
Review Article
Austin J Environ Toxicol. 2015;1(1): 5.
Can Plant - Herbivore Interaction be affected by Selenium?
Mechora Š1* and Ugrinović K2
1Department of biology, University of Maribor, Slovenia
2Agricultural Institute of Slovenia, Slovenia
*Corresponding author: Mechora Š, Department of biology, University of Maribor, Koroška cesta 160, 2000 Maribor, Sloveni
Received: September 03, 2014; Accepted: September 17, 2014; Published: February 12, 2015
Abstract
Selenium acts as an antioxidant, therefore in low concentrations increase the plant tolerance to drought, salinity, UV induced stress and metals. All these can be beneficial for the growth of plants and can positively affect their yield. In higher concentrations it can be toxic, but some plants can tolerate elevated concentrations. These are called Se accumulators. High selenium tissue concentration may affect other species, such are herbivores. Previous studies demonstrated that insects do accumulate selenium, but the effects of this element on insect growth and survival is limited. Laboratory studies have shown that selenate incorporated into the diet affects feeding site preferences and host plant selection, reduce growth and acts as antifeedant for larvae of the generalist herbivory beet armyworm (Spodoptera exigua). Selenium accumulation in tissues protect plants against caterpillars of cabbage looper (Trichoplusia ni), larvae of the cabbage white butterfly (Pieris rapae), green peach aphids (Myzus persicae), nematodes and prairie dogs. Therefore selenium can reduce herbivore's growth, cause toxic effects or deter herbivores, and at the same time positively affects plant growth.
Keywords: Selenium; Se accumulator; Herbivore; Pest; Nematode
Introduction
Selenium (Se) is a trace element, which is essential for humans and animals, while its essentiality for higher plants has not yet been proven. Many plant species grow on soils with moderate concentrations of Se, while some can tolerate high concentrations and even accumulate it. High Se concentrations occur naturally in some soils, especially those derived from Cretaceous shale parent material. Se content of soils ranges from almost zero up to 1250 mg Se kg-1 in some seleniferous soils in Ireland [1], but Se accumulator plants can tolerate elevated concentrations and even need it for their growth [2]. Se accumulators are predominantly found on Se-rich soils as seen in the western United States [3]. Many hypotheses for the functional significance of metalloid accumulation by plants have been proposed, including allelopathy, drought resistance and protection from both herbivores and pathogens [4]. The latter hypothesis is termed the elemental defense hypothesis and many studies confirmed it.
Higher concentrations of Se can be toxic for organisms. Se pollution of soil and water is a major environmental problem in many areas of the world [5]. Se can leak in the environment from soil with the irrigation of crops. Other anthropogenic activities also lead to Se pollution of water. The oxidized forms of Se, selenite and selenate, are the most soluble and predominate in natural water systems. Because of their high solubility, these forms are more available for plants [6] and are potentially toxic to organisms. Once in the aquatic environment, Se can rapidly attain levels that are toxic to wildlife because of its bioaccumulation in food chains and the resulting dietary exposure [7]. Aquatic insect larvae, as a significant component of the benthos, can be important carriers of trace elements (including Se) from sediment into the water column and to the terrestrial food chains.
Insects have great importance in most ecosystems and a significant contribution to the biological cycling of trace elements. Se accumulator plants obtain high levels of this element and since they are a part of the food chain, it is possible that Se in the tissue affect herbivores. This paper gives first a short background on the Se and its effects on plants and then brings a detailed overview of studies dealing with the Se effect on herbivores.
Selenium - the powerful versatile trace element
Se was discovered in 1817 by the Swedish chemists Jöns Jakob Berzelius. The name for this trace element comes from the Greek word for moon - selene. Se is one of metalloids, which is found in the Earth's crust, soils, minerals, in freshwater, seawater, and in sediments. It is present in organic and inorganic compounds in four oxidation states - elemental (Se0), selenite (Se+4), selenate (Se+6) and selenide (Se-2) [8], while in nature it is found in various volatile (dimethylselenide, dimethyldiselenide) and other organic compounds (SeMet, SeCys, etc.) [9]. The form of Se present in a given system depends on soil properties, including pH, redox potential, salinity and the content of calcium carbonate (CaCO3) [10]. Se is unevenly distributed over the globe. Some parts of the world contain deficit Se concentrations of 0.01 mg kg-1 at the Russian Plane to heavily toxic values of 1200 mg kg-1 in organic soils at Meath, Ireland [11]. In acid soils it is mainly present in the form of selenite, while in alkaline soils it is present in the form of selenate, which is more soluble and more available for uptake [12].
The perception of the element evolved since its discovery in 19th century. At first, Se was known as toxic and carcinogenic element. This perception changed when Schwarz and Foltz discovered its essentiality for humans and animals in 1957 [13]. Since then it is used in industry, pharmacy as well as in agronomy. Agricultural applications of Se include an additive and dietary supplement in animal feeds. In part of the world where soils are deficient with Se, the addition of Se to fertilizers and top dressings [14] is a good way to improve Se status. In Finland, Se-enriched fertilizers have been used in plant production since 1984 to increase Se content in agricultural plants [15].
The margin between Se essentiality and toxicity is rather narrow. At higher concentrations, Se can be toxic due to its chemical similarity to sulfur (S), leading to nonspecific replacement of S by Se in proteins, causing toxicity [16]. Still today, chronic or acute Se poisoning is responsible for the loss of thousands of head of livestock every year in the western United States [3], where Se is prevalent in soils. Selenosis in animals mostly is due to ingestion of Se accumulator plants. There are many studies regarding Se toxicity in farming animals, but only few investigated the effect of Se on invertebrate herbivores.
Beneficial effects of selenium on plants
Se has been found an essential element for green algae Chlamydomonas reinhardtii [17]. For higher plants, it has not yet been shown to be an essential but it is considered a beneficial nutrient [18]. In plants, it functions as an antioxidant [19].
Many plant species grow on soils with moderate concentrations of Se, while some plants can tolerate high concentrations and even accumulate it. These plants are so called accumulators of Se, among which are Stanleya pinnata (Brassicaceae) and Astragalus bisulcatus (Fabaceae). They can accumulate from hundreds to several thousand milligrams of Se kg-1 dry weight [2]. Primary accumulators accumulate more than 1000 mg Se kg-1 dry weight, while secondary Se accumulators accumulate up to 1000 mg Se kg-1 dry weight [2], among which is Brassica g-1 (Brassicaceae). Non accumulator plants are able to concentrate toxic selenate and Se proteins as a result of fortification with Se [20], but to a lower extent as Se accumulators. Especially plants containing a lot of S can accumulate considerable amounts of Se without suffering substantial damage. Plants from Brassicaceae family are known to be rich in S, therefore they can absorb greater amount of Se from their surroundings. For this the family has been widely studied for supplementation of inorganic Se to cultivated plants; i.e. turnip (Brassica campestris) [21], cabbage (B. oleracea (capitata group)) [22] and broccoli (B. oleracea (italica group)) [23].
In moderate concentrations Se can increase the plant tolerance to UV-induced oxidative stress, delay senescence and promote the growth of aging seedlings [24]. It also increases plant tolerance to drought [25], salinity [26] and metals [27]. All these can contribute to higher yield of better quality. There is mounting evidence that Se can also protect plants from biotic stress.
How does selenium affect herbivores?
Although there are many studies investigating the effect of Se on plants, not many are focusing on the influence of plant accumulated Se to herbivores. The elemental defense hypothesis explains that some elements, including Se, hyper-accumulate in plants; can act as chemical defense against herbivores [4]. Several studies have confirmed this hypothesis and demonstrated that these elements can deter herbivores from feeding on plants, inhibit their growth, affect their reproduction or cause their death. Same protective effect has been confirmed also for Se (Figure 1) within the studies that have focused on the interactions between insect herbivores and Se accumulator plants, Stanleya pinnata, Brassica g-1 (Brassicaceae) and Astragalus bisulcatus (Fabaceae) [28-31]. Se accumulation has been found to have an effect on aphids, caterpillars, moths, grasshoppers, crickets, nematodes and prairie dogs [28,30-35]. Yet, Se distribution in the food chains of regions with optimal Se content in the environment as well as in the regions with Se deficiency is extremely poorly understood [20].
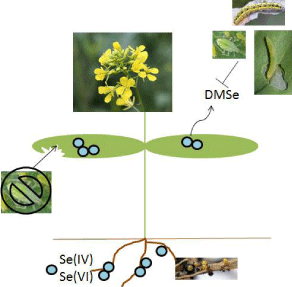
Figure 1: The mechanisms of Se deterrence effect on herbivores. Se(IV) - selenite, Se(VI) - selenate, DMSe - dimethylselenide.
Protective mechanism of Se accumulating plants is based either on 1) tissues containing toxic concentrations of Se or 2) volatile forms of Se deterring the pests (Figure 1). The toxicity of the plant tissue is the result of inorganic Se forms in the plant. Inorganic forms of Se are shown to be antifeedants (particularly selenate, despite the fact that selenite is the most toxic form) compared to organic Se compounds (selenocysteine and selenomethionine) [20]. The lack of selenomethionine avoidance may also have substantial environmental implications [28, 36].
Deterrence effect may in part be the result of the volatile compounds (dimethylselenide, dimethyldiselenide), which are known to be emitted from Se accumulator plants [2].
Some insect species, diamondback moth (Plutella xylostella), evolutionarily have adapted to toxic concentrations of Se in Se accumulator plants and thrive on them using specific physiological mechanisms of detoxification - they accumulate methylselenocysteine instead of selenocysteine which is accumulated with related moths [37]. Nevertheless, the protective role of Se in plants against herbivores seems to be more common.
Studies on selenium effect on herbivores
The interest in Se effects on herbivore, predominantly insect herbivore, has increased at the end of 20th century. First studies demonstrated that insects can accumulate Se, but did not consider the effects of this element on insect growth and survival [38,39]. Subsequent studies were stimulated by growing interest in plants used for phytoremediation of seleniferous soils. They have focused on biotransfer of Se from accumulator plants to selected herbivores, predominately insect pests such as Trichoplusia ni [29,32], Spodoptera exigua [28,29,36,40], Pieris rapae [30], one investigated also prairie dogs [34] and one nematodes [35].
Laboratory studies have shown that Se incorporated into the diet affects feeding site preferences and host plant selection and acts as antifeedant for larvae of the generalist herbivory beet armyworm Spodoptera exigua [28,36]. S. exigua (Hüber) (Lepidoptera: Noctuidae) is an agriculture important pest insect which is generalist feeder [28]. Its host range includes plants from families Liliaceae, Fabaceae, Solanaceae, Malvaceae, Chenopodiaceae, Apiaceae, Asteraceae and Amaranthaceae. This specie has highly mobile larvae, which selects feeding sites by moving between plants [41].
In the study of Trumble et al. [28] the dietary concentrations of 12 μg g-1 Se(VI) or Se(IV) reduced pupal weights. In the same study the toxic dose LC50 for S. exigua was set at 21.1 μg Se(IV) g-1 for selenite and 49.5 μg Se(VI) g-1 for selenate. The time required to complete the larval stage increased by over 25% and the time from egg to adult emergence was increased by up to 30% in the presence of Se. Low concentration of Se(VI) (4 μg g-1) depressed growth rate, while at 12 μg Se(VI) g-1 reduced the growth by 30%. Addition of Se(IV) severely affected growth rate of the pest, which dropped by over 90% at a concentration of 12 μg g-1. The important conclusion was that inorganic Se added to the time needed for development of S. exigua, while organic Se (selenomethionine) had no measurable effect at any concentration up to 30 μg g-1 [28].
In another study, Se(VI) incorporated into the diet affected feeding site preferences and host plant selection and acted as antifeedant for larvae of S. exigua [36]. A mean of 20% of the larvae were found on the treated diet (34.4 μg Se g-1) throughout the test period comparing to diet without Se. At concentrations 42.7 to 57.3 μg Se(VI) g-1 in the diet approximately 15% of larvae were found on treated diets in comparison to control [36]. In Se(IV) treatedment diet 30% of instar larvae were recorded at concentration 11.1 and 16.2 μg g-1, decreasing to less than 25% at higher concentrations (21, 27.5 μg g-1) [36]. In contrast, early instars demonstrated no consistent avoidance of diets with 49 μg g-1 of selenomethionine. Older larvae selectively avoided diet with Se(IV) and Se(VI), but there was no antifeedant activity to selenocysteine and selenomethionine. It can be concluded that inorganic Se was an antifeedant [36].
Alfalfa irrigated with high levels of Se(VI) with high Se accumulation exhibited significantly reduced S. exigua growth, development, and survival [40]. In another study of Vickerman et al. [42] Atriplex plant lines treated with 1 mg Se(VI) L-1, accumulated high amount of Se and S. exigua mortality mostly occurred during larval stage, rather than during pupation. The smaller population of S. exigua on Atriplex plant lines was also observed in the study of Bañuelos et al. [29]. Accumulated Se in plants B. g-1 (465 μg Se g-1) transferred to pest T. ni which resulted in higher mortality of the pest and consequently fewer pupae were counted on Se containing plants comparing to control [29].
The accumulated Se protected plants B. g-1 against caterpillars Pieris rapae and aphid Myzus persicae [30,31] due to both deterrence and toxicity. Caterpillar is a representative Brassica-specific herbivore. In the choice feeding experiment larvae of caterpillar preferred to feed on leaf without Se [30]. When newly hatched larvae were placed on plants containing 1.3 mg Se g-1, the larvae did not grow and died within 9 days. Nine days old larvae of caterpillars placed on Se containing plants (Se amount 1.6 mg g-1) lost 20% of weight in the first day and after one more day all caterpillars died [30].
The addition of Se to nutrient solution and foliar application effectively protected plants B. g-1 against Myzus persicae [31], which is generalist phloem feeder. Its host ranges over 40 plant families including Brassicaceae. B. g-1 that contained 10 μg Se g-1 pronounced negative effect on aphid colonization, while Se concentration above 125 μg g-1 appeared to be lethal for aphids. Further on the effect of Se as aphid control agent was observed [31]. In one approach Se was supplied systematically to aphid-harbouring plants in a hydroponic system while in the other Se was applied topically via spraying. In the first application treatment Se reduced aphid population growth up to 75% for 0.053 μg Se(VI) L-1 treatment while Se applied via spraying reduced aphid population growth by 20% for 0.11 μg Se(VI) L-1 treatment [31].
Popham et al. [32] investigated herbivore moth Trichoplusia ni (Lepidoptera: Noctuidae). The dietary supplementation with greater than 25 mg Se(IV) kg-1 inhibited growth of T. ni larvae by 40% based on larval weight. Feeding on the diet containing 50 mg Se(IV) kg-1 or 100 mg Se(IV) kg-1 resulted in 62% and 75% inhibition of larval growth, respectively [32]. The growth of larvae T. ni continuously fed with dietary supplementation of 10 and 20 mg Se(IV) kg-1 lag behind larvae fed with 1 or 5 mg Se(IV) kg-1. When larvae T. ni was firstly fed with 10 or 20 mg Se(IV) kg-1 and in four instar changed to the diet without Se, the growth lag significantly behind larvae fed without Se from the beginning [32]. On the contrary larvae, which firstly fed without Se and later on fed with 10 or 20 mg Se(IV) kg-1 lagged behind the larvae on Se free diet but began to catch up by the fifth instar.
Orthopterans represent a major group of insect herbivores. Brown crickets (Acheta domestica) are generalist orthopteran herbivores. Freeman et al. [33] exposed S. pinnata and B. juncea to Se(VI). Crickets and grasshoppers avoided plants with Se in choice feeding experiment, while crickets and grasshoppers died in nonchoice feeding experiment, eating plants containing 447 μg Se g-1 and 230 μg Se g-1, respectively [33]. Crickets on the diet with high Se (0.2 μg L-1) had greater mortality than crickets on low Se diet (0.01 μg L-1).
In the field study Quinn et al. [34] investigated Se accumulator Astragalus bisulcatus and B. juncea against herbivory of prairie dogs. Prairie dogs (Cynomys ludovicianus) live in large colonies and act as ecosystem engineers. They are voracious herbivores, both consuming and clipping vegetation. In the experiment Quinn et al. [34] compared the percentage of clipped plants and found out that A. bisulcatus, Se accumulator plant was clipped less often than Ranunculus crispus, Se non accumulator plant. In addition, A. bisulcatus appeared to be much less palatable, since on average only 3% of its clippings were apparently consumed compared with 65% for R. crispus. In 24 hours experiment the prairie dogs were given a choice between plants of B. juncea with or without Se. Prairie dogs preferentially fed on plants with less Se, avoiding plants with high Se levels [34]. These Se accumulators suffered little herbivory, suggesting that accumulated Se deterred prairie dogs [34].
Prins [35] in master thesis focused on nematodes. They are one of the many pests faced by farmers, and particularly in subsistence and sustainable agriculture systems, pesticides may not be an option [43]. In the study S. pinnata was treated twice a week with 0.11μg Se(VI) L-1 and after 24 weeks these plants had fewer nematodes as plants without added Se [35]. Instead of treating crops with organic pesticides, perhaps Se fertilization could be used to fortify roots with Se and reduce nematode levels. This result shows on possible use of Se as a pesticide for nematodes.
Only few studies considered the plant - herbivore interactions of cultivated plants, which on general are not Se accumulators, nevertheless some of them can accumulate considerable amount of Se.
Se toxicity to insects has been demonstrated already at the end of 1930s. It has been noted that the aphids do not inhabit the wheat with a high Se content, and cotton growing on soil supplemented with sodium selenate becomes toxic to bugs and moths [44 cited after 20]. Kastori and Kadar [45] reported that with increased application of Se(IV) to the soil (0, 90, 270, 810 kg ha-1), the Se concentration in the triticale grain increased and resulted in significantly lower percentage of damaged grains because of grain weevil (Sitophilus granaries).
An indirect evidence of the protective Se effect against Colorado potato beetle (Leptinotarsa decemlineata, Coleoptera) was obtained by detecting the anomalous Se accumulation by leaves of genetically modified potato resistant to the beetle pests [46].
Since it has been shown that broccoli can accumulate high concentrations of Se [21] our own field survey was conducted to test the influence of Se enriched broccoli on its pests Phyllotreta spp. and Delia radicum. The Se(VI) treated plants were slightly less damaged by Phyllotreta spp. and had less pupae of Delia radicum at harvest as control plants (unpublished results).
Conclusion
Despite the fact that Se has been shown to be an essential nutrient for animals and algae, the effects of this element on invertebrate herbivores is limited. The above review indicates that Se can affect plant - herbivore interactions. Plant - herbivore interactions are affected by the levels of Se in the soil and consequently its levels in the plant. Se accumulator plants contain high levels of this particular element in the tissues. Previous studies on Se accumulator plants S. pinnata, A. bisulcatus and B. juncea showed that Se protected plants against invertebrate pests as well as mammal herbivores. These results shed light on the possible selection pressures that driven the evolution of Se accumulation. Broccoli, Se non accumulator plant, which efficiently absorbed supplied Se, was less affected against Brassica pests. Since the diet is the predominant exposure pathway for Se, herbivores can be affected by feeding on the plant. The level of tolerance to Se by herbivores is dependent on the ability of herbivores to assimilate, accumulate and detoxify Se. It was shown that the element also accumulates in their bodies, but diamondback moth (Plutella xylostella) have adapted to toxic concentrations of Se in Se accumulator plants. Nevertheless, the protective role of Se in plants against herbivores is more common.
References
- Hartikainen H. Biogeochemistry of selenium and its impact on food chain quality and human health. J Trace Elem Med Biol. 2005; 18: 309-318.
- Terry N, Zayed AM, De Souza MP, Tarun AS. Selenium in Higher Plants. Annu Rev Plant Physiol Plant Mol Biol. 2000; 51: 401-432.
- Quinn CF, Galeas ML, Freeman JL, Pilon-Smits EA. Selenium: deterrence, toxicity, and adaptation. Integr Environ Assess Manag. 2007; 3: 460-462.
- Boyd RS, Martens SN. The raison d'être for metal hyperaccumulation by plants. In: The vegetation of ultramafic (Serpentine) soils. Baker AJM, Proctor J, Reeves RD, editors. Intercept, Andover, UK, 1992; 279-289.
- Terry N, Zayed AM. Phytoremediation of selenium. In: Environmental chemistry of selenium. Frankenberger WT Jr., Engberg RA, editors. Marcel Dekker, Inc, New York - Basel - Hong Kong. 1998.
- Carvalho KM, Martin DF. Removal of aqueous selenium by four aquatic plants. J aquat plant manage. 2001; 39: 33-36.
- Lemly AD. Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol Environ Saf. 2004; 59: 44-56.
- Canton SP, Van Derveer WD. Selenium toxicity to aquatic life: An argument for sediment-based water quality criteria. Environ toxicol chem. 1997; 16: 1255-1259.
- Uden PC, Boakye HT, Kahakachchi C, Tyson JF. Selective detection and identification of Se containing compounds--review and recent developments. J Chromatogr A. 2004; 1050: 85-93.
- Kabata Pendias A. Trace elements in soils and plants, 3rd edn. Boca Raton, FL, CRC Press. 2001: 241-252.
- Nowak J, Kaklewski K, Ligocki M. Influence of selenium on oxidoreductive enzymes activity in soil and in plants. Soil Biol Biochem. 2004; 36: 1553-1558.
- Navarro-Alarcon M, Cabrera-Vique C. Selenium in food and the human body: a review. Sci Total Environ. 2008; 400: 115-141.
- Schwarz K, Foltz C. Selenium as an integral part of factor 3 against necrotic dietary liver degeneration. J Am Chem Soc. 1957; 79: 3292-3293.
- Reilly C. The nutritional trace metals. Blackwell publishing Ltd. 2004.
- Eurola M, Ekholm P, Ylinen M, Koivistoinen P, Varo P. Effects of selenium fertilization on the selenium content of selected Finnish fruits and vegetables. Acta agr Scand. 1989; 39: 345-350.
- Stadtman TC. Selenium biochemistry. Annu Rev Biochem. 1990; 59: 111-127.
- Novoselov SV, Rao M, Onoshko NV, Zhi H, Kryukov GV, Xiang Y, et al. Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J. 2002; 21: 3681-3693.
- Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M. Physiological functions of beneficial elements. Curr Opin Plant Biol. 2009; 12: 267-274.
- Hartikainen H, Xue T, Piironen V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant soil. 2000; 225: 193-200.
- Golubkina N, Sheshnitsan S, Kapitalchuk M. Ecological Importance of Insects in Selenium Biogenic Cycling. Int J Ecol. 2014: 6.
- Sugihara S, Kondo M, Chihara Y, Yuji M, Hattori H, Yoshida M. Preparation of selenium-enriched sprouts and identification of their selenium species by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. Biosci Biotech Bioch. 2004; 68: 193-199.
- Mechora Š, Stibilj V, Kreft I, Radešcek T, Gaberšcik A, Germ M. Impact of Se (VI) fertilization on Se concentration in parts of red cabbage plants. J food agric environ. 2011; 9: 357-361.
- Finley JW, Ip C, Lisk DJ, Davis CD, Hintze KJ, Whanger PD, et al. Cancer-protective properties of high-selenium broccoli. J Agric Food Chem. 2001; 49: 2679-2683.
- Xue TL, Hartikainen H, Piironen V. Antioxidative and growth-promoting effects of selenium on senescing lettuce. Plant Soil. 2001; 237: 55-61.
- Kuznetsov VV, Kholodova VP, Kuznetsov VV, Yagodin BA. Selenium regulates the water status of plants exposed to drought. Dokl Biol Sci. 2003; 390: 266-268.
- Hawrylak-Nowak B. Changes in anthocyanin content as indicator of maize sensitivity to selenium. J plant nutr. 2008; 31: 1232-1242.
- Pedrero Z, Madrid Y, Hartikainen H, Cámara C. Protective effect of selenium in Broccoli (Brassica oleracea) plants subjected to cadmium exposure. J Agric Food Chem. 2008; 56: 266-271.
- Trumble JT, Kund GS, White KK. Influence of form and quantity of selenium on the development and survival of an insect herbivore. Environ Pollut. 1998; 101: 175-182.
- Bañuelos GS, Vickerman DB, Trumble JT, Shannon MC, Davis CD, Finley JW, et al. Biotransfer Possibilities of Selenium from Plants Used in Phytoremediation. Int J Phytorem. 2002; 4: 315-329.
- Hanson B, Garifullina GF, Lindblom SD, Wangeline A, Ackley A, Kramer K, et al. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol. 2003; 159: 461-469.
- Hanson B, Lindblom SD, Loeffler ML, Pilon-Smits EAH. Selenium protects plants from phloem-feeding aphids due to both deterrence and toxicity. New Phytol. 2004; 162: 655-662.
- Popham HJR, Shelbya KS, Popham TW. Effect of dietary selenium supplementation on resistance to baculovirus infection. Biol Control. 2005; 32: 419-426.
- Freeman JL, Lindblom SD, Quinn CF, Fakra S, Marcus MA, Pilon-Smits EA, et al. Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence. New Phytol. 2007; 175: 490-500.
- Quinn CF, Freeman JL, Galeas ML, Klamper EM, Pilon-Smits EA. The role of selenium in protecting plants against prairie dog herbivory: implications for the evolution of selenium hyperaccumulation. Oecologia. 2008; 155: 267-275.
- Prins C. Effect of elevated plant selenium levels on reproduction and root-nematode interactions [master thesis]. 2011.
- Vickerman DB, Trumble JT. Feeding preferences of spodoptera exigua in response to form and concentration of selenium Arch Insect Biochem Physiol. 1999; 42: 64-73.
- Freeman JL, Quinn CF, Marcus MA, Fakra S, Pilon-Smits EA. Selenium-tolerant diamondback moth disarms hyperaccumulator plant defense. Curr Biol. 2006; 16: 2181-2192.
- Hogan GR, Razniak HG. Selenium-induced mortality and tissue distribution studies in Tenebrio molitor (Coleoptera: Tenebrionidae). Environ Entomol. 1991; 20:790-794.
- Lalitha K, Rani P, Narayanaswami V. Metabolic relevance of selenium in the insect Corcyra cephalonica. Uptake of 75Se and subcellular distribution. Biol Trace Elem Res. 1994; 41: 217-233.
- Vickerman DB, Young JK, Trumble JT. Effect of selenium-treated alfalfa on development, survival, feeding, and oviposition preferences of Spodoptera exigua (Lepidoptera: Noctuidae). Environ Entomol. 2002a; 31: 953-959.
- Berdegué M, Reitz SR, Trumble JT. Host plant selection in Spodoptera exigua: do mother and offspring know best? Entomol Exp Appl. 1998; 89, 57-64.
- Vickerman DB, Shannon MC, Bañuelos GS, Grieve CM, Trumble JT. Evaluation of Atriplex lines for selenium accumulation, salt tolerance and suitability for a key agricultural insect pest. Environ Pollut. 2002; 120: 463-473.
- Bridge J. Nematode management in sustainable and subsistence agriculture. Annu Rev Phytopathol. 1996; 34: 201-225.
- Moxon AL. Selenium: its occurrence in the rocks and soils, absorption by plants, toxic action in animals, and possible essential role in animal nutrition. In: Lamb CA, Bentley OG, Beattie JM, editors. Trace Elements, Proceedings of the Conference. Academic Press, New York, NY, USA. 1958; 175-191.
- Kastori R, Kadar I. Effect of Selenium, Molybdenum and Zinc on Seedling Growth and Frequency of Grain Weevil(Sitophilus granarius) in Triticale. Pesticidi i fitomedicina. 2009; 24: 133-138.
- Golubkina N, Skriabin K. Anomalous accumulation of selenium by genetically modified potato, stable to Colorado beetle. J Food Compos Anal. 2010; 23: 190-193.