
Research Article
Austin J Environ Toxico. 2024; 10(1): 1050
Drugs Removal from Wastewater with Activated Carbon from Coffee Waste
Aryam Shams Mohammad Al Marzooqi; Hamda Salman Sayah Almazrouei; Hend Ali Mohamed Alhammadi; Maera Saeed Humaid Almansoori; Mazanah Khalil Mohamed Alhosani; Wadima Ali Hamad Al Mazrouei; Afef Dellai*
Applied Technology High School, Al Dhafra Campus - Abu Dhabi, United Arab Emirates
*Corresponding author: Afef Dellai Applied Technology High School, Al Dhafra Campus, United Arab Emirates. Email: dellai.afef79@gmail.com
Received: March 18, 2024 Accepted: April 15, 2024 Published: April 22, 2024
Abstract
Today, pharmaceutical residues constitute most of the problem in the water contamination because they are active principles which are able to act at low quantities, and they are usually recalcitrant to the conventional wastewater treatment. Hospital effluent is the main source of the environmental contamination by pharmaceuticals due to their excessive use in medical establishments. In our study, we collected the coffee grounds from Dubai cafes which was washed and then carbonized, and this carbonized product will be cooled and sieved. we will evaluate the ability of this unique activated carbon in decontaminating hospital effluents and eliminating pharmaceutical compounds. This filtration is carried out by the adsorption process on the pores generated by the activation of carbon in a prototype designed for this purpose. The chromatographic analysis will confirm the expected results. In fact, many pharmaceuticals were completely disappeared from hospital effluent after the treatment with coffee grounds and for other drugs the removal of the chemical compounds exceeded 90%. The developed technique which is based on the production of activated carbon from coffee grounds can have two major advantages: the recovery of one waste (coffee grounds) for the treatment of another waste hospital wastewater and completely clear of pharmaceuticals.
Keywords: Pharmaceuticals; Hospital wastewater; Coffee grounds; Chromatographic analysis
Introduction
Pharmaceuticals are a group of chemically active compounds used for diagnosis, treatment and prevention. They play an important role in the population’s well-being. Pharmaceutically active compounds (PhACs) are continuously released into the environment. Today, one of the major problems that concerns the whole world is the contamination of all kinds of water (fresh and marine water) by pharmaceutical substances [1,12,13]. Hospitals are considered the main source that generate these PhACs such as Antibiotics, Anti-Inflammatory, Analgesic, Anticonvulsants, Opioids, Diuretic, Terpenes…. [1,9] due to their excessive use in medical establishments. Thus, PhACs pose a serious concern due to their intrinsic ability to interact with the biological system, leading to their bioaccumulation in non-target organisms and the onset of harmful effects [3-8].
For this reason, the treatment of hospital discharges is a necessity. Several treatment processes (biological, physical, chemical treatment) have been studied and sometimes applied. However, these techniques are not satisfactory given the standards required. In fact, pharmaceutical residues are found at the exit of the stations without change [2,10]. One of the most effective techniques for wastewater treatment is adsorption using activated carbons from agricultural sources such as wood pulp, coconut or peanut shells, and olive pits. However, these conventional sources are disadvantageous and labor intensive. To solve this problem, an ecological, economical and above all feasible source of activated carbon is essential.
During our research project, we will try to activate the carbon obtained from coffee ground by firstly washing it with fresh water then dehydrating it in the open air. Secondly, carbonizing the pre-carbonized coffee grounds at 600°C for 4 h, then cooling and sieving the carbonized product. Thirdly, soaking the carbonized product in an acid solution. Finally drying it at 105°C. In the second part of our work, we will evaluate the ability of this unique activated carbon in decontaminating hospital effluents and eliminating pharmaceutical compounds. This filtration is carried out by the adsorption process on the pores generated by the activation of carbon in a prototype designed for this purpose. The chromatographic analysis with UPHPLC –MS/MS will confirm the expected results.
Material and Methods
Chemicals and Reagents
All the antibiotic standards are endowed with a high-purity grade (>90%), and they were obtained from Sigma-Aldrich (Bronem, Belgium) and Witega (Germany) (Table 1). HPLC grade methanol, ethanol and acetonitrile were purchased from HPLC grade (Biosolve, Germany). Formic acid 98% was from Merck (Germany). A Milli-Q-Advantage system from Millipore (Waters, USA) was employed to acquire HPLC-grade water. Low melting point (LMP) agarose and Normal Melting Point (NMP) agarose were purchased from Invitrogen. Sodium Chloride, EDTA, TRIS, DMSO, S9 mix (post-mitochondrial supernatant fraction), cytochalasin B, DAPI (DNA-staining), acridine-orange and triton X-100 were from Sigma and NaOH pellets from VWR. The positive controls Benzo(a)pyren (B(a)P), 4-Nitroquinoline 1-Oxide (4-NQO), Methyl Methanesulfonate (MMS), Ethyl Methanesulfonate (EMS)) in toxicological tests were purchased from Sigma-Aldrich.
Hospital Wastewater Sample Collection
The wastewater samples collection (2 L) was conducted between March 2023, effluents were collected from hospital of Beja in Tunisia.
Analytical Study
Samples extraction and method validation. A 100 mL of water sample was spiked with the internal standards at a concentration of 60 ng mL1. An Oasis® HLB cartridge was preconditioned with 6 mL of methanol and 6 mL of Milli-Q water. The collected sample with a flow rate of approximately 2 mL min1 was loaded into the cartridge and then the cartridge was rinsed with 6 mL of Milli-Q water and desiccated under vacuum for 20 minutes. Following the drying process, the analytes were eluted using 12 mL methanol and were evaporated to dryness at 40°C under a slow stream of nitrogen. The dry residue was dissolved in 1 mL of Milli-Q water, filtered through 1 μm and then an 8 μL aliquot was injected onto the UPLC column.
UPLC-MS/MS Analysis
The UPLC system comprised Waters ACQUITY UPLC (Waters, USA) associated with a TQ-S triple-quadrupole mass spectrometer (Waters Corp., Milford, MA). Separation was carried out on an ACQUITY UPLC® BEH C18 column (100 mm × 2.1 mm) with a 1.7 μm particle size (Waters, Ireland). As for the analytes, they were measured in the multiple reaction monitoring modes. The mobile phase was made up of a mixture of 0.1% formic acid in water.
(A) and in acetonitrile (B) and its composition switched linearly from 1% B at 0 min to 99% B at 10 min and then dropped again to 1% B at 14 min, after which the column was rinsed with 99% for 5 min and re-equilibrated to the initial conditions for 10 min. The established conditions were as follows; the flow rate was at 0.4 mL min1, whereas the column and the auto-sampler tray temperature were fixed at 50°C and 4°C, respectively. The mass spectrometer was handled in the positive electrospray ionization mode (ESIþ) with a capillary voltage of 1.00 kV, a source temperature of 140°C and desolvation temperature at 40°C. Employing Nitrogen as a desolvation gas, it was set at a flow of 800 L/h. The optimization of the parent and daughter ions, the cone voltage and the collision energy were determined by using a direct infusion of a 100 ng L-1 of working solution in water. Dwell times were adapted individually to acquire an optimal amount of data points to describe the peak. For each compound, two transitions were followed. One transition was employed for quantification, and the other for the monitoring of identification. The proportion of these two peak areas is a fixed value that is used for identification purposes. Data was obtained using MassLynx v.4.1 software and was processed using TargetLynx v.4.1 software (Waters Corp).
Prepration of the Filtration System
A physico-chemical treatment unit was designed to purify hospital effluent. This is an open activated carbon filtration system. The device consists of two compartments made of standard, thick clear glass and bonded with silicone. The whole thing is placed on a solid support. The choice of glass as the main material of the device is justified by the fact that it is non-toxic, water-repellent and resistant to corrosion and bad weather (temperature variations, rain, wind). The upper compartment represents the filtration basin (width: 40 cm, length: 80 cm and height: 40cm). The bottom of this basin was perforated with very small diameter holes (< 5 microns), then covered with a draining mat made up of a first layer of gravel, above which a second layer of activated carbon will be placed in powder. This multi-layer system allows the filtered effluent to percolate while preserving the active carbon layer as much as possible. The lower compartment represents the filtered water collection container. This container has a bottom inclined at an angle of 45° and is equipped at its base with a drain valve for recovering the treated water. This water will be loaded with possible activated carbon particles, hence the need for a tank equipped with a conical collector. This conical shape promotes the settling of activated carbon particles and allows the recovery of pure filtered water at a second drain tap.
Activated Carbon Production
The production of activated carbon is made from coffee grounds. Charcoal activation was carried out physically and chemically. First, a significant quantity of coffee residue was dried in the open air. Then, the carbonization process occurred slowly by heating the biomass at a high temperature of 600°C for 4 hours. This step allows the creation of initial pores on the structure of the coal layer. The carbonized product was then cooled and sieved through a 0.5mm sieve. The sifted activated coffee grounds are finally subjected to chemical activation by soaking in an acidic HCL solution (0.1 M) for 48 h. This step creates new pores and expands the specific surface area of the activated carbon produced. The acid solution is then evacuated, and the final product is placed in an oven at 105°C in order to dry it. The activated carbon produced is stored in the desiccator until the day of treatment.
Device Functionality
Taking into account the dimensions of the filtration basin, its total capacity is 12L. In this capacity, only a useful volume of 6 liters will be used to load hospital effluent. This loading will be filtered by a layer of powdered activated carbon. An adsorption phenomenon occurs, during which the micropollutants present in hospital liquid discharges react with the carbon. Molecules, atoms or ions adhere to the particle surface of the adsorbent through weak bonds such as van der Waals forces, electrostatic interactions and hydrogen bonds.
The quantity of adsorbent used depends on the volume of wastewater to be treated. Proportions ranging from 2g to 8g for each liter of raw wastewater have been tested. An optimal rate of 2g for each liter of raw water is observed and for which a significant increase in adsorption performance is noted. The activated carbon layer is renewed after 5 consecutive effluent filtration cycles. The treated water then passes through the granular bed made up of gravel, the perforated plate, the inclined collection container, to reach the tank fitted with the conical collector, where it will be freed of activated carbon particles and will eventually become pure.
Results
Obtention of Coffee Grounds
The results of the Scanning Electron Microscopy (SEM) analysis show a clear evolution in the surface state of the adsorbent (activated carbon). On the activated carbon sample before contact with the adsorbate, many visible and empty pores can be observed, as shown in Figure 1A. However, after contact with the adsorbate, we see that some visible pores become filled with substances but still remain not completely charged (Figure 1B).
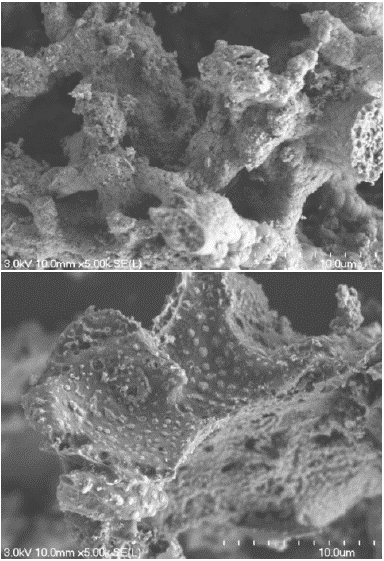
Figure 1: A) Microscopic observation by SEM of the activated carbon before the adsorption process at Magnification x5K. B) Microscopic observation by SEM of the activated carbon after the adsorption process at Magnification x5K.
Before proceeding to the adsorption step, the activated carbon produced is evaluated for its characteristics (Table 1). The results show a yield value of 17.8%, a water content of 6.42%, an ash content of 1.08% and an iodine adsorption capacity of 578.5 mg/g. These values comply with the quality standards according to SNI n° 06-3730-1995.
Treatment Process of Hospital Effluent
Physicochemical Parameters: The physicochemical analysis was carried out using conventional methods according to the AFNOR standard. The measurement of SS, COD, BOD, TOC, NO3 and AOX was carried out using a portable UV analyzer. Conductivity, pH and turbidity were measured using the conductivity meter, pH meter and AQUALITIC, respectively. Table 2 shows the main physicochemical parameters of hospital effluent before and after treatment with activated carbon. The water produced is less loaded with organic matter evaluated in terms of COD (556 ± 36), BOD (398 ± 16) in comparison with untreated hospital water COD (44.3 ± 8.4), BOD (8.5 ± 2.4). Suspended solids results were below 5 mg/l after treatment. TOC and turbidity and conductivity values were significantly decreased at a percentage reduction of 95.6%, 80.2% and 64% respectively.
Bacteriological Parameters: The microbiological analysis of the samples (hospital effluent before and after treatment) focused on the enumeration of microorganisms on an agar medium in order to highlight the level of reduction induced by activated carbon on the total bacterial load. Table 3 shows a significant elimination of bacteria at 91.82%.
Adsorption of Pharmaceuticals from Hospital Wastewater: Table 4 groups together the elimination efficiencies obtained by activated carbon. The invention makes it possible to obtain very effective results. In fact, 8 out of 20 pharmaceutical compounds were completely destroyed (100%) which are Propranolol, ibuprofen, salicylic acid, acetaminophen, carbamazepine, Triamterene, norgestrel and diazepam. For the remains of the compounds, a good elimination efficiency was also recorded with a percentage ranging from 72% to 99%.
Discussion
This present work draws its interest from its very interesting results in the elimination of emerging micropollutants considered to be the most recalcitrant for conventional treatments and also for their negative effects on the environment and human health. Indeed, during the activated carbon filtration process, the micropollutants adhere to the crevices formed on the activated carbon thanks to physicochemical bonds. A drainage mat made of gravel allows the percolation of filtered effluents while preserving the layer of powdered activated carbon as much as possible. However, a quantity of activated carbon may be carried with the effluent during treatment by filtration. For this, an additional tank equipped with a conical collector is put in place. The latter promotes the settling of any activated carbon particles and allows the recovery of pure filtered water, at a drain tap.
Activated carbon is produced from coffee waste in two activation stages: physical (carbonization) and chemical (soaking in an acid solution). These activations created cracks on the surface of the coal, making it possible to considerably improve the mic adsorption process.
This allowed, first of all, a very significant reduction in the polluting load, in particular the suspended matter, in the chemical oxygen and the biochemical oxygen demand. Also, a very significant reduction in turbidity, and this explains the elimination of bacteria which are considered colloidal (turbid) particles.
However, the elimination of pharmaceutical molecules remains the most striking results and allows us to say that this process is the most effective against this type of micropollutant in hospital effluents. Indeed, physical (filtration, denatation, etc.), chemical (advanced oxidation processes, choleration, etc.), physco-chemical (coagulation/flocculation) and biological (activated sludge, free bacteria, fungi, etc.) treatment types, have proven to be ineffective against pharmaceutical residues in hospital effluents [10,11,14,15].
The treatment of hospital effluent with activated carbon from coffee waste is therefore technically and economically viable and can take place in hospitals, the pharmaceutical and biotechnology industries.
Author Statements
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
The authors have no competing interests to declare that are relevant to the content of this article.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
The authors have no financial or proprietary interests in any material discussed in this article.
Data Availability Statement
Data and Materials are available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Afsa S, Hamden K, Lara Martin PA, Mansour HB. Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination. Environ Sci Pollut Res Int. 2020; 27: 1941-1955.
- Beltifa A, Alibi S, Mansour HB. Monitoring hospital wastewaters for their probable genotoxicity. J Water Health. 2020; 18: 1-7.
- Capolupo M, Franzellitti S, Kiwan A, Valbonesi P, Dinelli E, Pignotti E, et al. A comprehensive evaluation of the environmental quality of a coastal lagoon (Ravenna, Italy): Integrating chemical and physiological analysis in mussels as a biomonitoring strategy. Sci Total Environ. 2017; 598: 146–159.
- Capolupo M, Valbonesi P, Kiwan A, Buratti S, Franzellitti S, Fabbri E. Use of an integrated biomarker-based strategy to evaluate physiological stress responses induced by environmental concentrations of caffeine in the Mediterranean mussel Mytilus Galloprovincialis. Sci Total Environ. 2016; 563–564: 538–548.
- Cappello T, De Marco G, Oliveri Conti G, Giannetto A, Ferrante M, Mauceri A, et al. Time-dependent metabolic disorders induced by short-term exposure to polystyrene microplastics in the Mediterranean mussel Mytilus galloprovincialis. Ecotoxicol Environ Saf. 2021; 209: 111780.
- Cappello T, Giannetto A, Parrino V, Maisano M, Oliva S, De Marco G, et al. Baseline levels of metabolites in different tissues of mussel Mytilus galloprovincialis (Bivalvia: Mytilidae). Comp Biochem Physiol D. 2018; 26: 32–39.
- Cappello T, Vitale V, Oliva S, Villari V, Mauceri A, Fasulo S, et al. Alteration of neurotransmission and skeletogenesis in sea urchin Arbacia lixula embryos exposed to copper oxide nanoparticles. Comp Biochem Physiol C. 2017; 199: 20–27.
- Cappello T, Maisano M, Giannetto A, Parrino V, Mauceri A, Fasulo S. Neurotoxicological effects on marine mussel Mytilus galloprovincialis caged at petrochemical contaminated areas (eastern Sicily, Italy): 1H NMR and immunohistochemical assays. Comp Biochem Physiol C Toxicol Pharm. 2015; 169: 7–15.
- Emna Nasri, Ana Cristina Soler de la Vega, Carlos Barata Martí, Hedi Ben Mansour, Maria Silvia Diaz Cruz. Pharmaceuticals and personal care products in Tunisian hospital wastewater: occurrence and environmental risk. Environmental Science and Pollution Research. 2024; 31: 2716-2731.
- Klatt M, Beyer F, Einfeldt J. Hospital wastewater treatment and the role of membrane filtration - removal of micropollutants and pathogens: A review. Water Sci Technol. 2022; 86: 2213-2232.
- Liu A, Zhao Y, Cai Y, Kang P, Huang Y, Li M, et al. Towards Effective, Sustainable Solution for Hospital Wastewater Treatment to Cope with the Post-Pandemic Era. Int J Environ Res Public Health. 2023; 20: 2854.
- Tahrani L, Van Loco J, Anthonissen R, Verschaeve L, Ben Mansour H, Reyns T. Identification and risk assessment of human and veterinary antibiotics in the wastewater treatment plants and the adjacent sea in Tunisia. Water Sci Technol. 2017; 76: 3000-3021.
- Tahrani L, Mehri I, Reyns T, Anthonissen R, Verschaeve L, Khalifa ABH, et al. UPLC-MS/MS analysis of antibiotics in pharmaceutical effluent in Tunisia: ecotoxicological impact and multi-resistant bacteria dissemination. Arch Microbiol. 2018; 200: 553-565.
- Top S, Akgün M, Kipçak E, Bilgili MS. Treatment of hospital wastewater by supercritical water oxidation process. Water Res. 2020; 185: 116279.
- Pariente MI, Segura Y, Álvarez-Torrellas S, Casas JA, de Pedro ZM, Diaz E, et al. Critical review of technologies for the on-site treatment of hospital wastewater: From conventional to combined advanced processes. J Environ Manage. 2022; 320: 115769.