
Research Article
Austin J Environ Toxicol. 2016; 2(1): 1008.
Effects on Reproduction, Genotoxicity and DNA Methylation Pattern after Chronic Exposure of the Freshwater Snail Physaacuta (Gastropoda, Pulmonata) to Vinclozolin
Sanchez-Arguello P¹*, Aparicio N¹, Guevara MA², Díaz L², Cervera MT² and Fernandez C¹
¹Department of the Environment, Laboratory for Ecotoxicology, INIA, Spain
²Department of Forest Ecology and Genetics, INIACIFOR, Spain
*Corresponding author: Paloma Sanchez-Arguello, Department of the Environment, Laboratory for Ecotoxicology, INIA, Madrid, Spain
Received: January 20, 2016; Accepted: February 12, 2016; Published: February 12, 2016;
Abstract
The Fungicide Vinclozolin (VZ) is an endocrine disruptor with anti-androgenic activity whose potential for disrupting epigenetic mechanisms in mammals has also been shown. In this regard, this study combined reproductive endpoints and effect assessments at the sub-individual level associated with Genotoxicity and altered DNA methylation patterns. Physaacuta was exposed to VZ at nominal concentrations from 0.0005 to 5mg/L. Fecundity (production of F1 eggs) over 45 days and fertility (viability of F1 eggs) after further 21-dayembryonic development under exposure and non-exposure conditions were monitored. The Genotoxicity of VZ was evaluated by micronuclei scoring. Potential epigenetic alterations were measured by scoring DNA methylation patterns. Although acute exposure (96h prescreening test) at 5mg/L did not caused mortality, long-term exposure (45-day reproduction test) affected the survival of snails. Reduced effects on reproduction, micronuclei induction and DNA demethylation events were observed at 5mg/L. At the other VZ concentrations no effects on these endpoints were found. Thus reproduction endpoints were as sensitive as the effects at the sub individual level. This reproduction test, with additional assessments of the hatching success of the F1 recovered eggs, assessed reliable reproduction success in freshwater snails. The use of exposed and nonexposed F1 embryos allowed us to observe if damage due to parental exposure could be maintained or recovered when suspending treatment during embryonic development. Finally, the combination of long-term ecotoxicological effects and genotoxic/epigenetic biomarkers can broaden our understanding of pollutant impacts.
Keywords: Molluscs; Reproduction test; Micronucleus induction; DNA methylation
Introduction
Continuously increasing chemical pollution has led to a complex environmental scenario in which chronic tests on a wide range of species and specific endpoints are crucial tools for studying chemical effects. Chronic testing can provide information on processes such as growth, development and reproduction, which are relevant at the population level. Aquatic invertebrates are target organisms for different OECD chronic toxicity tests [1-4] including one lifecycle test [5]. Although no mollusk-based toxicity tests have been internationally standardized, an OECD project is developing a partial life-cycle test with gastropods [6,7]. The OECD/EDTA (Endocrine Disrupters Testing and Assessment) Conceptual Framework has indicated the need for mollusc-based tests, and the phylum offers species of ecological and economic relevance that are known to be uniquely sensitive to a number of Endocrine Disrupter Compounds (EDCs) [8,9] Some assays performed with freshwater gastropods have addressed the effects of xenobiotics their reproductive capacities (fecundity and fertility) after long-term exposure. Oehlmann et al. [10] reported malformations of the female genital system, and massive stimulation of oocyte and spawning mass production in Marisa cornuarietis induced by Bisphenol A and Octyl phenol with a complete life-cycle. These endpoints were also assessed on Lymnaeaacuminata after exposure to pyrethroid pesticides [11] Czech et al. [12] exposed adults of Lymnaeastagnalis to Tributyltin, β-sitosterol and 4-nonylphenol with a view to looking at the reproductive and histopathological effects on the F0 and F1 generations. Leung et al. [13,14] also exposed Lymnaeastagnalis and Physafontinalis from embryos to sexual maturity to Tributyltin. Recently, different approaches have been developed to perform embryo tests with freshwater snails to form part of mollusc lifecycle tests [8, 15-18]. All these studies have explored the life-stage specific effects of molluscs to xenobiotics, while other studies have investigated their genotoxic responses [19].
Presence of genotoxic compounds in the environment is a matter of concern given the transfer of effects across generations. The detection of chemical Genotoxicity has focused mainly on a direct change at the DNA level, such as point mutations. Cytogenetic alterations are widely accepted for detecting potential carcinogens.
The cytogenetic assay of Micronucleus (MN) induction has been applied successfully in ecotoxicological studies [20,21]. Exposure to chemicals can result in genetic alterations but many pollutants do not have the capacity to induce direct DNA damage. Non-genotoxic carcinogens are often assumed to possess an epigenetic mode of action which can induce heritable changes that cannot be explained by changes in DNA sequence [22,23]. Therefore, additional molecular mechanisms need to be considered, such as epigenetics, which has recently become a very promising target in molecular biology. DNA methylation has been the most extensively studied epigenetic mechanism. The evaluation of the global methylation status and the assessment of methylation in GC-rich regions of the genome have been proposed for both initial toxicity assessments of a compound’s toxicity potential and an earlier indication of its possible mechanism of action [24]. Genetic and epigenetic mechanisms are crucial for genome stability [25] and, regardless of the chemical-induced changes in DNA or in gene expression; both can have knock-on effects at higher biological organization levels. Therefore studying genotoxic/ epigenetics in Ecotoxicology can help clarify how these mechanisms can link to responses of ecological relevance.
Vinclozolin (VZ) is a nonsystemic fungicide used to control fungal pathogens. Concern about toxicity in mammals is due to its antiandrogenic activity [26,27]. VZ exerts its effects most dramatically during the development stages of animals, and ultimately result in reproductive effects [28]. VZ has been found to produce infertility of F1 males in multigenerational studies with rats [29]. Transgenerational effects in mammals through epigenetic mechanisms after VZ exposure have been observed [30]. Nevertheless, other studies have failed to find this mechanism of action [31]. The likelihood of VZ being released into surface waters, together with its known endocrine disruption effect, justifies undertaking chronic exposure studies with aquatic organisms. Martinovic et al. [32] studied the reproductive toxicity of VZ in fish and showed a concentration-dependent reduction in fecundity (production of fewer eggs) whereas hatching success of the deposited eggs (fertility) was not affected. VZ did not elicit an effect on the reproduction of the freshwater invertebrate Daphnia magna, whereas overall DNA methylation rate consistently decreased [33,34] previously observed an earlier sexual repose and morphological alterations of sex organs in the males of two species of prosobranch snails. Nevertheless, it was not possible to compare these data with fecundity data since spawning did not occur. Effects of VZ on the reproduction of the freshwater snail Lymnaeastagnalis were subsequently investigated by Ducrot et al., [35] who showed reduced in fecundity (cumulated number of eggs produced per individual) with a significant number of non-fertilized eggs as observed microscopically. Viability of eggs was not determined. Theseauthors recommended the complementary assessment of hatching success in the offspring of exposed snails (fertility) in the case of fecundity impairment when studying endocrine disruptors which may have epigenetic effects.
The present study evaluated the fecundity and fertility of the freshwater snail Physaacuta. The effects of VZ after 45-dayexposure, along with a complementary assessment of offspring embryonic development (F1 embryos) and hatching success were monitored. The F1 endpoints were evaluated for both non-exposed and exposed embryos in order to distinguish effects due to parental exposure. A combination of genetic and epigenetic endpoints to study the effects associated with mechanisms of inheritance was simultaneously assessed. The Genotoxicity of adults and F1 embryos was assessed by micronucleus test. The post-exposure profile alterations of global DNA methylation in adults was the epigenetic mechanism studied. The results were compared with our previous studies of VZ on Physaacuta embryos whose parents were not exposed [15]. Finally since long-term exposure was needed, actual VZ concentrations were measured.
Material and Methods
Acute and reproduction tests
Physaacuta individuals came from our own laboratory breeding stocks. Culture conditions are described by Sánchez-Argüello et al. [36].
In order to stablish the exposure range of the PLC test, a prescreening test with adults was performed at two concentrations (0.5 and 5mg/L) for 96h. Mortality and reproduction (only the number of egg masses) were monitored in triplicate.
For the definitive test, the adults from a single cohort cultured in our laboratory, and not previously exposed, were used for the PLC test. Groups of ten snails (size of test animals 7±2mm) were exposed to VZ in 60 ml of test medium using acetone (0.1%) as a solvent carrier at 20°C, with a photoperiod of 16h:8h light/darkness. Vessels were covered to prevent snail escaping. Maximal VZ concentration detected in surface water was 0.0005mg/L [35]. The range of exposure covered this measured environmental concentration and 1000 times higher. The VZ test treatments were: Control (reconstituted test water as used in 15); Acetone; 0.0005; 0.005; 0.05; 0.5 and 5mg/L. VZ was added by medium renewal and after spiking nominal concentrations on all the other days to maintain nominal concentrations. Test medium renewal, food supply (1.2 mg/snail of Shrimps food flakes -Sera®) and monitoring the endpoints (mortality and fecundity) were done twice-weekly over a 45-day period. Fecundity was assessed as the egg masses deposited per adult. The number of eggs inside each egg mass was also counted with a stereomicroscope (Olympus SZX12). Four replicates per treatment were used. Snails were recovered at the end of tests for the micronuclei induction study and DNA isolation and methylation analyses. Measures of chemical body burden were also taken.
F1 embryo toxicity tests
Oviposition was observed on the first day of monitoring (3 days after the test began) in all the treatments. Once a week spawned egg masses from Oviposition were sampled and washed through a stainless steel strainer tore move jelly-envelopment. This treatment allows single eggs to be obtained, which are easily observed under a stereomicroscope during the embryonic development instead of the three-dimensional arrangement of eggs in the egg mass [15]. Single eggs were used for testing embryo toxicity. F1 eggs from each treatment and control were divided into two batches: one batch was transferred to the 12-well plate with reconstituted water (nonexposed embryos), while the other batch was exposed to the same concentrations as their parents (exposed embryos). These differences in the exposure conditions for offspring help differentiate the effects due only to parental exposure from the effects observed by continuous exposure (F0-adults and F1-embryos), respectively. Embryo toxicity tests were performed in duplicate per treatment, and for both the embryos non-exposed and exposed to VZ, in accordance with the conditions described in Sánchez-Arguello et al. [15]. Briefly, a density of 30 eggs/well in 4ml/well was used. The embryo toxicity test lasted 21, for this time mal formed and dead embryos were monitored daily by stereomicroscope scoring. (Figure 1) shows differences between normal embryos, malformed embryos and dead embryos at different embryonic stages. Test medium was renewed twice weekly. Hatching success was measured until the test ended.
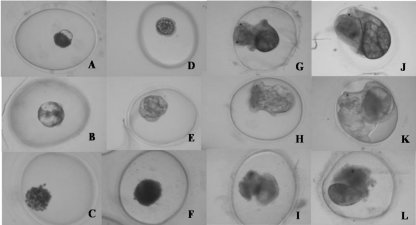
Figure 1: Physaacuta embryos at different stages: A) Normal embryo at 72-h
stage. B) Malformed embryo at 72-h stage, C) Dead embryo at 72-h stage.
D) Normal embryo at 96-h stage. E) Malformed embryo at 96-h stage. F)
Dead embryo at 96-h stage. G) Normal embryo at 8-day stage. H) Malformed
embryo at 8-day stage. I) Dead embryo at 8-day stage. J) Normal embryo
at 14-day stage. K) Malformed embryo at 14-day stage. L) Dead embryo at
14-day stage.
The second, third, fourth and fifth F1 broods were also recovered and embryo toxicity tests were conducted as described above.
Micronucleus test
Two replicates and five snails from each replicate were used for study Genotoxicity. Shells of were gently broken between two glass slides, and the mass was separated and immersed in 2ml HBSSBioWhittaker ® buffer (Lonza). Mechanical cell dissociation was carried out in a glass/glass tissue grinder (Sartorius).Pieces of tissue were separated by centrifugation (5min at 250g). The supernatant was incubated 2h with 6 mg/ml collagenase type IA (Sigma) at 37°C, and the cell suspension was passed through 70-μm gauze. At this point, the same procedure described by Sanchez-Arguello et al. [15] for micronucleus scoring with Physaacuta embryos was followed exactly, except that the dechorionation step was excluded.
Part of the recovered egg masses of the first Oviposition was used for testing micronuclei induction in the F1 embryos. Exposure continued in the 12-well plates (2wells/treatment) for 1 week under same treatment conditions as their parents. Then eggs were treated exactly as described by Sánchez-Argüello et al. [15] for the micronucleus test.
Preparations were coded to determine micronucleus frequencies. For each treatment two replicates were scored and five hundred cells were counted for each replicate, being scored a total of 1,000 cells/ treatment.
Global DNA methylation pattern analysis
DNA was extracted with the Master Pure TMDNA Purification kit (Epicentre® Biotechnologies). Restriction digestions of 1μg DNA were performed with 10 units of either Hpa II and Msp I in buffer SL (Sigma), and were incubated for 18h at 37°C. Hpa II and Msp I are isoschizomers that recognize the tetranucleotide sequence 5’-CCGG- 3’but show differential cleavage sensitivity to cytosine methylation. Digestion products were stored at -20°C until amplified by PCR.
Methylation Sensitive Amplified Polymorphism (MSAP) was performed using two primers (MGC0: 5’-AAC CCT CAC CCT AAC CGC GC + MGF2: 5’-AAC CCT CAC CCT AAC CCG CG) or three primers (MGC0 + MGF2+ MLG2: 5’-AAC CCT CAC CCT AAC CCCGG) (Stab Vida) which arbitrarily bound within the GC-rich regions of DNA. PCR reactions were performed in a total volume of 50μl that contained: 0.5μl of each primer (0.5μM), 25μl BioMixTM Red (Bioline) and 50mg of digested DNA. The reactions were carried out in a thermocycler (UnoCycler, VWR). The cycling conditions include five cycles of 30s at 94°C, 1min at 40°C, and 1.5min at 72°C [based on 37,24].
A high-resolution display of the IRDye-labelled PCR products was obtained using the primers labeled with 5’IRDye®-800 (Fisher Scientific). The IRDye-labelled DNA fragments were separated into denaturing polyacrylamide gels (16% Long Ranger®, 50% Gel Solution (Lonza), 7M urea and 1xTBE) at 1,500 V for 2h and 30min in a Li-Cor 4300 DNA Analysis System. Fragments of 100-400 bp were visually scored. The appearance or disappearance of amplified fragments was considered. Fragments were scored as present (1) or absent (0). The MSAP data were interpreted according to the review of Fulnecek and Kovarik [38]. (Table 1) summarizes the Msp I/Hpa II profile and the forms of the CCGG sites, which were considered [38]. The Msp I/ Hpa II profile observed in the controls was compared with the Msp I/ Hpa II profile of the treated samples, and the following interpretation was used:
Site
5’CCGG
GGCC5’
5’CmCGG
GGmCC5’
5’CmCGG
GGCmC5’
5’mCCGG
GGCmC5’
Msp I
1
1
0
0
Hpa II
1
0
0
0
Table 1: Effect on cleavage and the expected Methylated Sensitive Amplified Polymorphism (MSAP) profile according to Fulnecek and Kovarik [38] after digestion with isoschizomers Msp I and Hpa II. (1) A sequence is cut and a band is present in the electrophoretic profile of MSAP, (0) a sequence is not cut and a band is absent.
Control (Msp I/Hpa II: 1/1) and treatment (Msp I/Hpa II: 1/0) is a methylation event. Control (Msp I/Hpa II: 1/1) and treatment (Msp I/Hpa II: 0/0) is a methylation event. Control (Msp I/Hpa II: 1/0) and treatment (Msp I/Hpa II: 1/1) is a demethylation event. Control (Msp I/Hpa II: 0/0) and treatment (Msp I/Hpa II: 1/1) is a demethylation event.
Chemical analysis
VZ water concentrations were determined by the HPLC analysis with UV detection after SPE [based on 32]. Quantification was performed using external standards (fortified water samples 500- 15ng/ml). The limits of quantification and detection were established as15ng/ml and 5ng/ml respectively.
The adult snails collected at the end of the test were homogenized in acetone (Ultra-Turrax). The body burden measures of VZ were taken. The obtained extracts were analysed by GC-EC (HP-5 30m x 0.25 mm Agilent 19091J-413 column, 1.5 ml/min He and μECD). VZ recovery was higher than 95% when fortified blank snails were used.
Statistical analysis
The fecundity, fertility and micronuclei data were compared.
Student’s t-tests were performed to analyze differences in the fecundity (egg masses/adult) data of the pre-screening test. Nevertheless, fecundity expressed as egg masses and cumulated number of eggs produced per snail did not satisfy the normal distribution criteria. In this case, a Kruskal-Wallis test, followed by a Mann-Whitney test, was applied to study with the controls.
Hatching success data of the first Oviposition was tested for normality. Both the F1 non-exposed and exposed embryo toxicity data of the controls and solvents were normally distributed. Student’s t-tests were performed to study if the solvents differed significantly from the controls. Acetone did not produce significant embryo toxicity. Thus the data for the solvents and treatments were transformed to hatching percentages compared with their plate control wells. These transformed data were normally distributed and a two-factor ANOVA was performed. Differences due to the factor of treatments (Acetone; 0.0005; 0.005; 0.05; 0.5 and 5mg/L) and the factor of the exposure conditions (F1 non-exposed or exposed embryos) were studied. Finally, a one-way ANOVA, followed by a multiple comparison test (LSD), was applied to discern which treatments differed from the solvent controls.
The data of the subsequent Oviposition (percentage of embryo development completed, malformations and lethal effects) were set as the dependent variables, and the multivariate and multifactor GLM (General Linear Model) was used for statistical discriminations.
The micronuclei frequencies of adult snails and F1 embryos for controls and acetone were grouped. Data fitted normal distributions and Student’s t-tests were performed to study if the solvents differed significantly from the controls. Acetone produced no significant MN induction compared with the controls. Then MN frequencies were transformed and were represented only for the solvent and treatments in accordance with Sánchez-Argüello et al. [15]. MN data were transformed by dividing absolute frequencies by the average of their respective control assay, represented as MN induction above the control levels (relative MN induction). Transformed MN data were distributed normally and Student’s t-tests were applied to study the differences compared with their respective solvent controls.
Statistical analyses were performed by standard procedures with the SPSS 13.0package.
Results and Discussion
Acute and reproduction tests
Acute and reproduction tests No mortality was observed in either the pre-screening test or the lower treatment concentrations of the PLC test. Approximately 10% of mortality was found in the PLC test (Table 2), which is acceptable for assessing reproduction, and is as recommended for other standardized tests [i.e. 20% for the OECD Daphnia magna reproduction test, [4]. The highest treatment concentration produced a lower feeding rate and an avoiding behaviour in snails at the beginning of the test, which disappeared during the test. This derived ultimately in lethal effects. However, fecundity was also tested at the highest treatment concentration (5mg/L) in two replicates, where mortality was kept lower than 10% by replacing the dead snails from the other two replicates. A strong and statistically significant decrease of fecundity was observed for this highest treatment concentration (Kruskal-Wallis test p<0.01, df=6, n=312; Mann-Whitney test for the controls versus 5mgVZ/L p<0.01).None of the other VZ concentrations had any effects on fecundity, although a slight rise in Oviposition was noted, which increased according to concentration, but was not statistically significant.
Treatments (mg/L)
Control
Acetona (0.1%)
0.0005
0.005
0.05
0.5
5
Mortality (%) 96h test
0 (n=3)
0 (n=3)
0 (n=3)
0 (n=3)
0 (n=3)
0 (n=3)
0 (n=3)
Mortality (%) PLC test
10 ± 8 (n=4)
10 ± 8 (n=4)
2 ± 5 (n=4)
0± 0 (n=4)
12± 5 (n=4)
10± 11 (n=4)
40± 18 (n=4)
Fecundity (egg masses/snail) 96h test
1.4 ± 0.7 (n=3)
1.8 ± 0.5 (n=3)
1.4 ± 0.6 (n=3)
0.6 ± 0.3* (n=3)
Fecundity (egg masses/snail) PLC test
10 ± 2 (n=4)
11 ± 2 (n=4)
10 ± 1 (n=4)
11 ± 0.5 (n=4)
10 ± 2 (n=4)
12 ± 2 (n=4)
1 ± 0.3** (n=4)
Fecundity (egg number/snail) PLC test
223 ± 37 (n=4)
229 ± 19 (n=4)
241 ± 21 (n=4)
245 ± 26 (n=4)
244 ± 29 (n=4)
276 ± 28 (n=4)
16 ±4** (n=2a)
Table 2: Acute pre-screening test and reproduction test after VZ exposure of adult snails (Physaacuta). Cumulative mortality percentage (lethal effects) and the average of both cumulative egg masses and egg number per snail (fecundity). *p<0.05 Student’s t-test (96h test) **p<0.01 (Mann-Whitney Test). A n=2 corresponds to replicates with a mortality below 10%.
Previous research into the effects of VZ has indicated that this fungicide may alter the reproduction of molluscs. Tillmann et al. [34] described an earlier sexual repose of Marisa cornuarietis males at lower VZ concentrations (0.03 and 0.1μg/L) than we used herein. Lagadic et al. [39] pointed out the adverse effect of VZ as a result of endocrine disruption, whose nature and amplitude probably depended on the exposed life-stages and exposure conditions. In their preliminary investigations into VZ, these authors observed that the production of egg masses of L. staganalis was stimulated, but the hatching rate lowered after exposure to 0.25mg/L. Ducrot et al. [35] exposed young adults and adults of the pond snail Lymnaeastagnalis to a range of VZ concentrations (0.000025; 0.00025; 0.0025; 0.025; 0.25 and 2.5mg/L) to test fecundity. While fecundity significantly reduced in young snails at all tested concentrations, adult snails exhibited lower fecundity and mortality during the test, but only at 2.5mg/L. These authors concluded that age and maturation status influenced the sensitivity of the PLC experiments. The eggs produced by young snails were generally not fertilized, which the authors associated with impaired the male function. Using a similar range of concentrations for the adults of Physaacuta, we also observed effects on fecundity in adult snails at 5mg/L.
F1 embryo toxicity tests: Fertility
The fertility of the first Oviposition (Figure 2) was statistically affected by the factor of treatments (two-factor ANOVA; F= 3.40; p<0.05; df=5) but not by the factor of the non-exposed/exposed conditions (Two-factor ANOVA; F= 0.32; p>0.05; df=1). The highest treatment concentration produced statistically significant effects compared with the controls (One-way ANOVA/LSD; F= 3.8; p<0.05; DF=5). A sharp drop in hatching (32%) was observed when the F1 eggs were exposed during embryonic development (Figure 2), while the hatching percentage of the F1 non-exposed eggs was higher (54%). These results showed recovery trends for the embryos with parental exposure when exposure was suspended during embryonic development (Figure 2). Nevertheless, lower concentrations produced few differences in the effects between the F1 exposed and non-exposed embryos, as shown in (Figure 3) for the 0.5 mg/L treatment. This effect was observed in the following Oviposition (second, third, fourth and fifth F1 broods) for all the treatments below 5 mg/L. A similar slope of the curves for effects on embryos (normal embryonary development, mortality and malformations) in both F1 exposed and non-exposed embryos is observed in (Figure 4). Although the variability of data for F1 embryo toxicity was higher in the exposed embryos than in the non-exposed ones, the subsequent exposure conditions did not cause statistical differences for any variable (GLM; F= 0.66; p>0.05). The larger the brood number, the less successful hatching. The F1 hatching percentage lowered in the controls from 30% to 0% between second and fifth brood, respectively. Normal embryonary development was affected by both brood number (one-way ANOVA; F=23.04; p<0.05; DF=2) and treatments (one-way ANOVA; F= 4.65; p<0.05; DF=6). Malformations increased depending on brood number (oneway ANOVA; F=25.95; p<0.05; DF=2) and treatments (one-way ANOVA; F=3.12; p<0.05; DF=6). Nevertheless, lethal effects were affected by treatments (one-way ANOVA; F=3.01; p<0.05; DF=6) regardless of brood number (one-way ANOVA; F= 2.19; p<0.05; DF= 2). Therefore, lethal effects were caused mainly by treatments, while malformations were attributed to the quality of broods. These findings lead to the conclusion that for the next PLC experiments, the first brood would suffice to study fertility since its viability was the best.
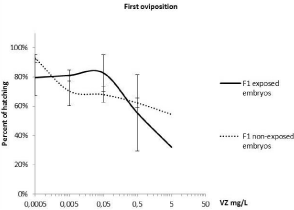
Figure 2: Percentage of hatching for offspring (F1 embryos) compared with
the control. The hatching percentages for solvents came close to 70% (67.6%
± 17.4 for exposed and 71.3% ± 20.6 for non-exposed). It was not possible
to represent them because the logarithmic scale was used (treatments=0).
Statistically analysis after a one-way ANOVA, followed by a multiple
comparison test (LSD), showed which treatments differed from the solvents
(*p<0.05).
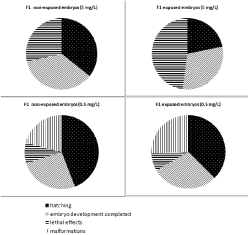
Figure 3: Embryonic effects (hatching, embryo development completed,
lethal effects and malformations) for both the F1 non-exposed and exposed
embryos after exposure to 5 and 0.5mg/L.
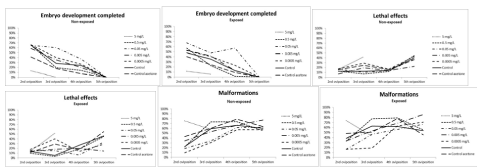
Figure 4: The embryonic effects (embryo development completed, lethal effects and malformations) observed in the second, third, fourth and fifth Oviposition after
the PLC tests. Both curves of the F1 non-exposed and exposed embryos showed similar slopes but higher uniformity was observed for the non-exposed embryos.
Lethal effects and decreased fecundity, both observed after adult VZ exposure to 5mg/L, also correlated with the lower fertility (hatching success) of both exposed and non-exposed F1 embryos. The embryo toxicity of VZ for this study did not correlate with our previous research since this treatment did not produce embryo toxicity for those eggs without parental exposure. More than 70% of the hatchability for the embryos without parental exposure was observed at 5mgVZ/L [15]. These differences in response sensitivity indicated an increasing sensitivity of embryos in this study (both exposed and non-exposed during embryonic development), probably due to the chronically stressed parents. Other studies conducted with freshwater snails have reported latent effects being expressed one generation later [40,41]. Oliveira-Filho et al. [42] evaluated the effects on the fecundity of mature F0 and F1 freshwater snails (Biomphalariatenagophila) and developmental toxicity in F1 and F2 embryos after exposure to endosulfan and ethanol. Their results indicated that while the reproductive effects of endosulfan did not apparently change as exposure extended to successive generations, ethanol effects became even more marked in the subsequent generation. The effect observed at 5mg/L herein, which did not correlate with the previous study [15] showed that early life stages (embryos) cannot be as sensitive as the adults of Physaacuta after chronic exposure. These results disagree that the short-term test with early life stages often shows the same effect concentrations as those observed with less sensitive life stages (i.e. adults) after long-term exposure [43]. Seeland et al [18] also observed less sensitivity of P acuta embryos than juveniles, which can be explained by egg integument acting against harmful environmental influences. Munley et al. [17] found that growth effects were predictive of reproduction effects when comparing a 28-day early life-stage test and a Full Life-Cycle (FLC) test on Lymnaeastagnalis, although the embryonic growth of F1 organisms was not included in the comparative data analysis.
Micronucleus tests
The adults exposed to 0.5 and 5mg/L showed statistically significant micronuclei induction. Although fungicide VZ has been ranked as essentially non-genotoxic [44,45] very few reports have indicated that it induces Genotoxicity (i.e. micronuclei, chromosome aberration and sister chromatid exchange) in mammalian cells in vivo and in vitro [46,47,31]. The highest concentration (5 mg/L) also produced a statistically significant induction of micronuclei in embryos without parental exposure, as previously observed by Sánchez-Argüello et al [15]. All the other treatments did not produce statistically significant MN induction in F1 embryos herein (Figure 5). Further research to make comparisons is required since the 0.5 mg/L treatment was not tested in embryos without parental exposure [15] and also because it was not possible to test 5mg/L in F1 embryos as it were embryo toxic.
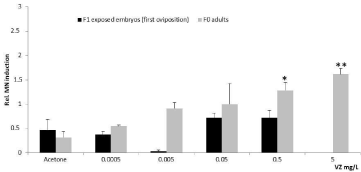
Figure 5: Relative induction of micronuclei in the cells isolated from adult
snails and the F1 exposed embryos. Data are shown as mean ± std (n=2;
1000 cells/n). Statistically significant differences after the Student’s t-tests
referred to the comparison made between treatments and solvents (*p<0.05;
**p<0.01).
Effects on DNA methylation
MSAP-PCR proved valuable for detecting that Physaacuta displays DNA methylation for the first time because we observed differences between profiles Msp I and Hpa II. Some modifications to the global DNA methylation pattern were also observed among the different treatments. Both methylation and demethylation events took place in treatments (Figure 6) compared with the control. Studies into the epigenetic effects of VZ exposure have focused on mammals. VZ induces both methylation and demethylation events in 25 regions in the rat genome [30]. A wide promoter analysis has identified 66 mouse promoters with 68 differential DNA methylation regions [48]. Studies on epigenetic biomarkers with exposed invertebrates are rare. When it comes to DNA methylation, insects have been the most widely studied invertebrate group [23]. One of the earliest investigations that studied the role of DNA methylation in invertebrate gene expression reported differences in patterns between insecticide-resistant aphid clones and those that had lost resistance [49]. Del Gaudio et al. [50] showed for the first time that the DNA of a polychaete annelid marine worm is methylated and that methylation is lower in adults than in embryos or sperm DNA. Krauss et al. [51] demonstrated that DNA methylation can play a key role in gene activity regulation during development in walking stick insects. Vandegehuchte et al. [33] demonstrated a reduction in the overall DNA methylation of Daphnia magna after exposure to 430μg/L of VZ, and also revealed that toxicant exposure can not only perturb the DNA methylation status of water fleas, but be transferred to two subsequent non-exposed generations. Very little work has been done to investigate DNA methylation in molluscs. The first evidence that supported a regulatory role of intragenic DNA methylation in invertebrates was done in a pacific oyster. The authors of this work suggested that DNA methylation in molluscs perform regulatory functions, including those involved in stress and environmental responses [52]. Their research showed an alteration to the DNA methylation profiles of treatments compared with the control, as expressed qualitatively in the events of both demethylation (five sites) and methylation (two sites).
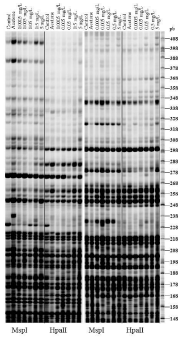
Figure 6: The methylation-sensitive arbitrarily primed PCR (MSAP-PCR)
analysis of the DNA digested with MspI and HpaII in polyacrylamide gels. Two
methylation profiles were obtained using two primers (a) and three primers
(b). Circles show demethylation events and squares denote methylation
events, according to Fulnecek and Kovarik (2014).
Chemical analysis
The exposure regime allowed concentrations to remain close to the nominal one during the experiment, as shown by the measurements taken before and immediately after medium renewal (Table3a). Previous experiments showed that VZ disappears rapidly in water (half-life of 10.8 h, 15] and is probably degraded to metabolites, but the presence of food could have conditioned its behaviour in this experiment. VZ bioavailability was expected to increase through dietary exposure since its log Kow is 3 [53]. VZ was measured in the snails recovered at the end of the PLC test (Table 3b). VZ might be adsorbed to living organisms but may also be bioconcentrated/ bioaccumulated by snails. VZ body burden peaked at 0.5 mg/L since similar concentrations in snails were observed for highest treatment concentration.
a) VZ water concentrations (mg/L)
b) VZ body burden (μg/g)
Nominal
Before medium renewal
After medium renewal
0.0005
0.0002
0.0002
0.0009
0.0009
0.049
0.076
0.005
0.0050
0.0052
0.0032
0.0039
0.206
0.600
0.05
0.0578
0.0625
0.0558
0.0691
4.72
4.94
0.5
0.4072
0.4225
0.5558
0.5166
18.60
25.46
5
3.909
3.370
5.336
nm
22.95
nm
Table 3: Actual VZ concentrations: a) Water concentrations before and after medium renewal. b) VZ body burden at the end of the reproduction test. No measured (nm).
Conclusion
This study showed that VZ affected the fecundity of Physaacuta, but only when effects on survival after long-term exposure were observed (5mg/L). The lower concentrations neither produced mortality nor affected fecundity. Similarly, fertility was affected only at 5mg/L. This concentration was not embryotoxic in a previous study conducted with embryos without parental exposure [15], which indicates that embryos were more sensitive when parents were exposed. Therefore, it can be concluded that a reproduction assessment in freshwater invertebrates should include both fecundity (eggs production) and fertility (offspring viability). Additionally comparisons between exposed and non-exposed offspring help indicate whether damage due to parental exposure can be maintained or recovered.
The highest treatment concentration (5mg/L) also resulted in Genotoxicity for adults. Recent studies have offered lines of evidence that MN formation is induced epigenetically. Luzhna et al. [25] have indicated that global DNA methylation loss correlates with MN formation. Demethylation was observed4 times at 5mg/L. This study provides information on the methylation pattern in a species representing a phylum where almost no information regarding this topic is available at the moment and show that a known epigenetic toxicant can cause alterations. Although data on the epigenetics of molluscs in an ecotoxicological context are scarce, this study shows that they are suitable for continuing with this research line. Both genetic and epigenetic molecular mechanisms are associated. The combination of genetic/epigenetic biomarkers for establishing mechanisms of inheritable molecular basis is recommended.
Reproduction success is ultimately the relevant endpoint to address effects at the population level. The linkage between reproduction effects and mechanisms capable of inducing heritable changes could broaden our understanding of pollutant impacts.
References
- OECD Guidelines for the Testing of Chemicals. Guideline 218: Sediment- Water Chironomid Toxicity Using Spiked Sediment. 2004a.
- OECD Guidelines for the Testing of Chemicals. Guideline 219: Sediment- Water Chironomid Toxicity Using Spiked water. 2004b.
- OECD Guidelines for the Testing of Chemicals.Guideline 225: Sediment- Water Lumbriculus Toxicity Test Using Spiked Sediment. 2007.
- OECD Guidelines for the Testing of Chemicals. Guideline 211. Daphnia magna reproduction test. 2012.
- OECD Guidelines for the Testing of Chemicals. Guideline 233. Sedimentwater chironomid life-cycle toxicity test using spiked water or spiked sediment. 2010a.
- Mathiessen P. Ecotoxicitytests methods for endocrine-disrupting chemicals. In Endocrine disrupters: Hazard testing and assessment methods. Published by John Wiley and Sons, Inc., Hoboken, New Jersey. 2013.
- Ducrot V, Askem C, Azam D, Brettschneider D, Brown R, Charles S, et al. Development and validation of an OECD reproductive toxicity test guideline with the pond snail Lymnaea stagnalis (Mollusca, Gastropoda). 2014; 70: 605-614.
- Bandow C, Weltje L. Development of an embryo toxicity test with the pond snail Lymnaea stagnalis using the model substance tributyltin and common solvents. 2012; 435-436: 90-95.
- OECD Guidelines for the Testing of Chemicals. Detailed review paper on molluscs life-cycle toxicity testing. ENV/JM/MONO (2010)9.2010b.
- Oehlmann J, Schulte-Oehlmann U, Tillmann M, Markert B. Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part I: bisphenol A and octtylphenol as xeno-estrogens. Ecotoxicology. 2000; 9: 383-397.
- Tripathi PK, Singh A. Toxic effects of cypermethrin and alphamethrin on reproduction and oxidative metabolism of the freshwater snail, Lymnaea acuminata. 2004; 58: 227-235.
- Czech P, Weber K, Dietrich DR. Effects of endocrine modulating substances on reproduction in the hermaphroditic snail Lymnaea stagnalis L. 2001; 53: 103-114.
- Leung KM, Morley NJ, Grist EP, Morritt D, Crane M. Chronic toxicity of tributyltin on development and reproduction of the hermaphroditic snail Physa fontinalis: Influence of population density. 2004; 58: 157-162.
- Leung KM, Grist EP, Morley NJ, Morritt D, Crane M. Chronic toxicity of tributyltin to development and reproduction of the European freshwater snail Lymnaea stagnalis (L.). 2007; 66: 1358-1366.
- Sanchez-Arguello P, Aparicio N, Fernandez C. Linking embryo toxicity with genotoxic responses in the freshwater snail Physaacuta: single exposure to benzo(a)pyrene, fluoxetine, bisphenol A, vinclozolin and exposure to binary mixtures with benzo(a)pyrene. Ecotoxicol EnvironSaf. 2012; 80: 152-160.
- Liu T, Koene JM, Dong X, Fu R. Sensitivity of isolated eggs of pond snails: a new method for toxicity assays and risk assessment. 2013; 185: 4183-4190.
- Munley KM, Brix KV, Panlilio J, Deforest DK, Grosell M. Growth inhibition in early life-stage tests predicts full life-cycle toxicity effects of lead in the freshwater pulmonate snail, Lymnaea stagnalis. 2013; 128-129: 60-66.
- Seeland A, Albrand J, Oehlman J, Muller R. Life stage-specific effects of the fungicide pyrimethanil and temperature on the snail Physellaacuta (Draparnaud, 1805) disclose the pitfalls for the aquatic risk assessment under global climate change. Environ Pollut. 2013; 174: 1-9.
- Bolognesi C, Hayashi M. Micronucleus assay in aquatic animals. 2011; 26: 205-213.
- Sanchez P, Llorente MT,Castao A. Flow cytometric detection of micronuclei and cell cycle alterations in fish-derived cells after exposure to three model genotoxic agents: mitomycin C, vincristine sulfate and benzo(a)pyrene. Mutat Res. 2000; 465: 113-122.
- Cakal Arslan O, Parlak H, Katalay S, Boyacioglu M, Karaaslan MA, Guner H. Detecting micronuclei frequency in some aquatic organisms for monitoring pollution of Izmir Bay (Western Turkey). 2010; 165: 55-66.
- Vandegehuchte MB, Janssen CR. Epigenetics and its implications for ecotoxicology. Ecotoxicology. 2011; 20: 607-624.
- Vandegehuchte MB, Janssen C. Epigenetics in an ecotoxicological context. Mutat Res. 2013; 764-765: 36-45.
- Watson RE, McKim JM, Cockerell GL, Goodman JI. The value of DNA methylation analysis in basic, initial toxicity assessments. Toxicol Sci. 2004; 79: 178-188.
- Luzhna L, Kathiria P, Kovalchuk O. Micronuclei in genotoxicity assessment: from genetics to epigenetics and beyond. 2013; 4: 131.
- Forget-Leray J, Landriau I, Minier C, Leboulenger F. Impact of endocrine toxicants on survival, development, and reproduction of the estuarine copepod Eurytemora affinis (Poppe). 2005; 60: 288-294.
- Zavala-Aguirre JL, Torres-Bugarin O, Zamora-Perez AL. Aquatic ecotoxicology approaches in Western Mexico. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007; 42: 1503-1511.
- Hutchinson TH, Odum J and Gourmelon A. Application of the OECD Conceptual Framework for Assessing the Human Health and Ecological Effects of Endocrine Disrupters. In Endocrine disrupters: Hazard testing and assessment methods. Published by John Wiley and Sons, Inc., Hoboken, New Jersey. 2013; 341-368.
- Van Ravenzwaay B, Kolle SN, Ramirez T, Kamp HG. Vinclozolin: a case study on the identification of endocrine active substances in the past and a future perspective. Toxicol Lett. 2013; 223: 271-279.
- Anway SL, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and males fertility.Science. 2005; 308: 1466- 1469.
- Schneider S, Marxfeld H, Groters S, Buesen R, Ravenzwaay B. Vinclozolinno transgenerational inheritance of anti-androgenic effects after maternal exposure during organogenesis via the intraperitoneal route. ReprodToxicol. 2013; 37: 6-14.
- Martinovi AD, Blake LS, Durhan EJ, Greene KJ, Kahl MD, Jensen KM, et al. Reproductive toxicity of vinclozolin in the fathead minnow: confirming an antiandrogenic mode of action. 2008; 27: 478-488.
- Vandegehuchte MB, Lemiere F, Vanhaecke L, Vanden Berghe W, Janssen CR. Direct and transgenerational impact on Daphnia magna of chemicals with a known effect on DNA methylation. Comp Biochem Physiol C Toxicol Pharmacol. 2010; 151: 278-285.
- Tillmann M, Schulte-Oehlmann U, Duft M, Markert B, Oehlmann J. Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part III: Cyproterone acetate and vinclozolin as antiandrogens. 2001; 10: 373-388.
- Ducrot V, Teixeira-Alves M, Lopes C, Delignette-Muller ML, Charles S, Lagadic L. Development of partial life-cycle experiments to assess the effects of endocrine disruptors on the freshwater gastropod Lymnaeastagnalis: a case-study with vinclozolin. Ecotoxicology. 2010; 19: 1312-1321.
- Sanchez-Arguello P, Fernandez C, Tarazona JV. Assessing the effects of fluoxetine on Physa acuta (Gastropoda, Pulmonata) and Chironomus riparius (Insecta, Diptera) using a two-species water-sediment test. 2009; 407: 1937- 1946.
- Gonzalgo ML, Liang G, Spruck CH 3rd, Zingg JM, Rideout WM 3rd, Jones PA. Identification and characterization of differentially methylated regions of genomic DNA by methylation-sensitive arbitrarily primed PCR. 1997; 57: 594- 599.
- FulneA ek J, KovaA™Ak A. How to interpret Methylation Sensitive Amplified Polymorphism (MSAP) profiles? 2014; 15: 2.
- Lagadic L, Coutellec MA, Caquet T. Endocrine disruption in aquatic pulmonate molluscs: few evidences, many challenges. 2007; 16: 45-59.
- Plautz SC, Guest T, Funkshouser MA, Salice CJ. Transgenerational crosstolerance to stress: parental exposure to predators increases offspring contaminant tolerance. Ecotoxicology. 2013; 22: 854-861.
- Kimberly DA, Salice CJ. If you could turn back time: understanding transgenerational latent effects of developmental exposure to contaminants. 2014; 184: 419-425.
- Oliveira-Filho EC, Grisolia CK, Paumgartten FJ. Effects of endosulfan and ethanol on the reproduction of the snail Biomphalaria tenagophila: a multigeneration study. 2009; 75: 398-404.
- Nebeker AV, Schuytema GS. Chronic effects of the herbicide diuron on freshwater cladocerans, amphipods, midges, minnows, worms, and snails. 1998; 35: 441-446.
- Kevekordes S, Gebel T, Pav K, Edenharder R, Dunkelberg H. Genotoxicity of selected pesticides in the mouse bone-marrow micronucleus test and in the sister chromatid exchange test with human lymphocytes in vitro. Toxicol Lett. 1996; 89: 35-42.
- Wu XJ, Lu WQ, Roos PH, Mersch-Sundermann V. Vinclozolin, a widely used fungizide, enhanced BaP-induced micronucleus formation in human derived hepatoma cells by increasing CYPA expression. Toxicol Lett. 2005; 159: 83- 88.
- Hrelia P, Fimognari C, Maffei F, Vigagni F, Mesirca R, Pozzetti L, et al. The genetic and non-genetic toxicity of the fungicide Vinclozolin. 1996; 11: 445- 453.
- Lioi MB, Scarfì MR, Santoro A, Barbieri R, Zeni O, Di Berardino D, et al. Genotoxicity and oxidative stress induced by pesticide exposure in bovine lymphocyte cultures in vitro. 1998; 403: 13-20.
- Guerrero-Bosagna C, Covert TR, HaqueMd M, Settles M, Nilsson EE, Anway MD, et al. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol. 2012; 34: 694-707.
- Field LM, Devonshire AL, French-Constant RH, Forde BG. Changes in DNA methylation is associated with loss of insecticide resistance in the peachpotato aphid Myzuspersicae (Sulz.). FEBS Lett. 1989; 243: 323-327.
- Del Gaudio R, Di Giaimo R, Geraci G. Genome methylation of the marine annelid worm Chaetopterus variopedatus: methylation of a CpG in an expressed H histone gene. 1997; 417: 48-52.
- Krauss V, Eisenhardt C, Unger T. The genome of the stick insect Medauroidea extradentata is strongly methylated within genes and repetitive DNA. 2009; 4: e7223.
- Gavery MR, Roberts SB. DNA methylation patterns provide insight into epigenetic regulation in the Pacific oyster (Crassostrea gigas). 2010; 11: 483.
- Tomlin CDS. The pesticide manual. Tomlin C.D.S. Editor.The British crop protection Council. 2000.
Citation: Sanchez-Arguello P, Aparicio N, Guevara MA, Díaz L, Cervera MT and Fernandez C. Effects on Reproduction, Genotoxicity and DNA Methylation Pattern after Chronic Exposure of the Freshwater Snail Physaacuta (Gastropoda, Pulmonata) to Vinclozolin. Austin J Environ Toxicol. 2016; 2(1): 1008. ISSN:2472-372X