
Research Article
Austin J Environ Toxicol. 2016; 2(2): 1014.
Genotoxicity Hazard Assessment of Industrial Effluent Discharge and Domestic Waste Discharge on Surface River Water
Mathur N¹*, Kaur K¹, Pareek S¹, Nepalia A¹ and Bhatnagar P²
¹Department of Zoology, University of Rajasthan, India
²Department of Life Sciences, IIS University, Jaipur, India
*Corresponding author: Mathur Nupur, Department of Zoology, University of Rajasthan, India
Received: June 07, 2016; Accepted: August 26, 2016; Published: August 31, 2016
Abstract
Surface water is utilized by humans for the purpose of drinking, domestic usage, industrial usage, irrigation etc. In many parts of the world this surface water is receiving indiscriminate dumping of industrial effluents, domestic sewage and agricultural runoff, thereby, deteriorating the quality of water. Untreated or improperly treated wastewater effluent discharges often contain mutagens especially when the proportion of industrial wastewater in comparison to municipal wastewater is high. Some of the substances found in waste water are genotoxic and are suspected to be possible case of cancers observed in the last decades. This restricts the usage of surface water for potability and direct consumption by human population. Even then, the polluted water is being utilized continuously in many areas as they are the only natural sources of water. Also, toxicity and risk associated with the usage of such waters is ignored.
The present study focuses on genotoxicity assessment of water samples taken from upstream and downstream potability sites of Chambal River and also of effluent discharged into Chambal River from two big industries located in Kota (Rajasthan), India. The water samples taken from both upstream and downstream sites of Chambal River and also from the two effluent treatment plants were found to be highly genotoxic. The assays used for genotoxicity assessment were Salmonella typhimurium reverse mutation assay and E. coli. WP2 assay.
Genotoxicity tests were found to be an excellent means to study the toxicity and associated risk with these anthropogenic activities on natural water resources.
Keywords: Salmonella typhimurium; E. coli WP2 assay; Genotoxicity; Mutagenicity; Surface water; Water pollution
Introduction
Water Pollution is one of the major consequences of urbanization. In the quest for higher standards for life, humans are deteriorating and depleting the natural resources. Anthropogenic urban-industrial effluents discharge, domestic waste discharge and agricultural waste discharge can add significant amounts of contaminants to surface water and sediments and, consequently, water pollution is becoming a serious problem for the aquatic biota and humans that interact with these aquatic ecosystems. It is a well known fact that the contamination of water resources by genotoxic compounds is a worldwide problem [1-5].
In India, there is a tendency of disposal of industrial effluents directly into municipal sewer system, which is further treated along with the domestic sewage in the municipal sewage treatment plant. However, many cities are still lacking municipal sewage treatment plants and are directly discharging raw sewage and industrial effluents in the surface waters of the rivers in their vicinity deteriorating its quality and adding to its pollution load and increasing its toxicity for the humans itself. Studies have shown that water quality is an important risk factor in cancer and relative risks have been estimated [6,7].
Many mutagenecity and genotoxicity tests have been used in combination with physical and chemical analysis in order to evaluate water quality [5,8-13]. The growing interest in these tests is due to the fact that despite the existence of different toxicity mechanisms for various organisms of different species, a substance that is toxic for an organism often demonstrates similar toxic effects on other organisms [14].
One of the most commonly used microbial bioassays is the Ames Salmonella mutagenicity assay. It has several advantages over the use of mammals for testing compounds. Also E. coli WP2 reverse mutation system is a valuable tool for mutagenesis research [15] by using a battery of different bioassay systems each with different mechanism of toxicity, the composite toxicological response to a waste water sample can be characterized.
Material and Methods
Sampling sites
Chambal River is an important water source that supplies drinking water for over 10 lakh habitants of the city of Kota in the Rajasthan state of India. Treated drinking water are received from Chambal itself and supplied to the houses of the city. Surface water samples were collected at three sampling sites, of the Chambal River.
Sampling was done during the months of June (summer) and December (winter) to account for seasonal variation.
The sample sites were as follows (Figure 1):
Site 1: Upstream site of the Chambal River, located in the vicinity of the city of Kota prior to Kota Barrage.
Site 2: Kota Super thermal power plant, situated on the left bank of Chambal river at upstream of Kota barrage. Thermal power station has set up its own treatment plant for treatment of effluents prior its discharge into Chambal River.
Site 3: Downstream site of river after Kota barrage receiving effluents from industrial areas of Kota.
Site 4: DCM Shriram Rayons, the Kota complex, consists of several manufacturing plants: power plant, calcium carbide plant, cement plant, chloralkali plant, fertilizer plant, PVC plant, PVC compounding plant and common supporting units.
Site 5: The Akelgarh pumping station, the only pumping station in Kota city supplying potable water to the inhabitants of the city the water sample was taken from the site from where the Chambal water is pumped into the pumping station.
Site 6: Potable water supplied by Akelgarh pumping station (Figure 1).
Sample collection
Wastewater samples from all the sites were collected in precleaned and sterilized glass bottles and refrigerated at 4°C until testing. No further fractionation or treatment of samples was done.
The samples were then tested for their genotoxic potential in the test doses 2 μl, 5 μl, 10 μl, 50 μl and 100 μl. These sample mixtures were treated as a single entity and were tested in their crude form.
Bioassays
Ames Salmonella/microsome reversion mutagenicity Assay: The Salmonella/microsome reversion assay was conducted using the plate incorporation procedure [16,17]. The tester strains of Salmonella typhimurium viz. TA98, TA100 and TA102 were obtained from Microbial Type Culture Collection & Gene Bank, Institute of Microbial Technology (IMTEC), Chandigarh (India). The samples were analyzed with and without the hepatic S9 fraction, which incorporates an important aspect of mammalian metabolism into the in vitro test. To prepare S9 mix, uninduced Swiss-Albino mice liver was used [18]. The S9 mix contains liver enzymes, from a rat. These enzymes can metabolize the agent being tested in order to predict the mutagenic properties within a living system. Five dose levels of individual samples were tested (2, 5, 10, 50 and 100 μl). The positive controls used in this assay were Sodium azide used for TA100 in absence of S9 mix, 2-Nitrofluorene, used for TA98 and TA 102 in absence of S9 mix and 2-Anthramine used as positive control for TA98, TA100 and TA102 in presence of S9 mix. All the plates were run in duplicate. Each set of experiment was repeated twice.
The S. typhimurium strains TA98, TA100 and TA102 were grown at 37°C, with shaking, for 10hrs to obtain final cell concentration of 109 bacterial cells. 0.1 ml of this fresh culture was mixed with 0.2 ml of histidine/biotin solution, 0.1 ml or less of test chemical, 0.5 ml of buffer or 0.5 ml of S9 mix and total volume was made up to 1.0 ml by autoclaved distilled water. This mixture was then shaken and poured on plates containing about 25 ml of minimal glucose agar medium. The test concentrations were selected from a set of standard test doses for liquids. The plates were immediately covered with paper to protect photosensitive chemicals present in the test compounds. Plates were then inverted and placed in a dark incubator for 48 h at 37°C. The revertant colonies were clearly visible in a uniform background lawn of auxotrophic bacteria. After 48 h the revertant colonies on the test and control plates were counted. All regents used were of analytical grade, supplied by Himedia Laboratories Limited (India) and Sigma- Aldrich (India).
E.coli. WP2 Bioassay: Escherichia coli strain WP2 and its repairdeficient derivatives are suitable strains for mutagen screening. In these strains, agents which cause base substitution mutations can be shown to increase the frequency of trp+ revertants. In addition, agents causing many types of DNA damage can be detected through increased killing of the repaired deficient derivatives. E.coli. tryptophan reversion system has been used extensively in microbial studies (including chemical screening, radiation studies and analysis of bacteria DNA-repair pathways and in numerous non-genetic applications). In contrast to the Salmonella strains that have different unique target DNA sequences in the Histidine operon, the four most commonly used WP2 strains carry the same tryptophan marker, trpE [19]. The assay is currently used by many laboratories in conjunction with the Ames Salmonella assay for screening chemicals for mutagenic activity [20]. The strain was obtained from Microbial Type Culture Collection and gene Bank (MTCC), Institute of Microbial Technology (IMTech), Chandigarh (India).
The cultures were grown overnight in 10 ml of growth medium and re-incubated with aeration for an additional 2.5 h at 37°C till the cell density reached 2x108 cells/ml. SA2 agar was used in this assay as it contains a trace of tryptophan. After solidification, 2, 5, 10, 50 and 100 μL of the test material was added and spread through spreader. The plates were incubated at 37°C for 48 h, permitting diffusion of the chemical into agar. All samples were tested in at least two independent experiments using five doses and three plates per dose. All reagents used were of analytical grade, supplied by Himedia Laboratories Limited (India) and Sigma-Aldrich (India).
Statistical analysis for mutagenicity assays
Non-statistical analysis: The most common method of evaluation of data from the mutagenecity assay is the ‘‘two fold rule’’ [20]. This rule specifies that if a test compound doubles or more than doubles mean spontaneous mutation frequency obtained on the day of testing, then the compound is considered significantly mutagenic. Using this procedure the following criteria were used to interpret results:
- Positive-A compound is considered a mutagen if it produces a reproducible, dose-related increase in the number of revertant colonies in one or more strains of S. typhimurium and E.Coli WP2. A compound is considered a weak mutagen if it produces a reproducible dose- related increase in the number of revertant colonies in one or more strains but the number of revertants is not double the background number of colonies.
- Negative-A compound is considered a non-mutagen if no dose-related increase in the number of revertant colonies is observed in at least two independent experiments.
- Inconclusive-If a compound cannot be identified clearly as a mutagen or a non-mutagen, the results are classified as inconclusive (e.g. if there is one elevated count). For this analysis the doserelated increases in the number of revertant colonies were observed for the test compounds and mutagenicity ratios were calculated. Mutagenicity ratio is the ratio of average induced revertants on test plates (spontaneous revertants plus induced revertants) to average spontaneous revertants on negative control plates (spontaneous revertants).
For all samples that showed dose dependent increase in the number of revertant colonies, mutagenicity ratios were calculated. Mutagenicity ratio is the ratio of average induced revertants on test plates (spontaneous revertants plus induced revertants) to average spontaneous revertants on negative control plates (spontaneous revertants) [4]. Mutagenicity ratio of 2.0 or more is regarded as a significant indication of mutagenicity.
Statistical analysis: The Quadratic regression model was used to observe the genotoxic effects of water samples from 7 sampling sites, during summer and winter seasons on 3 strains of S. typhimurium and 1 strain of E. coli WP2. The SPSS ver.2 program was used for the quadratic regression analysis [21]. Revertant colonies were taken as the dependent variable and dose as the independent variable; whereas the time and strains (TA98, TA100, TA 102 and E.coli WP2) were fixed for all the seven water samples. A comparison-wise P value of 0.05 was considered to be statistically significant and test was twotailed.
Results
The results of Salmonella mutagenicity assay for four different sampling sites are summarized in (Table 1) as the mutagenicity ratio of average induced reversions to spontaneous reversions.
Site
Sample aliquot (μl)
Mutagenicity ratio TA98
Mutagenicity ratio TA100
Mutagenicity ratio TA102
Mutagenicity ratio E.Coli. WP2
Dec-08
Jun-09
Dec-08
Jun-09
Dec-08
Jun-09
Dec-08
Jun-09
0
0
0
0
0
0
0
0
0
0
0
0
0
0
DSCL (DCM Shriram Rayons Ltd.)
2
+
+
+
+
+
+
+
+
+
+
+
+
+
5
+
+
+
+
+
+
+
+
+
+
+
+
+
+
10
+
+
+
+
+
+
+
+
+
+
+
+
+
+
50
+
+
+
+
+
+
+
+
+
+
+
+
+
+
100
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Thermal power plant, Kota
2
+
+
+
+
+
+
+
+
+
+
+
+
+
+
5
+
+
+
+
+
+
+
+
+
+
+
+
+
+
10
+
+
+
+
+
+
+
+
+
+
+
+
+
+
50
+
+
+
+
+
+
+
+
+
+
+
+
+
+
100
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Domestic Sewage
2
+
+
+
+
+
+
+
+
+
+
+
+
+
+
5
+
+
+
+
+
+
+
+
+
+
+
+
+
+
10
+
+
+
+
+
+
+
+
+
+
+
+
+
+
50
+
+
+
+
+
+
+
+
+
+
+
+
+
+
100
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Table 1: Mutagenicity ratio of Salmonella tester strains TA98, TA100 and TA102 in Ames test and E.Coli. WP2 strain in E.Coli WP2 assay on waste water samples from various sites of Chambal River.
Site
Sample aliquot (μl)
Mutagenicity ratio TA98
Mutagenicity ratio TA100
Mutagenicity ratio TA102
Mutagenicity ratio E.Coli. WP2
Dec-08
Jun-09
Dec-08
Jun-09
Dec-08
Jun-09
Dec-08
Jun-09
0
0
0
0
0
0
0
0
0
0
0
0
0
0
Upstream Chambal river water
2
+
+
+
+
+
+
+
+
+
+
+
+
+
+
5
+
+
+
+
+
+
+
+
+
+
+
+
+
+
10
+
+
+
+
+
+
+
+
+
+
+
+
+
+
50
+
+
+
+
+
+
+
+
+
+
+
+
+
+
100
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Downstre--am Chambal river Water
2
+
+
+
+
+
+
+
+
+
+
+
+
+
+
5
+
+
+
+
+
+
+
+
+
+
+
+
+
+
10
+
+
+
+
+
+
+
+
+
+
+
+
+
+
50
+
+
+
+
+
+
+
+
+
+
+
+
+
+
100
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Pumping station, Akelgarh
2
-
-
-
-
-
+
-
+
+
+
-
+
-
-
5
+
+
-
+
+
+
-
+
+
+
+
+
-
-
10
+
+
+
+
+
+
+
+
+
+
+
+
-
-
50
+
+
+
+
+
+
+
+
+
+
+
+
+
+
100
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Potable water supplied by Akelgarh
2
-
-
-
-
-
-
-
-
-
-
-
-
-
-
5
-
-
-
-
-
-
-
-
-
-
-
-
-
-
10
-
+
-
+
-
-
-
-
-
-
-
-
-
-
50
+
+
-
+
-
-
-
+
-
-
-
+
-
-
100
+
+
+
+
-
+
-
+
+
+
+
+
-
-
Table 1 & off:
Upstream river water
Upstream river site receives effluents from industries, domestic sewage from adjoining areas and agricultural runoff. Water samples taken from this site showed positive mutagenicity with mutagenicity ratios much higher than 2.0 when tested using the two bioassays (Table 1). These samples showed 400-500 induced TA98 revertants without S9 (Graph 1); 500-600 induced TA100 revertants without S9 (Graph 2); 800-900 induced TA102 revertants without S9 (Graph 3) and 500- 900 induced E.Coli WP2 revertants per 100 μl of sample without S9 (Graph 4). Upon addition of S9 mix the number of revertant colonies obtained were increased to 950-1000 induced TA98 revertants with S9 (Graph 5); 920-930 induced TA100 revertants per 100 μl of sample with S9 (Graph 6); 1885-1900 induced TA102 revertants per 100 μl of sample with S9 (Graph 7).
graph 1: Dose-response curve of different water samples with strain TA98
without S9.
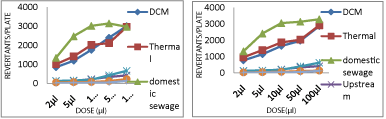
graph 2: Dose-response curve of different water samples with strain TA100
without S9.
graph 3: Dose-response curve of different water samples with strain TA102
without S9.
graph 4: Dose-response curve of different water samples with strain E.Coli
without S9.
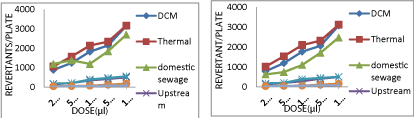
graph 5: Dose-response curve of different water samples with strain TA98
with S9.

graph 6: Dose-response curve of different water samples with strain TA100
with S9.
graph 7: Dose-response curve of different water samples with strain TA102
with S9.
Effluents discharged by the Kota Super thermal power plant
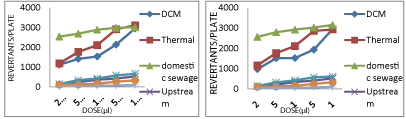
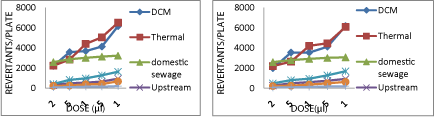
The Thermal power plant discharges its treated effluent sample in the Chambal river water at upstream site. During all the years of sampling, the effluent samples from the thermal plant showed positive mutagenicity with mutagenicity ratio much higher than 2.0. With the effluent coming out of the Thermal effluent treatment plant, the number of revertant colonies obtained were very high, (2900-3000 induced TA98 revertants per 100 μl of sample without S9 (Graph 1), (3100-3200 induced TA 100 reverttants per 100 μl of sample without S9 (Graph 2), (3300-3700 induced TA102 revertants per 100 μl of sample without S9 (Graph 3) in Ames bioassay. These high numbers of revertant colonies are indicators of strong mutagenic potential.
In E.Coli WP2 assay also the number of revertant colonies obtained were very high (3172-3214 induced E.Coli.WP2 revertants per 100 μl of sample without S9 (Graph 4). Addition of S9 mix further increased the number of revertant colonies obtained to two fold value (6000-6100 induced TA98 revertants per 100 μl of sample with S9 (Graph 5) indicating the presence of such metabolisable mutagens in discharge from Thermal plant which when metabolized by enzymes present in S9 fraction are transformed to highly potent mutagens. Also effluent coming out from the thermal plant did not show any seasonal variations when observed under two varied seasons.
Downstream river water
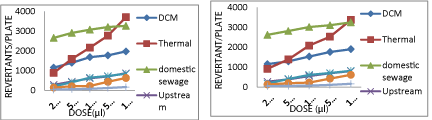
Downstream river site which passes through densely populated areas and industrial area receives effluents from many industries and a heavy load of domestic sewage. All these probably may contribute to its genotoxicity. The number of revertants found in these samples were much higher as compared to the upstream water samples (600- 700 induced TA98 revertants without S9 (Graph 1); 603-666 induced TA100 revertants without S9 (Graph 2); 800-1000 induced TA102 revertants without S9 (Graph 3); 500-1000 induced E.coli. WP2 revertants without S9 (Graph 4). Addition of S9 mix also showed increase in the number of revertants.
Also seasonal variations were observed in both the river water samples. In June, which is the dry and hot season of summer, the number of revertant obtained was higher than in month of December.
Effluents discharged by DCM Shriram Rayons
DCM Shriram Rayons discharges its treated effluents in the Chambal River at its downstream site. During both the years, the effluent samples from the DSCL showed positive mutagenicity with mutagenicity ratio much higher than 2.0. With DSCL samples when Ames assay was carried out with tester strain battery of TA98, TA100 and TA 102 the number of revertant colonies obtained were also very high (2800-3000 induced TA98 revertants per 100 μl of sample without S9 (Graph 1), (2500-3000 induced TA100 revertants per 100 μl of sample without S9 (Graph 2), (1500-2000 induced TA102 revertants per 100 μl of sample without S9 (Graph 3). Surprisingly these effluents from DSCL are being discharged by an industrial waste water treatment plant. These high numbers of revertant colonies are a strong indication of positive mutagenic potential of the effluent from DSCL. With E.Coli WP2 strain in E.Coli. WP2 assay also very high number of revertant colonies was obtained (3000-3300 induced E.Coli. WP2 revertants per 100 μl of sample without S9 (Graph 4). Addition of S9 mix obtained a much higher number of revertants (5900-6200 induced TA98 revertants per 100 μl of sample with S9 (Graph 5), (6400-6500 induced TA100 revertants per 100 μl of sample with S9 (Graph 6), (4100-4300 induced TA102 revertants per 100 μl of sample with S9 (Graph 7) indicating the presence of such mutagenic compounds in the effluent which when metabolized by river enzymes yielded metabolites of much higher mutagenic potential. Similar response of S9 mix was observed from both the industries. Also effluent coming out from the industry did not show any seasonal variations when observed under two varied seasons.
Water sample getting in from Chambal River to Akelgarh pumping station and potable water supplied
Chambal River water from the pumping site when analyzed for genotoxicity using bacterial bioassays showed positive mutagenicity with mutagenicity ratio higher than 2.0 on higher doses only. This shows that although at lower doses the water being pumped out from pumping site at Chambal River is non-mutagenic, the same is not true at higher doses. As seen from the dose response curve number of revertant colonies obtained at higher doses i.e. 50 μl (120-200 number of revertants obtained with TA 98 (Graph 1) and 100 μl (200- 400 number of revertants obtained with TA98 without S9 (Graph 1) are strongly indicating the presence of genotoxic compounds in this water sample. It is an indication of moderate mutagenicity of sample. When assayed on strain TA98, TA100, TA102 and E.Coli. WP2 the sample showed mutagenic response especially in the presence of mammalian liver enzymes.
The water from Chambal River is pumped at Akelgarh; the only pumping station of city and here after chlorination and filtration, the water is pumped to the houses of the city to be utilized for potable and other purposes. The water sample taken from this site showed negative mutagenicity at lower doses, but at the higher doses, the mutagenicity ratio obtained was higher than 2.0. This indicates that the mutagenicity is present in the water consumed directly by the people of Kota (Table 1). The number of revertant colonies obtained were 130-140 TA98 revertants without S9 (Graph 1); 100-120 TA100 revertants without S9 (Graph.2); 160-180 TA102 revertants without S9 (Graph 3) and 80-90 E.Coli. WP2 revertant without S9 (Graph 4). It indicates the presence of both frame shift and base pair mutagens in the potable water.
Discussion
The Chambal River receives untreated wastes from agricultural operations, industrial plants and domestic sewage and is the main water source for the city inhabitants. Results of this study showed that the effluents being discharged by the industries and domestic sewerage of Kota have components that can induce mutagenic responses. On comparing the number of revertant colonies obtained by effluents of both the industries it was observed that Thermal power plant produces much higher number of revertant colonies in comparison to DSCL industries. Similar observations for industrial effluents have also been reported by other researchers [2,21-25]. Discharge of such treated and untreated effluents have contaminated the receiving surface waters of Chambal river which when analyzed for mutagenic potential at two different sites, upstream and downstream of the river yielded positive results indicating the presence of potent mutagens in the Chambal river water. The samples taken from Akelgarh pumping station of Kota city prior to pumping and after treatment were also found to be mutagenic. The treatment protocol of the river water to be used for potable purposes includes only chlorination and filteration which removes the microbial content of the water but has no or very less effect on the chemical content which in turn is responsible for genotoxicity. Chlorination is a common water disinfectant method which is able to reduce microbial water pollution, but which can also produce genotoxic and toxic compounds if precursors are present in the water to be treated and the level of chlorine is high [26]. Surface water can contain variable levels of organic matter, including humic acids that are the main source of potentially toxic by-products of disinfection with chlorine, which can react with such compounds. The major chlorination by-products that have been the object of intensive evaluation are the trihalomethanes, halogenated acetic acids and chlorinated furanones, most of which are known carcinogens, although the cellular mechanisms of their carcinogenicity are poorly understood [26-30].
The mutagenic potential of river water is low when compared to the wastes and the effluents discharged into it. This can be due to the dilution of the effluent when it enters the river. The addition of S9 hepatic fraction has shown an increase in the number of revertants in all the strains (TA98, TA100, TA 102, E. Coli WP2). This indicates the presence of such compounds in the water samples, which gets metabolized into mutagenic compounds.The seasonal variation in mutagenecity found in the samples, is because in summer season, there is more evaporation leading to scarcity of water. Therefore the wastes in the water get concentrated and show more mutagenic potential as compared to that in winters (which follows rainy season).
Among the assays that have been used for evaluating water quality, bacterial systems have proved to be sensitive biomonitors of the genotoxic effects of environmental chemicals and can be used for the detection of environmental mutagens in vivo as well as in vitro. As the bacterial mutagenicity assays can be carried out in 48 hours, they can be used as rapid pre-screen for distinguishing between carcinogenic and non carcinogenic chemicals, allowing many thousands of components in our environment, not previously tested, to be screened for potential hazards. A good correlation has been obtained by several groups, for a number of carcinogenic compounds in their ability to induce mutation in the above strain and the ability to induce a response in animals (Ames et al., 1973). Short term genetic bioassays have proved to be an important tool in genotoxic studies because of their simplicity, sensitivity to genetic damage, speed, low cost of experimentation and small amount of sample required [21]. The present study also emphasizes the importance of the Ames Salmonella mutagenicity assay and E.Coli. WP2 assay as a shortterm test. These can be used as a complement to other ecological, toxicological and conventional chemical tests for establishing priorities of pollution control. However, along with the S9 mix used on prokaryotic systems, further animal studies should also be performed to actually assess the adverse effects of domestic sewage and industrial effluents discharge into surface water.
Conclusion
The indiscriminate dumping of improperly treated industrial effluents and domestic sewage is deteriorating the quality of natural water resources to a level restricting their usage. If this continues and in ignorance if human population consumes potable water having mutagenic potential on regular basis, it can result in infection, genotoxicity, chemical toxicity and may increase the possible risk of cancer. Along with the physic chemical analysis, biological analyses of liquid effluents generated should be ensured so that the liquid waste generated from industries is completely safe for disposal in environment. Intensive research in this area is further required. There is an urgent need to build common sewerage treatment plant in every city which is indiscriminately dumping domestic sewage without any treatment in the natural water resource to protect water resources from pollution and to safely utilize these natural water bodies for innumerable never ending needs.
This study also builds up a basic framework to acquire more information about the prevalence and levels of mutagenic agents in industrial effluents and domestic sewage. Furthermore, Ames test and E.Coli. WP2 assay being simple, quick and relatively easy to perform can be used as a initial screening test to assess the suitability of industrial effluents to be released into the environment. Complementary studies should be undertaken in the analytical field in the order to try to identify and quantify the compounds responsible for the genotoxicity. This difficult task will be necessary to identify the sources of toxic contaminants and thus to take preventive and/or curative measures in order to limit the toxicity of the effluents.
Although the treated water from potable site did not produce a significant genotoxic response, there was some indication of genotoxic potential. This is however a warning indication and if no measures are taken to rectify this ever increasing contamination of Chambal river it would lead to dire consequences.
Although a number of bioassays are available for genotoxicity testing, these two assays were chosen because of their simplicity, wide usage, low cost and wide acceptance for such monitoring studies. They can be used for day to day screening of complex environmental samples. They can thus be used as important pre-screening bioassays.
References
- Buschini A, Martino A, Gustavino B, Monfrinotti M, Poli P, Rossi C, et al. Comet assay and micronucleus test in circulating erythrocytes of Cyprinus carpio specimens exposed in situ to lake waters treated with disinfectants for potabilization. Mutat Res. 2004; 557: 119-129.
- Claxton LD, Houk VS, Hughes TJ. Genotoxicity of industrial wastes and effluents. Mutat Res. 1998; 410: 237-243.
- Kong IC. Metal toxicity on the dechlorination of monochlorophenols in fresh and acclimated anaerobic sediment slurries. Water Science and Technology. 1998; 38: 143-150.
- Mathur N, Bhatnagar P, Bakre P. Assessing mutagenicity of textile dyes from Pali (Rajasthan) using Ames bioassay. Applied Ecology and Environmental Research. 2006; 4: 111-118.
- Ohe T, White PA, DeMarini DM. Mutagenic characteristics of river waters flowing through large metropolitan areas in North America. Mutat Res Genet Toxicol Environ Mutagen. 2003; 534: 101-112.
- Koivusalo M, Vartiainen T, Hakulinen T, Pukkala E, Jaakkola JJ. Drinking-water mutagenicity and leukemia, lymphomas and cancers of the liver, pancreas and soft-tissue. Arch Environ Health. 1995; 50: 269-276.
- Tao XG, Zhu HG, Matanoski GM. Mutagenic drinking water and risk of male esophageal cancer: A population-based case-control study. Am J Epidemiol. 1999; 150: 443-452.
- Isidori M, Lavorgna M, Nardelli A, Parrella A. Integrated environmental assessment of Volturno River in South Italy. Sci Total Environ. 2004; 327: 123-134.
- Reinecke SA, Reinecke AJ. The comet assay as biomarker of heavy metal genotoxicity in earthworms. Arch Environ Contam Toxicol. 2004; 46: 208-215.
- Russo C, Rocco L, Morescalchi MA, Stingo V. Assessment of environmental stress by the micronucleus test and the Comet assay on the genome of teleost populations from two natural environments. Ecotox Environ Safety. 2004; 57: 168-174.
- Umbuzeiro GD, Roubicek DA, Sanchez PS, Sato MIZ. The Salmonella mutagenicity assay in a surface water quality monitoring program based on a 20-year survey. Mutat Res Genet Toxicol Environ Mutagen. 2001; 491: 119-126.
- Vargas VMF, Migliavacca SB, de Melo AC, Horn RC, Guidobono RR, Ferreira I, et al. Genotoxicity assessment in aquatic environments under the influence of heavy metals and organic contaminants. Mutat Res Genet Toxicol Environ Mutagen. 2001; 490: 141-158.
- Verschaeve L. Genotoxicity studies in groundwater, surface water and contaminated soil. Scientific World Journal. 2002; 2: 1247-1253.
- Kaiser KLE. Correlation of Vibrio fischeri bacteria test data with bioassay data for other organisms. Environ Health Prospect. 1998; 106: 583-91.
- Gupta P, Mathur N, Bhatnagar P, Nagar P, Srivastava S. Genotoxicity evaluation of hospital waste waters, Ecotoxicol. Environ. Saf. 2009; 72: 1925-1932.
- Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella mammalian microsome mutagenicity test. Mutation Research. 1975; 31: 347-364.
- Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mut. Res. 1983; 113: 173-215.
- Prival MJ, Bell SJ, Mitchell VD, Vaughan VL. Mutagenicity of benzidine and benzidine congener dyes and selected monoazo dyes in a modified Salmonella assay. Mut. Res. 1984; 136: 33-47.
- Wilcox P, Naidoo A, Wedd DJ, Gatehouse DG. Comparison of Salmonella typhimurium TA102 with Escherichia coli WP2 tester strains ; Mutagenesis. 1990; 5: 285-291.
- Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutation Research. 2000; 455: 29- 60.
- Mathur N, Bhatnagar P, Mohan K., Bakre P, Nagar P, Bijarnia MK. Mutagenicity evaluation of industrial sludge from common effluent treatment plant. Chemosphere. 2007; 67: 1229-1235.
- Gana JM, Ordoneza R, Zampinia C, Hidalgob M, Meonib S, Islaa IM. Industrial effluents and surface water genotoxicity and mutagenicity evaluation of a river of tucumana, Argentina. Journal of hazardous material. 2008; 155: 403-406.
- Houk VS, Demarini DM. Use of the microscreen phage- induction assay to assess the genotoxicity of 14 hazardous industrial wastes. Environ. Mol. Mutagen. 1998; 11: 13-29.
- McGeorge LJ, Louis JB, Atherholt TB, McGarrity GJ. Mutagenicity analysis of industrial effluents: Backgrounds and results to date, New Jersey, Department of environment protection, Trenton, NJ. 1983.
- Soni H, Bakre PP, Bhatnagar P. Assessment of teratogenicity and embryotoxicity of sludge from textile industries at Pali (India) in Swiss alvino mice exposed during organogenotoxic period. J. Environ. Biol. 2008; 29: 965-969.
- Komulainen H. Experimental cancer studies of chlorinated by-products. Toxicology. 2004; 198: 239-248.
- Draper NR, Smith H. Applied Regression Analysis, Wiley. New York. 1966.
- Garner RC, Nutman CA. Testing of some azo dyes and their reduction products for a mutagenicity using Salmonella typhimurium TA1538. Mut. Res.1977; 44: 9-19.
- McCann J, Choi E, Yamasaki E, Ames BN. Detection of carcinogens as mutagens bacterial tester strains with R factor plasmids. Proc. Natl. Acad. Sci., USA. 1975; 72: 979-983.
- Moore D, Felton JS. A microcomputer program for analyzing Ames test data. Mutation Research.1983; 119: 95-102.