14.5
0.92
0.98
S7
56.8
137
0.06
0.06
3.63
8.30
0.78
0.81
0.50
0.62
5.85
10.1
0.98
0.98
S12
312
357
0.57
0.51
178
182
0.99
0.98
0.51
0.53
76.8
83.9
0.98
0.99
S13
222
232
0.02
0.03
5.24
6.68
0.27
0.36
0.66
0.68
8.05
9.20
0.93
0.96
S15
149
222
0.01
0.02
2.16
4.19
0.64
0.69
0.92
0.86
2.17
4.43
0.97
0.97
S17
78.7
161
0.05
0.04
3.72
5.84
0.94
0.91
0.74
0.78
3.95
6.22
0.97
0.98
Table 2: Langmuir and Freundlich adsorption parameters for the Mo sorption process in the investigated soils at 20°C and 30°C.
Freundlich equation is one of the widely used adsorption equations to describe the experimental data on the adsorption of ions or molecular species in soil. This mathematical model is based on the hypothesis that the bonding energy of adsorption decreases logarithmically as the fraction of covered surface increases [27]. The main disadvantage of Freundlich adsorption isotherm is it can’t predict any adsorption maxima. In our present investigation, the Freundlich adsorption isotherms were fitted very well (R²>0.9) for all eleven soils under two different temperature conditions. Kf and N are two empirical constants derived from the Freundlich equations that were sensitive to the given adsorbent-adsorbate system and temperature conditions. The Kf in Freundlich equation represents the affinity value or relative exchangeability of ions. The affinity constant (Kf) in the Freundlich equation ranged from 2.17 (S15) to 219 (S3) and 4.43 (S15) to 263 (S3) under 20 and 30 °C, respectively. The N (1/n) value in the Freundlich equation is always in between 0 to 1. If the N value is approached to zero, it indicates that sites have been saturated. On the other hand, if the N value approaches 1, it suggests a linear curve where adsorption continues proportionately with the concentration (Sanyal, 2001). In our investigation, the N value of the Freundlich equation ranges from 0.49 (S4) to 0.92 (S15) and 0.51 (S4, S5) to 0.86 (S15) under 20 and 30°C, respectively.
Correlation of Mo Sorption Parameters with Initial Soil Properties
Pearson’s correlation matrix was presented in Fig 3 between soil physicochemical properties and the molybdenum sorption parameters to understand the role of soil parameters in the molybdenum sorption process. It was observed that pH had a significant negative correlation with the Langmuir and Freundlich adsorption parameters. The soil pH recorded a significant negative correlation of -0.82**, -0.80** with the Smax, -0.82**, -0.89** with ω, -0.84**, -0.90*** with Kf and -0.82**, -0.88*** with MBC at 20 and 30 °C, respectively. The soil organic carbon recorded significant positive correlation of 0.72*, 0.69* with the Smax, 0.70*, 0.68* with ω, 0.68*, 0.65* with Kf and 0.68*, 0.68* with MBC at 20 and 30 °C, respectively. Amorphous Fe, Mn and soil texture did not show any significant correlation with the adsorption parameters in Mo sorption process. The result of this study is consistent with the previous experiments by Sun and Salim, 2020. The findings of our experiment suggested that the adsorption of molybdenum in soil is a function of soil’s pH. The decreased absorption of molybdenum in the alkaline soil is because of the low activity of Fe and Al oxides [15], along with increased competition of OH- for the adsorption sites [30]. The positive correlation with the soil organic matter suggesting that soil organic matter can also significantly impact molybdenum availability through ligand exchange and specific adsorption processes [29]. Other studies have also pointed out the potential importance of organic matter-Mo associations [31]. Yang and Wang [33] conducted molybdenum sorption experiment by collecting 11 different soil series from Taiwan with diverse soil physicochemical properties and observed that soil pH had significant negative correlation with all the adsorption parameters and positively correlated with amorphous iron and aluminum oxides and no significant correlation with soil organic carbon and clay.
Evaluation of Thermodynamic Parameters
Evaluation of thermodynamic parameters i.e., Kº, ΔGº, ΔHº, ΔSº provide an insight into mechanism of Mo sorption in the soils. The data in Table 3 indicate that value of Kº increased with the rise in temperature from 20 to 30 ºC in all the studied soils. The ΔGº values for Mo were negative in all the soils (Tables 3) at two temperature conditions. These negative values indicate that the sorption process was spontaneous in nature. The ΔGº at 20 ºC and 30 ºC ranged from -0.80 (S17) to -3.14 (S3) and -0.88 to -3.35 kcal mol-1, respectively. In all the soils, the free energy (ΔGº) of the Mo sorption was more negative at higher temperature which suggested that the spontaneity of the process increased with rise in temperature [16]. The values of change in enthalpy (ΔHº) of Mo sorption were positive and ranged from 1.56 to 19.17 kcal mol-1 (Table 3). This indicates that sorption reaction was endothermic. The values of ΔSº for molybdenum sorption were positive and ranged from 0.0121 to 0.0729 kcal K-1 mol-1 (The values of ΔSº at 20 and 30 C were found similar). The positive values of ΔSº indicates an increased randomness/disorder at solid-solution interface during the adsorption of molybdenum. Adhikari and Singh [1] conducted sorption experiments for two contaminants i.e., Pb and Cd to evaluate their thermodynamic behaviour. They observed that ΔGº for both Pb and Cd were spontaneous, however ΔHº were positive for Pb suggesting endothermic and negative for Cd suggesting exothermic reaction.
Soils
K0
ΔG
(kcal Mol-1 )ΔH
(kcal mol-1)ΔS
(kcal-1 K-1Mol-1)20°C
30°C
20°C
30°C
20°C
30°C
S1
58.1
153
-2.36
-3.03
17.15
0.066611
0.06661
S2
43.9
130
-2.20
-2.93
19.17
0.072944
0.072944
S3
219
263
-3.14
-3.35
3.25
0.021802
0.021802
S4
153
196
-2.93
-3.18
4.38
0.024955
0.024955
S5
137
156
-2.86
-3.04
2.32
0.017707
0.017707
S6
8.04
14.5
-1.21
-1.61
10.47
0.039901
0.0399
S7
5.85
10.1
-1.01
-1.39
9.64
0.036429
0.036428
S12
76.9
84
-2.53
-2.67
1.56
0.013952
0.013952
S13
8.05
9.20
-1.21
-1.34
2.34
0.012146
0.012146
S15
1.96
4.30
-0.39
-0.88
13.90
0.048768
0.048768
S17
3.95
6.22
-0.80
-1.10
8.01
0.030071
0.030071
Table 3: Thermodynamic parameters of the molybdenum sorption process in the investigated soils.
Desorption of Molybdenum from Soils
The desorption process of molybdenum at two different temperature conditions presented in Figure 4. The desorption curves exhibited deviations from the corresponding sorption curves (i.e., sorption hysteresis), revealing the sorption irreversibility of molybdate in soils. To quantify the sorption irreversibility, the hysteresis indexes (HI; θ) of the desorption curves were determined [33]. The deviation of the θ values from unity indicates sorption irreversibility; the lower or more the θ value from the unity, the more difficult it is for molybdate to desorb [5]. The hysteresis index of the investigated soil was presented in the table 4 at two temperature conditions. The HI (θ) ranged from 0.93 to 8.12 at 20°C and 0.67 to 6.78 at 30°C condition. In the present investigation, acidic soils recorded the higher value of hysteresis index suggesting ligand exchange or specific adsorption. In alkaline soils lower value of hysteresis index might be due to anion exchange or non-specific adsorption.
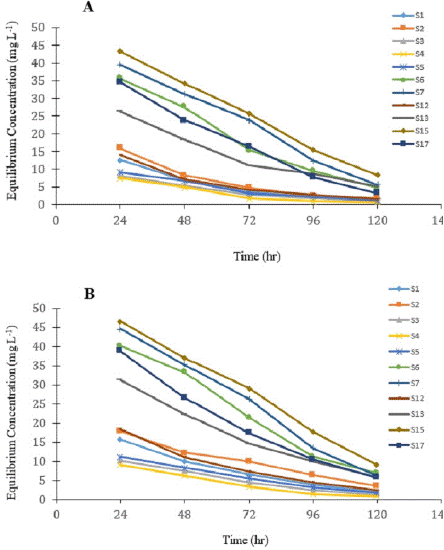
Figure 4: Desorption of Mo from the studied soils at two different temperature condition A 20°C and B. 30°C.
S3, S4, S5: pH 4-5; S1, S2: pH 5-6; S6, S7, S12, S13, S17: pH 6-7; S15: pH 7-8.
Soils
20°C
30°C
S1
5.24
3.98
S2
4.30
2.62
S3
8.12
5.93
S4
8.09
6.78
S5
5.93
4.51
S6
0.99
1.02
S7
0.75
0.68
S12
3.72
2.70
S13
1.73
1.39
S15
0.93
0.67
S17
1.76
1.19
Table 4: Hysteresis index (θ) of molybdenum sorption in different soils.
Conclusion
The goal of this investigation is to better understand molybdenum's sorption behaviour. Twenty distinct physical and chemical characteristics of soils across the country have been collected. Out of the twenty soils, only eleven exhibited molybdenum adsorption behaviour. The highest Mo adsorption was detected in soils with pH values between 4-5, whereas negative adsorption was detected in alkaline soils (pH > 8). In a wide range of the soil's pH, Freundlich isotherm fitted better than Langmuir isotherm. The adsorption parameters and soil pH showed a highly significant negative correlation, but the adsorption parameters and SOC showed a significant positive correlation. It was discovered that molybdenum sorption in soil is an endothermic reaction that occurs spontaneously while analyzing the thermodynamic characteristics. The study found that the soil's sorption capacity increased as the temperature rose. Neutral to alkaline soils showed HI value close to 1, suggesting reversible adsorption. Acid soils recorded higher deviation than unity suggesting hysteretic desorption behaviour. The importance of soil characteristics in controlling molybdenum mobility within a soil's chemical environment was highlighted in this study. Comprehending the molybdenum sorption mechanisms is beneficial in formulating appropriate approaches against heavy metal contamination.
Author Statements
Acknowledgements
This research was carried out as a part of Ph.D. dissertation by the first author. The author is thankful to the Indian Council of Agricultural Research for the ICAR-SRF fellowship for the above mention period.
Data Availability
Raw data that support the results of this study are available from the first and corresponding author upon reasonable request.
Competing Interest
The authors have no known competing financial or non-financial interest to disclose.
References
- Adhikari T, Singh MV. Sorption characteristics of lead and cadmium in some soils of India. Geoderma. 2003; 114: 81-92.
- Arora CL, Bajwa MS. Comparative study of some methods of oxidation of plant materials for elemental analysis. Current Science. 1994; 25: 314-7.
- Bohn HL, McNeal BL. O Connor, GA Soil Chemistry. New York. 1985; 329.
- Dandanmozd F, Hosseinpur AR. Thermodynamic parameters of zinc sorption in some calcareous soils. Journal of American science. 2010; 6: 298-304.
- Datta SP, Bhadoria PB. Boron adsorption and desorption in some acid soils of West Bengal, India. Journal of Plant Nutrition and Soil Science. 1999; 2: 183-91.
- Ellis BG, Knezek BD. Adsorption reactions of micronutrients in soils. 1972; 59-78.
- Gengelbach GP, Ward JD, Spears JW. Effect of dietary copper, iron, and molybdenum on growth and copper status of beef cows and calves. Journal of Animal Science. 1994; 72: 2722-2727.
- Goldberg S. Influence of soil solution salinity on molybdenum adsorption by soils. Soil science. 2009; 174: 9-13.
- Gupta SS, Saini T, Misra S, Ghosh A. Waste to wealth: Agricultural prospect. In: Waste Management for Sustainable and Restored Agricultural Soil. Academic Press. 2024; 311-321.
- Gupta UC, editor. Molybdenum in agriculture. Cambridge University Press. 1997.
- Gustafsson JP, Tiberg C. Molybdenum binding to soil constituents in acid soils: An XAS and modelling study. Chemical Geology. 2015; 417: 279-288.
- Halmi MIE, Ahmad SA. Chemistry, biochemistry, toxicity and pollution of molybdenum: A mini review. Journal of Biochemistry, Microbiology and Biotechnology. 2014; 2: 1-6.
- Jackson ML. Soil chemical analysis: advanced course: a manual of methods useful for instruction and research in soil chemistry, physical chemistry of soils, soil fertility, and soil genesis. UW-Madison Libraries parallel press. 2005.
- Jacobs JA. The Earth’s core. Academic Press. 1987.
- Jiang W, Yang Z, Yu T, Hou Q, Zhong C, Zheng G, et al. Evaluation of the potential effects of soil properties on molybdenum availability in soil and its risk estimation in paddy rice. Journal of Soils and Sediments. 2015; 15: 1520-30.
- Jurinak JJ, Bauer N. Thermodynamics of zinc adsorption on calcite, dolomite and magnesite-type minerals. Soil Science Society of America Journal. 1956; 20: 466-71.
- Kabata-Pendias A, Szteke B. Trace elements in abiotic and biotic environments. Taylor & Francis. 2015; 468.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4247040/
- Manuel TJ, Alejandro CA, Angel L, Aurora G, Emilio F. Roles of molybdenum in plants and improvement of its acquisition and use efficiency. Hossain M A, Kamiya T, editors. In: Plant micronutrient use efficiency. Academic Press. 2018; 137-159.
- Marks JA, Perakis SS, King EK, Pett-Ridge J. Soil organic matter regulates molybdenum storage and mobility in forests. Biogeochemistry. 2015; 125: 167-183.
- Miltimore JE, Mason JL. Copper to molybdenum ratio and molybdenum and copper concentrations in ruminant feeds. Canadian Journal of Animal Science. 1971; 51: 193-200.
- Neunhäuserer C, Berreck, M, Insam H. Remediation of soils contaminated with molybdenum using soil amendments and phytoremediation. Water, air, and soil pollution. 2001; 128: 85-96.
- Sanyal SK. Colloid chemical properties of soil humic substances: A relook. Journal of the Indian Society of Soil Science. 2001; 49: 537-69.
- Smith GM, White CL. A molybdenum–sulfur–cadmium interaction in sheep. Australian journal of agricultural research. 1997; 48: 147-154.
- Sparks DL, editor. Soil physical chemistry. CRC press. 1998.
- Sun W, Selim HM. Kinetic modeling of molybdenum sorption and transport in soils. Environmental Science and Pollution Research. 2020; 27: 20227-34.
- Tan KH. Principles of soil chemistry. CRC press. 2010.
- Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil science. 1934; 37: 29-38.
- Wichard T, Mishra B, Myneni SC, Bellenger JP, Kraepiel AM. Storage and bioavailability of molybdenum in soils increased by organic matter complexation. Nature Geoscience. 2009; 2: 625-9.
- Wu CH, Kuo CY, Lin CF, Lo SL. Modeling competitive adsorption of molybdate, sulfate, selenate, and selenite using a Freundlich-type multi-component isotherm. Chemosphere. 2002; 47: 283-92.
- Wurzburger N, Bellenger JP, Kraepiel AM, Hedin LO. Molybdenum and phosphorus interact to constrain asymbiotic nitrogen fixation in tropical forests. PloS one. 2012; 7: e33710.
- Xu NA, Braida W, Christodoulatos C, Chen J. A review of molybdenum adsorption in soils/bed sediments: speciation, mechanism, and model applications. Soil and Sediment Contamination: An International Journal. 2013; 22: 912-29.
- Yang PT, Wang SL. Sorption and speciation of molybdate in soils: Implications for molybdenum mobility and availability. Journal of Hazardous Materials. 2021; 408: 124934.
