
Research Article
Austin J Environ Toxicol. 2016; 2(1): 1009.
Antibiotic Pollution Pressure on Bizerte Lagoon Isolated Bacteria
Ben Said O*, Souissi M, Ben Khelil M, Aissa P and Beyrem H
Faculty of Sciences of Bizerte, Department of Sciences of Bizerte, University of Cathage, Tunisie
*Corresponding author: Olfa Ben Said, Faculty of Sciences of Bizerte, Laboratory of Environment Biomonitoring, Coastal Ecology and Ecotoxicology Unit, University of Cathage, Tunisia
Received: November 13, 2015; Accepted: February 24, 2016; Published: March 01, 2016
Abstract
Present work aims firstly to identify the most sold ATBs in Bizerte city. Its objectives are also to quantify the total and fecal bacteria at four lagoon stations near the outlet of Bizerte city Wastewater Treatment Plant (WWTP), to isolate, characterize and study their antibiotic resistance profiles. According to the census, the ß-lactams represented the most sold ATBs (99.8 %) in Bizerte city. Fecal bacterial biomass was higher in sediments mainly in upstream of WWTP discharge (3 106 fecal streptococci/mg). The outfall sediment appeared rich in fecal coliforms (2 105 bacteria / mg). Altogether, 88 bacterial strains have been isolated and characterized. The antibiotic resistance study has identified 100 % of resistance to ATBs tested at WWTP discharge point. Resistant phenotype was omnipresent in all sampling sites, in particular ß- lactam resistance profile.
Keywords: ATBs; Sold; Bacteria; Antibiotic resistance; Wastewater treatment plant
Background
According to a study conducted in 1999 and 2000 [1], 82 chemicals including 31 drugs (such as ATBs) were found in 80% of Canadian rivers. Antibiotics, defined as natural or synthetic compounds produced by a microorganism and prohibiting the growth of another [2], are widely used in human and veterinary medicine [2]. One of the consequences of their presence in the receiving environment is that many bacterial species develop transferable mutations, which allow them to escape the unfavorable external conditions [3] but also to develop antimicrobial resistance increasingly problematic for the environment. This resistance could be an aggravating factor, assuming that it would reduce the therapeutic options in case of infection. Indeed, antibiotic resistance, which leads to the spread of multi-resistant strains, is distinguished by its natural or acquired character, its mechanism and genetic support [4]. Antibiotics, once administered, are excreted and eliminated in untreated and treated wastewater [2]. Present work represents the first census of antibiotics consumed in Bizerte city. Its second objective is to quantify and isolate indicator bacteria of fecal contamination from Bizerte lagoon water and sediment samples collected upstream and downstream releases of Bizerte city wastewater treatment plant WWTP in order to study their antibiotic resistance profiles.
Materials and Methods
ATBs census
Survey site: Bizerte is a town in north-eastern Tunisia, located 65 km north-west of the capital (Figure 1). Its population was 536.0 people since the 2007 census [5]. Bizerte has 22 private pharmacies (including 2 night pharmacies), a regional hospital, a military hospital and polyclinic of the National Social Security Fund (CNSS) (Figure 1).
Figure 1: Location of Bizerte (a) and pharmacies (b) in Bizerte. (■) pharmacy,
A: Regional Hospital; B: Military Hospital; C: Polyclinic CNSS.
Survey at pharmacies: The investigation in pharmacies aims to make qualitative and quantitative assessment on ATBs sold in Bizerte city. The qualitative study is intended to focus on the top selling Bizerte city ATBs in 2008. The quantitative study is to quantify the most-consumed ATBs in 2008. Only 40.9% of Bizerte city pharmacies participated in this study that 86.4% of them correctly answered the questionnaire. A survey was also conducted among consumers considering 100 people of different ages and social backgrounds, to determine the real percentage of ATBs consumed (C = Ci / 100 with c: percentage of individual i consumption). The estimate of excreted ATBs amount (in g) was then performed by a calculation procedure that uses the data provided by the qualitative study, dosages and modes of selling drug action [6] and the quantitative survey data. Antibiotic excreted quantity is expressed by the following formula:
QE (g / boxes) = Qi (g) x P x n (%), where:
QE: Amount of ATBs excreted in grams per can or bottle of syrup
Qi: Amount of ATBs ingested in g per tablet or spoon syrup
n: Number of tablets per box or number of scoops a bottle of syrup
P: Percentage of active ATBs excreted compared to the amount initially consumed
Thus the total amount of ATBs excreted during 2008 was estimated by the following
Formula behind: QEt (g) = QEx C x Nt knowing that Nt = (N / 40.9) x 100
Nt: Total cans or bottles ATBs sold in 2008 in all pharmacies
N: Total number of cans or bottles ATBs sold in 2008 40.9% of pharmacies
C: Real percentage of consumed ATBs
Bacterial strains isolation from Bizerte lagoon
Sample collection: Collecting samples of water and sediment was carried out in the bay of Sebra (S1, S2, S3) and Chaara station (S4) from the Bizerte lagoon (Figure 2). Back to laboratory, sediments were directly used to bacterial quantification and bacterial strain isolation.
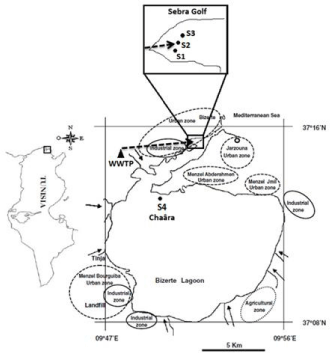
Figure 2: Location of sampling stations in Bizerte lagoon (September,
2009). WWTP: Bizerte city wastewater treatment plant; S1: upstream WWTP
discharge; S2: outfall WWTP discharge; S3: downstream WWTP discharge;
S4: WWTP discharge away station (Chaara); →: direction of WWTP
discharge.
Quantification and isolation of total and fecal bacterial strains
Enumeration of water and sediment total and fecal bacteria was performed by the method of the Most Probable Number (MPN), using the liquid culture medium suitable for each bacterial group. The isolation of fecal coliforms, fecal streptococci, pathogen staphylococci and non-Enterobacteriaceae was performed after plating saline suspensions (1g sediment or 1ml of water) on selective culture media. After incubation for 24 hours at 44 ° C, the morphologically different bacterial colonies were isolated and purified by the method of transplanting streaks. After isolated bacterial strains purification, we performed morphological characterization, Gram stain [7] and biochemical characterization by performing enzyme tests (oxidase, catalase) on crops youth.
Antibiotic susceptibility isolated fecal bacteria
Antibiotics tested were chosen by exploiting the results of carried antibiotic surveys and with reference to the literature [8,9]. Antibiotic resistance was determined by disk diffusion method [10] in Mueller- Hinton agar medium. Measuring the diameter of the bacteria growth inhibition zone was conducted for each antibiotic after 18-24 h incubation at 37 ° C.
Results
Census of ATBs
Survey in pharmacies: The survey highlights the marketing of a wide range of antibiotics, administered by several routes (oral, injectable, other channels). (Table 1) shows that the oral route is by far the most important route of ATB administration (49.3%) and the ß-lactams are the predominant family of antibiotics (Table 1). According to the sales frequency histogram of oral administration antibiotics, more likely to be discharged into the lagoon environment (Figure 3), the best-selling ATB are Clamoxyl® (100%), Augmentin® (89.5%), the Zithromax (84.2%), and the Oroken® Zinnat® (63.2%).
Administration routes
Oral routes
injectable solutions
Other routes
% most sold
49.3
13.3
37.4
ATB families
ß-lactam
ß-lactam
Aminosides
Table 1: Families of 2008 most sold ATBs in Bizerte city for each route of administration with the percentage (%) of sales.
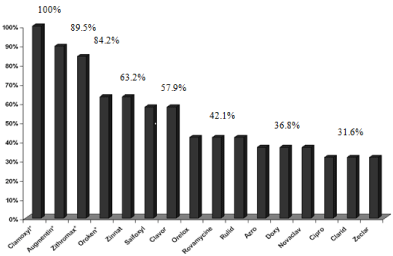
Figure 3: Sales frequency* (%) of the main oral antibiotics sold in Bizerte
city in 2008. Only represented antibiotic that % sales frequency> 30%. (*)
Percentage of pharmacies that sell the antibiotic in question.
Consumer survey: Whatever the age and social environment, consumers are generally conscious of the need to complete their antibiotic treatment, as evidenced by the value of the average percentage of Consumption (C) equal to 80.7%. According to the quantitative study, the family of ß-lactam containing Amoxicillin (AMX), Clavulanic Acid (CLA), Cefixime (CFM) and the Cefuroxime (CXM) represents 99.77% of the ATB total consumption (Figure 4), wherein the AMX alone represents 95.5% of antibiotics eliminated while Azithromycin (AZM), which is a macrolide represents only 0.20%.
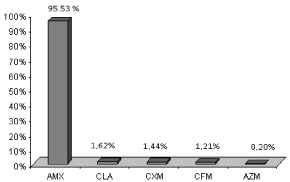
Figure 4: Relative importance (%) of excreted amounts of most consumed
ATB in Bizerte city in 2008. AMX: amoxicillin; CLA: clavulanic acid; CXM:
cefuroxime; CFM: cefixime; AZM: azithromycin.
Enumeration, isolation and antibiotic resistance of bacterial
Bacterial counts: Fecal bacterial abundance were highest in sediment collected at the upstream of WWTP discharge (3 106 bacteria / mg for fecal streptococci, 2 105 bacteria / mg for staphylococci and 104 bacteria / mg for total coliforms). In contrast, the water column of WWTP discharge point appeared to be most contaminated by fecal coliforms (6 103 bacteria / ml). At this station, sediments showed a maximum abundance of fecal coliforms (2 106 bacteria / mg).
Isolation of bacterial strains: From water and sediment samples, 88 bacterial strains were isolated and identified. The results of characterization showed that 64% of them belong to Gram+, with a predominance of Gram+ cocci (55%), against only 36% for Grambacteria. Gram bacilli isolated from water and sediment were 50% Enterobacteriaceae and 50% non-Enterobacteriaceae. In addition, among the Gram + cocci isolated (all stations and compartments combined), 48% were staphylococci and 52% were streptococci.
The biodiversity of streptococci was the most important at S2 (33% of streptococci strains) followed by the station S3 (29%), while the station S4 presented the lowest level of biodiversity (17%). Also, the biodiversity of staphylococci strains was higher in S2 (52%) and then in S3 (22%) although it was less interesting in S1 (9%). Enterobacteriaceae was particularly diversified at S2 (43%), also they were well represented at S1 (31%) and less diversified at S3 and S4 (only 13%). The non-Enterobacteriaceae strains also revealed a maximum biodiversity at S2 (49%), less biodiversity (19%) at S1 and S3 and a minimum of 13% at S4 (Figure 5).
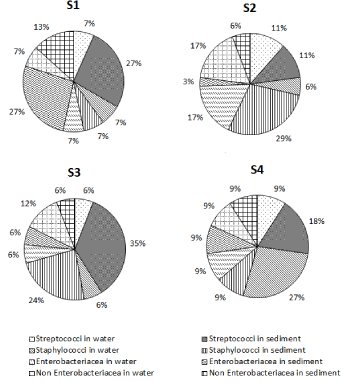
Figure 5: Spatial Biodiversity of fecal contamination bio-indicator bacteria
which were isolated from the Bizerte Lagoon (September 2009). S1:
upstream of the discharge station; S2: discharge station; S3: downstream of
the discharge station; S4: chemically clean station.
Antibiotic resistance: Resistance to antibiotics tested (all stations combined) (Figure 6), which shows the different resistance patterns to ATBs tested as well as their frequencies, highlights the resistant phenotype and the phenotype of sensitive bacterial strains. Our results show that i) the resistant phenotype is dominant for a majority of ATBs, including ß-lactam antibiotics (68% for the CTX 52% for AMX), but also to aminosides TM (79%) and GM (42%) ii) susceptible phenotype is well represented for several antibiotics, especially the CIP (80%).
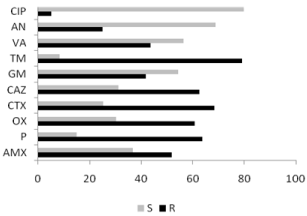
Figure 6: Frequency of antibiotic resistance phenotypes (%) among
bacterial strains isolated from Bizerte lagoon (September 2009). R:
resistant phenotype; S: sensitive phenotype; AMX: amoxicillin; P: penicillin
G; OX: oxacillin; CTX: cefotaxime; CAZ: ceftazidime; GM: gentamicin; TM:
tobramycin; VA: vancomycin; AN: amikacin; CIP: ciprofloxacin.
ß-lactam resistance spatial variation
From (Figure 6), a clear ß-lactams resistance was observed, whatever the sampling station. Indeed, 87% of bacterial strains isolated in station S1 were resistant to ß-lactam antibiotics against 94% in S2, 88% in S3 and 100% in S4.
Discussion
According to ATB survey results, oral administration is the predominant method of ATB treatment (49.3% of sales). The diversity of ATBs consumed is closely linked with that of bacterial infections and the development of antibacterial resistance [11], especially for ß-lactam antibiotics which represents the most important antibiotic family grouping penicillins, cephalosporins, carbapenems and monobactams [12]. Of the five ATBs most sold in Bizerte city in 2008, we have identified four ß-lactams (Clamoxyl®, Augmentin®, Zinnat® and Oroken®). This confirms the importance of marketing of this group. ATBs partially metabolized, and excreted in the drained wastewater beings finally discharged into Bizerte lagoon of after or without treatment. Furthermore, the significant commercialization of ß-lactam antibiotics, coupled with their high consumption (80.7%), can lead to what this family of ATBs is predestined to be released in large quantities in Bizerte lagoon. Indeed, 99.8% of the excreted ATBs are ß-lactam antibiotics including amoxicillin alone accounts for 95.5% of the amount released. According to antibiotic resistance phenotypes frequencies observed in isolated bacterial strains, we found a remarkable antibiotic resistance to all ATBs, especially of ß-lactam antibiotics (68% for CTX, 64% P, 63% for the CAZ, 61% to 52% for OX and AMX) and the TM (79%). This result point to the importance of the data obtained from the census of ATBs marketed in Bizerte city. The impact of the use of ATBs on the evolution of resistance [13] can explain our results which are consistent with those obtained by [14], indicating Bizerte lagoon bacterial resistance to OX 97%. Moreover, the high frequency of sensitivity t° CIP (80%) was not surprising since about 90% of fluoroquinolones (CIP) are usually removed in sewage stations and their concentrations in purified water are very low less than 20 ng / l [15,16]. Resistance to ß-lactam antibiotics has been important in all sampling stations (> 87%). It can be explained, including S1, S2 and S3, that these sampling sites are close to WWTP discharge. Furthermore, ß-lactam antibiotics absolute resistance in S4, however furthest WWTP discharge, can be attributed to the emergence of antibiotic resistance by transfer of resistance genes among bacterial strains during their transportation to the S4 station [4]. According to [17,18], the emergence of multiresistant bacteria and their rapid dissemination severely disrupts antibacterial therapy; resistance genes can be either produced by spontaneous mutations of existing genes, is added in the bacterial population from other microbes resistant [19-23]. ATB producing strains, equipped with genes encoding protective functions are potential sources of resistance genes [21]. Indeed, in the vast majority of cases, the cause of bacterial resistance is acquired by horizontal transfer mechanism of genetic material resistance [19,23].
Conclusion
This study focused on the problems associated with antibiotic therapy and the question of its effectiveness. Pharmacies selling movements of Bizerte city in 2008 showed consumption of various types of ATBs. The family of ß-lactam antibiotics has emerged as the most consumed and probably the most excreted through the wastewater discharged in Bizerte Lagoon. The study of antibiotic resistance of the isolated strains in Bizerte lagoon showed a remarkable antibiotic resistance to ß-lactam antibiotics and TM. The frequency of resistance to ß-lactam antibiotics, still high, however, varied spatially to reach 100% in S4, the farthest station WWTP of Bizerte city. The results of antibiotic resistance are consistent with those of ATB census, reflecting probably a selective ATB pressure on Bizerte lagoon ecosystem. It would be interesting to study, among bacterial isolated strains, genetic determinism involved in ATBs resistance belonging to the family of ß-lactam, especially the AMX and confirm ATB presence in Bizerte Lagoon sediment by analytical methods.
Funding Information
A mass consumption of antibiotic namely ß-lactam ATBs in Bizerte city in 2008. Bacterial strain isolated from water and sediment samples of Bizerte lagoon near WWTP of Bizerte city in 2009 showed 100 % of ß-lactam resistance.
Acknowledgment
This work was supported by a funding the Faculte des Sciences de Bizerte (FSB) and the Hopital regional de Bizerte.
References
- Water Pollution. Safe Drinking Water Foundation SDWF. 2009.
- Kummerer K. Antibiotics in Environment in Pharmaceuticals in the Environment Sources, Fate, Effects and Risks. Springer edition. Berlin Heidelberg. 2008; 75-93.
- Schluter A, Szczepanowski R, Puhler A, Top EM. Genomics of IncP-1 antibiotic resistance plasmids isolated from wastewater treatment plants provides evidence for a widely accessible drug resistance gene pool. FEMS Microbiol Rev. 2007; 31: 449-477.
- Yala D, Merad AS, Mohamedi D, Ouarkorich MN. Resistance bacterienne aux antibiotiques. Medecine du Maghreb. 2001; 91: 13-14.
- Institut national de la statistique. Tunisie. 2009.
- CD dictionnaire Vidal. 2009. Base de donnees medicamenteuses.
- Perrier R, Auffret T, Kemp VD, Zonszain F. Experiences faciles et moins faciles en sciences biologiques. Doin editor. Paris. 1997; 173-178.
- Turano A, Ravizzola G, Peroni L, Ceruti T, Greco LM, Pitzus E, et al. A multicentre study: Staphylococcus and Enterococcus susceptibility to antibiotics. Eur J Epidemiol. 1994; 10: 567-572.
- Coudert C, Beau F. Resistance des principaux germes responsables d’infection, isoles au laboratoire d’analyse de biologie medicale de l’institut Louis Malarde (annee 2003). Inform’Action. 2004; 17-21.
- Bonnet R, Cavallo JD, Chardon H, Chidiac C, Courvalin P, Dabernat H, et al. Comite de l’antibiogramme de la societe Francaise de microbiologie - Recommandations 2010. Societe francaise de microbiologie SFM. 2010; 15-17.
- Seppala H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997; 337: 441-446.
- Essack SY. The development of beta-lactam antibiotics in response to the evolution of beta-lactamases. Pharm Res. 2001; 18: 1391-1399.
- Guillemot D. Antibiotic use in humans and bacterial resistance. Curr Opin Microbiol. 1999; 2: 494-498.
- Ben Said O, Goni-Urriza MS, El Bour M, Dellali M, Aissa P, Duran R. Characterization of Aerobic Polyaromatic Hydrocarbon-Degrading Bacteria From Bizerte Lagoon Sediments, Tunisia. J Appl Microbiol. 2008; 104: 987- 997.
- Golet EM, Alder AC, Giger W. Environmental exposure and risk assessment of fluoroquinolone antibacterial agents in wastewater and river water of the Glatt Valley Watershed, Switzerland. Environ Sci Technol. 2002; 36: 3645- 3651.
- Golet EM, Xifra I, Siegrist H, Alder AC, Giger W. Environmental exposure assessment of fluoroquinolone antibacterial agents from sewage to soil. Environ Sci Technol. 2003; 37: 3243-3249.
- Cohen ML. Epidemiology of drug resistance: implications for a postantimicrobial era. Science. 1992; 257: 1050-1055.
- Neu HC. The crisis in antibiotic resistance. Science. 1992; 257: 1064-1073.
- Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994; 264: 375-382.
- Mazel D, Davies J. Antibiotic resistance in microbes. Cell Mol Life Sci. 1999; 56: 742-754.
- Martinez JL, Baquero F. Mutation frequencies and antibiotic resistance. Antimicrob Agents Chemother. 2000; 44: 1771-1777.
- Ochman H, Lawrence JG, Groisman EA. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000; 405: 299-304.
- Levy SB. Antibiotic resistance: consequences of inaction. Clin Infect Dis. 2001; 33: S124-129.