
Case Report
Austin J Environ Toxicol. 2016; 2(1): 1010.
Methemoglobinemia & Hemolytic Anemia Secondary to Hematite (Geru Powder) Ingestion in a G6PD Deficient Patient
Naidu S*, Datta K, Das I, Patel M, Verma S and Kole T
Department of Emergency Medicine, Max Hospital, India
*Corresponding author: Sarat Naidu, Department of Emergency Medicine, Max Hospital, Shalimar Bagh, India
Received: March 15, 2016; Accepted: May 02, 2016; Published: May 03, 2016
Abstract
A 3 years old previously healthy boy presented with h/o fever, gross hematuria and vomiting after ingesting a locally popular ayurvedic drug formulation called GERU which contains Ferric oxide.
On investigations he was found to be G6PD deficient which lead to Intravascular hemolysis and Methemoglobinemia due to oxidative stress.
He was aggressively managed and was discharged in a stable condition after 5 days.
Keywords: G6PD Deficiency; Glucose-6-phosphatase dehydrogenase; Hemolysis; Hemoglobinuria; Methemoglobinemia; Geru; Hematite; Toxicity; Ayurvedic medication; Ferric oxide; India
Introduction
Hematite is a chemical compound containing silicate of alumina and iron oxide (ferric oxide, Fe3+).
It is a mineral, coloured black, brown, grey or red. In India it is popularly called as GERU and is widely used for multiple purposes. In some areas of India it is used for jewelry making, painting earthenware pots, medicinal use topically in allergic rash, headache, bleeding, constipation etc.
G6PD (Glucose-6-Phosphatase Dehydrogenase) is an intracellular enzyme which plays an important role in protection of RBCs against oxidative stress like oxidant drugs, infections, fava beans etc [1-8].
G6PD deficient patients can develop acute hemolysis during oxidative stress [1].
The presence of methemoglobinemia has significant diagnostic and therapeutic consequences in G6PD deficient patients who are exposed to oxidative stress [1].
We report a previously healthy 3 yrs old boy who presented with severe acute hemolytic anemia and methemoglobinemia after ingesting a locally popular ayurvedic drug formulation called GERU (Hematite). He was subsequently diagnosed to have G6PD deficiency and was managed with conservative treatment successfully.
Case Study
A previously healthy 3 years old boy of Indian origin was brought to the emergency department at around 10 pm by his parents with h/o passing grossly dark coloured urine (hematuria), multiple episodes of vomiting and mild fever since 1 day.
They also mentioned that the child had some allergic skin rash 2 days back for which he was given orally some ayurvedic drug formulation locally known as Geru powder, following which he developed the above symptoms.
He otherwise had no known medical or surgical conditions and was not known to be allergic to any medications.
There was no h/o jaundice and was not on any medications.
There was no family h/o jaundice and blood disorder.
Physical examination revealed the child was conscious and oriented but was in mild to moderate respiratory distress with respiratory rate of 30/min.
He was pale, mildly jaundiced and febrile (101.5 deg F) but was not cyanosed.
His pulse rate was 100/min, BP was 100/50 mmHg, RBS was 120 mg%, SpO2 was only 75% at RA.
He had no hypothermia or rash.
Neurological, Cardiovascular, Respiratory and per abdominal examinations were insignificant.
Patient was immediately taken to the resuscitation bay and O2 supplementation was started @15LPM through non-rebreathing face mask but the SpO2 did not increase beyond 80%.
Arterial blood gas analysis showed pH = 7.442, PO2 = 32 mmHg, PCO2 = 27.9 mmHg, Lactate = 1.23 mmol/L, Methemoglobin levels = 21.3%, Hb = 6.4 gm%, O2 sat = 90%.
Chest x ray did not show anything significant.
In view of above findings, IV fluids were started with Dextrose 10% 100ml + Sodabicarb 8.4% 35ml @ 75ml/hr.
Nephrology and Hematology consultations were requested and the patient was shifted to Paediatric ICU after 2 hrs of aggressive management in the ER.
Course in the hospital and outcome
The diagnosis of acute severe hemolysis with methemoglobinemia was made.
Investigations revealed hemoglobin 6.8 gm/dl, TLC 24,000/cmm, raised unconjugated bilirubin, total lron levels 497 mcg/dl [Normal adult = 50-170, Children = 50-120].
G6PD activity showed 1.36 IU/gm Hb [Normal range 3.8 - 5.9 IU/ gm Hb in a child > 3 months age].
Blood Peripheral Smear exam showed Normocytic normochromic anemia with e/o hemolysis and no e/o basophilic stippling.
Kidney function test and Direct Coomb’s test were within normal limits.
Forced alkaline dieresis was started and Inj Frusemide was 20mg + 10mg given IV. Blood transfusion was started with packed red blood cells after which Hb increased to 12gm/dl.
He was started on Furosemide infusion @ 0.2mg/kg/hr and Vitamin C 500mg/day orally.
In view of fever, raised TLC and raised CRP, Piperacillin + Tazobactum antibiotic was started @ 1gm IV TDS.
After 12 hrs of presentation, patient’s respiratory distress decreased and MethHb levels reduced to 7.1%. Patient started maintaining SpO2 with minimal O2 requirement.
On day 3, MethhZb levels reduced to 2.6%.
On day 4, urine colour started improving (became lighter coloured) and finally became normal coloured and Bilirubin levels also became normalized.
Total iron levels reduced to 120mcg/dl.
Subsequently he was shifted to ward after 4 days of ICU stay and was discharged in stable condition after 5 days of hospitalization.
During the entire hospital stay, the child had normal higher mental functions and was on the improving trend since the start of the treatment.
Discussion and Therapeutic Considerations
This case report illustrates that severe intravascular hemolysis [9,1,4,10,7] and methemoglobinemia [9,1,2,11,12,10,13,5,6,8] can occur in a G6PD deficient patient following ingestion of ironcontaining (Fe3+) substances like hematite (geru powder) (Figures 1 & 2).

Figure 1: GERU mineral stone.

Figure 2: GERU in powder form.
The patient here developed the symptoms after ingesting a locally popular ayurvedic medication formulation called GERU powder which is actually HEMATITE that contains Silicate of Aluminate and Ferric oxide. In some parts of Northern India, geru powder is traditionally used for allergic skin rash as a topical form. But this child was given this chemical orally which triggered the symptoms eventhough it was taken in a small amount (less than a teaspoon).
This ferric oxide [Fe3+] in G6PD deficient patient leads to intravascular hemolysis and methemoglobinemia [1,2,11,12].
G6PD deficiency (Glucose-6-phosphate dehydrogenase deficiency) is an X-linked recessive genetic condition that predisposes to hemolysis (spontaneous destruction of red blood cells) and resultant jaundice in response to a number of triggers, such as certain foods (fava beans containing divicine [14]), illness, drugs (antimalarials) or some heavy metals [2].
It is commonly prevalent in people of Mediterranean and African origin [2].
It can manifest at any age when the trigerring factors come into play.
The condition is characterized by abnormally low levels of glucose-6-phosphate dehydrogenase, an enzyme involved in the pentose phosphate pathway/Hexose monophosphate shunt that is especially important for the red blood cell functioning [1].
There is no specific treatment, other than avoiding known triggers.
From (Figures 3 & 4), it is shown that the antioxidant defence of RBCs is dependent on G-6PD enzyme which catalysis the first reaction in HMP shunt [1].
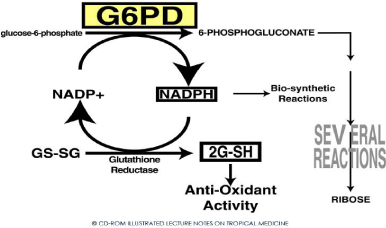
Figure 3: Hexose Monophosphate shunt (HMP) and G-6-PD.
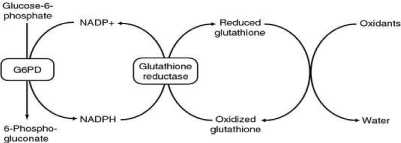
Figure 4: Hexose Monophosphate shunt (HMP) and G-6-PD.
This reaction produces NADPH which donates electrons to glutathione thereby producing reduced glutathione. This reduced glutathione neutralizes the reactive oxygen species thereby protecting RBC proteins including hemoglobin [1].
Methemoglobin (metHb) is a form of hemoglobin that contains ferric [Fe3+] iron and has a decreased affinity to bind oxygen [1,2].
Methemoglobinemia is a disorder characterized by the presence of a higher than normal level of methemoglobin in the blood [1].
Normal methemoglobin levels are <1% of total hemoglobin, as measured by the co-oximetry test [2].
MetHb is formed when ferrous [Fe2+] iron of heme is oxidized to ferric form [Fe3+] in presence of oxygen [2]. But under normal conditions, metHb is maintained below 1% of total Hb mainly by the enzyme NADH-dependant Cyt b5 - methemoglobin reductase. However if an exogenous oxidizing agent overwhelms this reducing system, metHb will rise.
Spontaneously formed methemoglobin is normally reduced (regenerating normal hemoglobin) by protective enzyme systems, e.g., NADH methemoglobin reductase (cytochrome-b5 reductase) (major pathway), NADPH methemoglobin reductase (minor pathway) and to a lesser extent the ascorbic acid and glutathione enzyme systems. Disruptions with these enzyme systems lead to methemoglobinemia.
The ferrous iron [Fe2+] in normal hemoglobin has an increased affinity for oxygen.
The binding of oxygen to methemoglobin results in an increased affinity of oxygen to the three other heme sites (that are still ferrous) within the same tetrameric hemoglobin unit. This leads to an overall reduced ability of the red blood cell to release oxygen to tissues, with the associated oxygen-hemoglobin dissociation curve therefore shifted to the left [2].
Elevated levels of methemoglobin in the blood are caused when the mechanisms that defend against oxidative stress within the red blood cell are overwhelmed and the oxygen carrying ferrous ion (Fe2+) of the heme group of the hemoglobin molecule is oxidized to the ferric state (Fe3+). This converts hemoglobin to methemoglobin, resulting in a reduced ability to release oxygen to tissues and thereby hypoxia. This can give the blood a bluish or chocolate-brown color.
Hypoxia occurs due to the decreased oxygen-binding capacity of methemoglobin, as well as the increased oxygen-binding affinity of other subunits in the same hemoglobin molecule, which prevents them from releasing oxygen at normal tissue oxygen levels.
The presence of met-Hb should be suspected when the oxygen saturation as measured by pulse oximetry is significantly different from the oxygen saturation calculated from arterial blood gas analysis (saturation gap), as evident in this case report [2].
Methemoglobinemia was indicative when the Pulse oximetry SpO2 was 75% and the ABG O2 saturation was 90% [2]. There was a significant gap between the 2 values.This was confirmed when the MethHb levels was shown to be raised in the lab values.
The patient was managed conservatively and aggressively with supplemental oxygen, forced alkaline diuresis, blood transfusion, antibiotics, adequate hydration and he was treated successfully.
The treatment of methemoglobinemia depends on the metHb levels [1].
In assymptomatic patients, levels above 30% needs treatment.
Symptomatic patients require treatment if levels are above 10%.
Methemoglobinemia is usually treated with 100% oxygen and 1% methylene blue solution @ 0.1-0.2 ml/kg [1-2 mg/kg] infusion IV over 3-5 mins [1,2,11].
MetHb is an oxidant which is reduced by NADPH to its active metabolite leukomethylene blue.
But in G6PD deficient patients, methylene blue cannot be reduced due to insufficiency of NADPH and eventually it will act as an oxidant, infact worsening the hemolytic anemia [15,16].
As a result, methylene blue may not only be ineffective but is also potentially dangerous, since it has an oxidant potential that may induce hemolysis in G6PD deficient subjects [1,2].
Methylene blue therefore, is contraindicated in G6PD deficiency since the reduction of met-Hb by methylene blue is dependent upon NADPH generated by G6PD [1,2].
However, if the methemoglobinemia is very severe and lifethreatening, methylene blue can still be used with caution at a lower dose @ 0.3-0.5mg/kg [11,13], with monitoring for any persisting or increased hemolysis.
If methylene blue is contraindicated, moderate doses of ascorbic acid (300 to 1000 mg/day orally in divided doses) should be given, as it may also cause oxidant hemolysis in G6PD-deficient patients when given in very high doses [2].
Blood transfusion with PRBCs is what is needed in severe hemolytic anemia [11].
Conclusion
Glucose-6-phosphatase dehydrogenase deficient patients can present with severe hemolytic anemia and methemoglobinemia even with exposure to mild oxidative stress, like in our report where the child had consumed a small amount of ferric oxide.
Methylene blue therapy is contraindicated or used with caution in G6PD deficient patients for methemoglobinemia.
Conservative treatment and blood transfusion and removal of the triggering factor remain the mainstay of treatment.
References
- Schuurman M, van Waardenburg D, Da Costa J, Niemarkt H, Leroy P. Severe hemolysis and methemoglobinemia following fava beans ingestion in Glucose-6-Phosphatase dehydrogenase deficiency: case report and literature review. Eur J Pediatr. 2009; 168: 779-782.
- Methemoglobinemia in an Elderly Patient with Glucose-6-Phosphate Dehydrogenase Deficiency: A Case Report. Oman Med J. 2014; 29: 135-137.
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008; 371: 64-74.
- Lau HK, Li CH, Lee AC. Acute massive haemolysis in children with glucose-6- phosphate dehydrogenase deficiency. Hong Kong Med J. 2006; 12: 149-151.
- Mullick P, Kumar A, Dayal M, Babbar S, Kumar A. Aniline-induced methaemoglobinaemia in a glucose-6-phosphate dehydrogenase enzyme deficient patient. Anaesth Intensive Care. 2007; 35: 286-288.
- Rosen PJ, Johnson C, McGehee WG, Beutler E. Failure of methylene blue treatment in toxic methemoglobinemia. Association with glucose-6-phosphate dehydrogenase deficiency. Ann Intern Med. 1971; 75: 83-86.
- Sarkar S, Prakash D, Marwaha RK, Garewal G, Kumar L, Singhi S, et al. Acute Intravascular Haemolysis In Glucose-6-Phosphate Dehydrogenase Deficiency. Ann Trop Paediatr. 1993; 13: 391-394.
- Verma M, Aggarwal A. Glucose-6 phosphate dehydrogenase deficiency with methemoglobinemia. Indian Pediatr. 1977; 14: 831-836.
- Tintinalli JE, Stapczynski S, Cline DM. Emergency Medicine. A Comprehensive study guide. 7th - edition. New York: Mc Grew Hill. 2011; 48: 1326-1329, 1487-1493.
- Liao YP, Hung DZ, Yang DY. Hemolytic anemia after methylene blue therapy for aniline-induced methemoglobinemia. Vet Hum Toxicol. 2002; 44: 19-21.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999; 34: 646- 656.
- Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J. 2011; 104: 757-761.
- MaddaliMM, Fahr J. Postoperative methemoglobinemia with associated G-6-P-D deficiency in infant cardiac surgery enigmas in diagnosis and management. Paediatr Anaesth. 2005; 15: 334-337.
- Arese P, Mannuzzu L, Turrini F. Pathophysiology of favism. Folia Haematol Int Mag Klin Morphol Blutforsch. 1989; 116: 745-752.
- Beall SN, Moorthy SS. Jaundice, oximetry, and spurious hemoglobin desaturation. Anesth Analg. 1989; 68: 806-807.
- Benatti U, Guida L, Grasso M, Tonetti M, De Flora A, Winterbourn CC. Hexose monophosphate shunt-stimulated reduction of methemoglobin by divicine. Arch Biochem Biophys. 1985; 242: 549-556.