
Research Article
Austin J Environ Toxicol. 2016; 2(2): 1016.
Ultrastructural Alterations in Oreochromis niloticus Exposed to Glyphosate-Based Herbicide, Excel Mera 71
Samanta P1,2, Pal S3, Mukherjee AK4, Senapati T1 and Ghosh AR1*
1Department of Environmental Science, The University of Burdwan, India
2Department of Environmental Science and Ecological Engineering, Korea University, Republic of Korea
3Department of Environmental Science, Aghorekamini Prakashchandra Mahavidyalaya, India
4Department of Conservation Biology, Durgapur Govt. College, India
*Corresponding author: Ghosh AR, Department of Environmental Science, The University of Burdwan, India
Received: November 11, 2016; Accepted: December 08, 2016; Published: December 15, 2016
Abstract
Oreochromis niloticus was exposed to Excel Mera 71 for 30 days both at field and laboratory conditions to investigate ultrastructural responses in stomach and intestine through light, Scanning and Transmission Electron Microscopy (SEM and TEM). Stomach showed damage in mucosal folds and gastric glands, fusion of gastric folds under laboratory condition but clumping of nucleus at the base of epithelial cell was observed in field. SEM study displayed cellular lysis, fragmentation of epithelial cells under laboratory condition, while excessive mucin droplets were observed in field. TEM displayed severe deformed mitochondria and Endoplasmic Reticulum (ER), vacuolation but under field condition vacuolations were prominent. Degenerative changes in Columnar Epithelial Cells (CEC) and lamina propria in intestine were observed under light microscopy but under field condition only damage in CEC were seen, while SEM study showed degeneration in CEC and microridge and huge mucus secretion under both conditions. Swelling of mitochondria, deformation and fragmentation in ER were seen under TEM in both conditions, but in field the degree of damages were comparatively less. Results clearly disclosed that responses were more profound in laboratory condition than field and these responses could be considered as potential biomarkers of exposure of agrochemicals like Excel Mera 71.
Keywords: Oreochromis niloticus; Columnar epithelial cells; Excel mera 71; Scanning electron microscopy; Transmission electron microscopy
Introduction
Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) is one of the most important commercially cultured fish species. They are very good species for aquaculture especially in developing countries such as Asia, Africa and America where there are high levels of animal protein deficiencies and integrated paddy-cum-fish-culture system has gained considerable attention in earlier times [1,2]. But recently in India such integrated paddy-cum-fish-culture system is almost non-existent because of increasing use of inorganic fertilizers and pesticides in rice fields causing deleterious effects on fish [3].
Weeds in crop fields are responsible for lowering production of rice and reducing the yield of approximately 60-70% [4]. Therefore, for protection of the crop health and increasing its productivity in terms of biomass use of herbicides becomes inevitable in one hand that considered as a beneficial tool or a pollutant, in other hand, due to its bioaccumulation and non-biodegradable properties. Simultaneously, its indiscriminate use in the agricultural fields might endanger the ecosystem as they are ultimately reach to the nearby aquatic ecosystem as well as fish farm which are in close proximity to the agricultural fields and also posing threats to the non-target aquatic organisms such as fin-fish and shell-fish having great economic importance to the human society. This may also contribute long term effects in the environment by changing the water quality [5].
Excel Mera 71, a glyphosate-based commercial herbicide formulation, is one of the most extensively used agrochemicals for controlling the broad leaved weeds and sedges both in the rice fields and aquatic ecosystem [6]. It is a non-selective and post-emergent modern, third generation herbicide of organophosphate group. They are highly efficacious, cost effective, practically non-toxic and degrade readily in the environment [7]. Glyphosate is a weak organic acid and chemical name is N-(phosphonomethyl) glycine and is usually formulated as the isopropylamine or trimethylsulfonium salt of glyphosate. The half-life of glyphosate in soil ranges between 2 and 197 days; a typical field half-life is of 47 days. Soil and climatic conditions also affect the glyphosate's persistence in soil. The median half-life of glyphosate in water varies from 4 to 91 days [8].
Use of cytopathological biomarkers in evaluation of the health status of fish exposed to xenobiotic substances has gained much attention throughout the world both in the laboratory [9,10] and field studies [11-13]. Histological analysis provides a rapid and efficient process to investigate the health of organisms exposed to a contaminated environment. Histopathological alterations are very sensitive to determine the cellular changes and allow examining the specific target organs, including stomach and intestine [14]. Furthermore, the changes observed in these organs are normally easier to identify than the functional ones [15] and serve as warning signs of damage to animal health [16]. Some study relating to use and effects of agrochemicals including the glyphosate in fish and aquatic invertebrates are available [17-24]; however, a little or no information is available on the effects of glyphosate on histopathological and ultrastructural responses in Oreochromis niloticus (Linnaeus) in the field condition. Nevertheless, field studies using histopathology and electron microscopy of fish as biomarker of aquatic contamination have not yet been reported. In the present study, an attempt has been made to investigate the histopathological and ultrastructural responses in stomach and intestine of the air-breathing, omnivorous freshwater teleost, O. niloticus to an exposure of Excel Mera 71 under field and laboratory conditions to establish some baseline information on the application of this herbicide.
Materials and Methods
Fish
Freshwater teleostean fish, Oreochromis niloticus (Linnaeus) of both the sexes with an average weight of 38.57±2.47 g and total length of 13.59±0.50 cm were acquired from local market and were acclimatized under congenial laboratory conditions for 15 days in aquaria of 250 L capacity. Fish were kept in continuously aerated water with a static system under natural photoperiod of 12-h light/12-h dark. During the acclimatization period, the average value of water parameters were as follows; temperature: 26.49±0.127oC, pH: 7.94±0.040, electrical conductivity: 392.22±0.62 µS/cm, total dissolved solids: 279.33±0.69 mg/l, dissolved oxygen: 6.44±0.05 mg/l, total alkalinity: 204.00±7.30 mg/l as CaCO3, total hardness: 180.44±3.74 mg/l as CaCO3, sodium: 24.45±0.56 mg/l, potassium: 5.33±1.02 mg/l, orthophosphate: 0.03±0.001 mg/l, ammoniacal-nitrogen: 1.66±0.21 mg/l and nitrate-nitrogen: 0.21±0.030 mg/l. Fish were fed once a day with commercial fish pellets (32% crude protein, Tokyu) during both acclimation and exposure periods. The experiment was carried out in accordance with the guidelines of the University of Burdwan and approved by the Ethical Committee of this University.
Field experimental design
After acclimatization, one set of fish were transferred to the field ponds situated at Crop Research Farm premises of the University of Burdwan. Fish were again divided into two groups as follows: control group containing 10 fish in three separate cages and exposure group also with 10 fish in separate cages for 30 days. The desired dose of 750 g/acre corresponds to concentration recommended for use in rice culture was dissolved in water and applied once [25,26]. It was sprayed on first day of the experiment on the surface of each glyphosate-treated cage. Glyphosate concentration in water was monitored according to Jan, et al. [27] during 30 days and it was 1.20±0.47 mg/l. For field experiment a special type of cage was prepared and installed separately at pond of Burdwan University Crop Research Farm, The University of Burdwan. The cages were prepared for the culture of the experimental fish as per Chattopadhyay, et al. [28] with some modifications. All the cages were square in shape having an area of 2.5x1.22 m2 and height of the cage was 1.83 m (submersed height was 0.83 m). The cages were framed by light strong bamboo. The four-sided wall, floor of the cage and top of the cage cover was fabricated with nylon net and was embraced by two PVC nets: the inner and outer bearing mesh sizes of 1.0x1.0 mm2 and 3.0x3.0 mm2 respectively. During the experimentation period (30 days) in the field, pond water had the following average values; temperature: 24.03±0.203oC, pH: 6.56±0.087, electrical conductivity: 347.00±1.15 µS/cm, total dissolved solids: 247.67±1.45 mg/l, dissolved oxygen: 7.00±0.157 mg/l, total alkalinity: 221.33±3.53 mg/l as CaCO3, total hardness: 140.00±2.31 mg/l as CaCO3, sodium: 63.40±2.67 mg/l, potassium: 15.96±2.10 mg/l, orthophosphate: 0.24±0.026 mg/l, ammoniacal-nitrogen: 0.74±0.111 mg/l and nitrate-nitrogen: 1.66±0.035 mg/l.
Laboratory design
After acclimatization, another set of fish were transferred to the laboratory aquarium. Fish were again divided into two groups (control and glyphosate-treated) and maintained in six aquaria, containing 10 fish in each aquarium in the Ecotoxicology Lab, Department of Environmental Science, The University of Burdwan: three for control and another three for treatment. Fish were exposed to sub-lethal dose of glyphosate, i.e., 17.20 mg/l in 40 L aquaria for a period of 30 days [29,30]. Doses were applied every alternate day. Glyphosate concentration in water was 16.88±1.69 mg/l. During experimentation, glyphosate-treated and control aquariums were subjected to same environmental conditions. During experimentation period, the average water parameters were as follows; temperature: 26.63±0.120oC, pH: 7.93±0.075, electrical conductivity: 426.00±5.93 µS/cm, total dissolved solids: 302.89±4.69 mg/l, dissolved oxygen: 5.06±0.43 mg/l, total alkalinity: 209.80±10.50 mg/l as CaCO3, total hardness: 163.11±3.04 mg/l as CaCO3, sodium: 37.76±1.02 mg/l, potassium: 7.26±1.12 mg/l, orthophosphate: 0.04±0.002 mg/l, ammoniacal-nitrogen: 7.09±2.15 mg/l and nitrate-nitrogen: 1.78±0.263 mg/l.
Sampling
During experimentation period the quality of the water was assessed as per APHA [31]. After completion of the experiment i.e., 30 days the fish were collected both from the aquarium and pond as well as from control condition and were anesthetized with tricaine methanesulphonate (MS 222) and stomach and intestine were taken immediately after dissection and proceeded specific ways for histological, scanning and transmission electron microscopic study.
Histopathological analysis
Fish tissues namely stomach and intestine from control and treatment were collected and fixed in aqueous Bouin’s fluid solution, dehydrated through graded series of ethanol and finally embedded in paraffin. Paraffin sections were cut at 3-4 µm using Leica RM2125 microtome. These sections were then stained with Haematoxylin-Eosin (H&E). Histopathological observations were made under Leica DM2000 light microscope.
Ultrastructural analysis
For scanning electron microscopic study, tissues were fixed in 2.5% glutaraldehyde in phosphate buffer (0.2 M, pH 7.4) for 24 h at 4oC and then post-fixed with 1% osmium tetraoxide in phosphate buffer (0.2 M, pH 7.4) for 2 h at 4oC, dehydrated through graded acetone, subsequently followed by amyl acetate and subjected to critical point drying with liquid carbon dioxide. Tissues were then mounted on metal stubs and sputter-coated with gold with thickness of approximately 20 nm. Tissues were examined with a scanning electron microscope (Hitachi S-530) at University Science Instrumentation Centre of the University of Burdwan.
For transmission electron microscopic study, tissues were fixed in Karnovsky fixative (mixture of 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M phosphate buffer) for 12 h at 4oC and then post-fixed with 1% osmium tetraoxide in phosphate buffer (0.2 M, pH 7.4) for 2 h at 4oC, dehydrated through graded acetone, infiltrated and embedded in epoxy resin, araldite CY212. Ultrathin sections (0.5-1 μm) were then cut by using a glass knife on an "Ultracut E Reichart-Jung" with the thickness of 70 nm, collected on naked copper-meshed grids and contrasted with uranyl acetate and lead citrate. The tissues were examined under TECHNAI G2 high resolution transmission electron microscope at Electron Microscope Facility, Department of Anatomy, AIIMS, New Delhi.
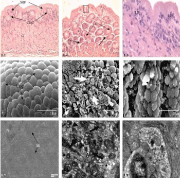
Figure 1: Histopathological photomicrographs of stomach of O. niloticus under control condition (C), glyphosate treated laboratory condition (GL), glyphosate treated field condition (GF). 1.1 Showing regular arrangement of Columnar Epithelial Cell (CEC), Lamina Propria (LP), Gastric Gland (GG) and Sub-Mucosa (SM) under light microscopy (Cx400). 1.2 Mucosal folds showing fusion (square), degeneration in gastric gland (white arrow) and vacuolation in lamina propria (broken arrow) (GLx400). 1.3 Showing almost normal structure of CEC with prominent nucleis under light microscopy (GFx1000). 1.4 Scanning electron microscopy showing normal arrangement of Mucosal Folds (MF) supported by oval or rounded CEC with stubby Microvilli (MV) (Cx4000). 1.5 Showing fragmentation of CEC (bold arrow) and severe mucus secretion on CEC under SEM (GLx3000). 1.6 Showing almost normal structure of mucosal folds and CEC and presence of Mucin (M) droplets under SEM (GFx3000). 1.7 Normal appearance of gastric glands and Nucleus (N) with prominent Mitochondria (M) and Rough Endoplasmic Reticulum (RER) (Cx9900). 1.8 Showing deformed mitochondria (bold arrow) and endoplasmic reticulum (arrow head) and severe vacuolation (broken arrow) under TEM study (GLx6300). 1.9 Showing normal appearance of gastric glands and Nucleus (N) but presence of vacuolation (broken arrow) under transmission electron microscopy (GFx8000).
Results
Stomach
Histological observations: Light microscopic observations of stomach of control fish showed four histological layers viz., mucosa, submucosa, muscularis and serosa. The gastric mucosa is lined with a single layer of compactly arranged columnar epithelial cells with centrally placed nuclei. A thin layer of top plate externally covers the gastric columnar epithelium. The mostly tubular as well as rounded gastric glands are present at basal portion of gastric mucosa. The cells of the gastric glands are closely arranged within the lumen and are provided with centrally placed nuclei. The well-vascularised submucosa is a thick layer of connective tissues, which was extended as narrow strip in the mucosal fold and separates the gastric gland. The submucosa layer is projected into the lamina propria (Figure 1.1).
Stomach of Oreochromis niloticus exposed to 17.20 mg/l of glyphosate under laboratory condition showed severe pathological alterations such as damage in mucosal folds, degenerative changes in gastric glands showing necrosis, vacuolation in lamina propria and fusion in between mucosal folds (Figure 1.2) but no marked pathological changes was observed except clumping of nucleus at the base of the epithelial cells in the field exposure (Figure 1.3).
Scanning electron microscopic observations: Topographic study through SEM depicted that in stomach of control fish columnar epithelial cells showed short with stubby microvilli. Prominent gastric pits were surrounded by the epithelial cells (Figure 1.4).
The ultrastructural changes in stomach as viewed under SEM exhibited severe damages such as cellular lysis, fragmentation of epithelial cells and less amount of mucin droplets over epithelial cells in the laboratory condition (Figure 1.5), while damages were comparatively less in field condition and excess mucin droplets were seen over the epithelial surface (Figure 1.6).
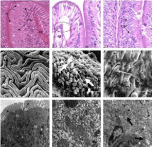
Figure 2: Histopathological photomicrographs of intestine of O. niloticus under control condition (C), glyphosate treated laboratory condition (GL), glyphosate treated field condition (GF). 2.1 Showing normal appearance of Lamina Propria (LP), Columnar Epithelial Cells (CEC) with prominent nucleus under light microscopy (Cx1000). 2.2 Showing degenerative CEC (white arrow) and distortion of lamina propria (oval) under light microscopy (GLx1000). 2.3 Light microscopy showing almost normal structure of CEC with prominent nucleus but some places showing damage in CEC (white arrow) (GFx1000). 2.4 Showing normal mucosal folds packed with oval or rounded CEC under SEM observation (Cx100). 2.5 Showing degenerative changes in CEC (bold arrow), damage in MV (arrow) and excess mucus secretion (M) (GLx1500). 2.6 SEM observation showing damage in MV (arrow) (GFx4000). 2.7 Showing normal appearance of CEC with prominent Mitochondria (M) and Glycocalyx (G) under transmission electron microscopy (Cx3200). 2.8 RER showing deformation and fragmentation (square) and severe vacuolation (broken arrow) under TEM (GLx6300). 2.9 Intestine showing less vacuolation (broken arrow) and deformed mitochondria (bold arrow) under transmission electron microscopy (GFx6300).
Transmission electron microscopic observations: Transmission electron microscopic observation showed the normal structure of stomach with abundant mitochondria and rough endoplasmic reticulum. The gastric glands show the presence of tubular network along with heterochromatic nucleus (Figure 1.7).
Due to glyphosate exposure in the laboratory condition, severe deformed mitochondria and endoplasmic reticulum, necrosis and vacuolation were observed in stomach of O. niloticus (Figure 1.8) but no significant alterations were observed except vacuolations and damages were comparatively less than laboratory condition as viewed under TEM study in the present findings (Figure 1.9).
Intestine
Histological observations: Histologically, intestine of control fish composed of four histological layers viz., mucosa, submucosa, muscularis and thin serosa. The mucosa of intestine is projected as finger like projections, the villi which is made up of simple, long absorptive columnar epithelial cells each with basally and centrally placed nucleus. Mucous cells are present all over the intestinal mucosa. Lamina propria is formed of loose connective tissue fibres of submucosa layer (Figure 2.1).
In the laboratory condition, intestine of exposed fish showed degenerative changes in villi and its columnar epithelial cells and lamina propria (Figure 2.2), while after glyphosate exposure in the field condition damage of columnar epithelial cells in some places was also observed in O. niloticus (Figure 2.3), but most of other layers were remain unaffected.
Scanning electron microscopic observations: SEM study of intestine of control fish showed that mucosal folds were supported by irregular wavy folds packed with rounded or oval columnar epithelial cells and epithelial cells were embraced with short and closely packed microvilli on its apical surface. Distinct mucous cells were also found on luminal surface of the intestine (Figure 2.4).
Topographic study of intestinal folds after glyphosate exposure in the laboratory condition exhibited degenerative changes in columnar epithelial cells and microvilli and huge secretion of mucus under SEM study (Figure 2.5) and in the field condition alterations such as damage in columnar epithelial cells and mucosal folds were also observed but the degree of damages were comparatively less than laboratory condition (Figure 2.6).
Transmission electron microscopic observations: Transmission electron microscopic study of intestine showed normal appearance of columnar epithelial cells under control condition. Abundant mitochondria are observed in the epithelial cells along with prominent nucleus. Apical surface of the intestinal epithelial cells show normal appearance of microvilli and are made up of glycocalyx (Figure 2.7).
After glyphosate exposure in the laboratory condition, severe degenerative changes were observed such as swelling in mitochondria, deformation and fragmentation of endoplasmic reticulum and dilation of nuclear envelope in intestine of O. niloticus (Figure 2.8). On the other hand, comparatively less damage in intestinal epithelium of O. niloticus was observed in field study than laboratory condition which included fewer amounts of vacuolations and fewer damages in endoplasmic reticulum with deformed mitochondria after glyphosate exposure (Figure 2.9).
Discussion
The present study is a maiden attempt to report the toxicity of the glyphosate-based commercial herbicide formulation, Excel Mera 71 with regard to histopathological and ultrastructural observation through light, scanning and transmission electron microscopic study in freshwater teleostean fish, O. niloticus both under field and laboratory study, although Senapati, et al. [32] reported some histopathological alterations in gill, oesophagus, stomach and intestine of Channa punctatus after Excel Mera 71 exposure in the laboratory condition and Samanta et al. [29,30] in their previous study reported that this herbicide caused alterations on several biochemical parameters in different fish species including O. niloticus.
In the present study, histopathological and ultrastructural effects in gastrointestinal tract namely stomach and intestine of freshwater omnivorous fish, O. niloticus were investigated both under natural and laboratory conditions after glyphosate intoxication. The study aimed at assessing the suitability of histopathological and ultrastructural responses as biomarkers of environmental contamination in aquatic ecosystem. The organ namely stomach and intestine investigated under present study are major sites of digestion and absorption of food materials in fish. All organs showed significant cytopathological lesions due to glyphosate exposure under different conditions. Generally, the responses were more pronounced in the laboratory condition than field condition might be assumed that in case of field experiments existence of fish in natural environment along with dilution effects of herbicidal action may induct general metabolic adaptation under this natural environment.
The present results exhibited severe histopathological lesions in stomach of O. niloticus including damage in mucosal folds, degenerative changes in gastric glands along with necrosis, vacuolation in lamina propria and fusion in between mucosal folds under laboratory condition. Similar results were also reported by Mohamed [33] who reported severe degenerative and necrotic changes in the intestinal mucosa and submucosa, aggregation of necrotized cells in the intestinal lumen, haemorrhage in the submucosa and dilation in intestine of T. zillii and S. vulgaris collected from Lake Qarun which was contaminated with agricultural runoff and drainage effluents. Haloi, et al. [34] in their study also reported similar results in Channa punctatus after endosulfan exposure. The pathological lesions observed under the present study in the intestine of O. niloticus were also in agreement with other authors who observed similar effects of different toxicants on fish intestine [35,36]. Epithelial degeneration, inflammatory cells infiltration in the submucosa as well as submucosal oedema was also reported by Soufy, et al. [37] in the intestine of tilapia following chronic exposure to carbofuran pesticide. Clumping of nuclei at the base of the epithelial cells was observed in the field exposure. Ghanbahadur and Ghanbahadur [38] also reported vacuolization in submucosa, shrinkage of mucosal folds in stomach of larvivorous fish Rasbora daniconius after endosulfan exposure. Scanning electron microscopic study showed severe damages such as cellular lysis, fragmentation of epithelial cells and less amount of mucin droplets over epithelial cells. Similar study of fragmentation and lysis of columnar epithelial cells and severe mucus secretion on epithelial surface of stomach of Notopterus notopterus after arsenic exposure was observed through scanning electron microscope by Ghosh and Chakrabarti [39]. Bose [40] and Senapati, et al. [32] in their study also reported similar type of pathological alterations in the alimentary canal of Anabas testudineus after lead and cadmium exposure and in Channa punctatus after glyphosate exposure respectively. In the field condition, excess mucin droplets were observed over epithelial surface indicating enhanced protection ability of the fish against the herbicidal stress. Transmission electron micrograph showed severe deformed mitochondria and endoplasmic reticulum, necrosis and vacuolations in stomach of O. niloticus after glyphosate exposure under both conditions but lesions were comparatively less in case of field experiment. Deformed mitochondria and endoplasmic reticulum, necrosis and vacuolations in cytoplasm as observed in the present investigation were also reported by Rebolledo and Vial [41] in stomach of Elasmobranch species, Halaelurus chilensi. They also reported dilation in mitochondria, appearance of vast amount of endoplasmic reticulum and damage in tubular network. On the other hand, Carrasson, et al. [42] in their study observed abundant smooth endoplasmic reticulum, mitochondria and rough endoplasmic reticulum, tubule-vascular network and heterochromatic nuclei in stomach of Dentex dentex. Therefore, these changes in stomach can be used as indicator to determine the damages caused by xenobiotic exposure in aquatic ecosystem.
In intestine, glyphosate intoxication caused severe pathological alterations both under laboratory and field conditions, although the pathological responses were more severe under laboratory condition. Degenerative changes in columnar epithelial cells and lamina propria were prominent alterations. Mandal and Kulshrestha [43] in their study reported similar pathological lesions such as degenerative columnar epithelial cells and lamina propria along with lifting of columnar epithelial cells, severe mucus secretion, vacuolations in lamina propria and complete disappearance of brush border in intestine of Clarias batrachus after exposure of sumithion herbicide. Sharma, et al. [44] also reported severe mucus secretion, vacuolations in lamina propria in intestine of Cirrhinus mrigala after pesticide exposure. Cytopathological alterations such as degenerative changes in columnar epithelial cells and microridge structures and severe mucus secretion were prominent under SEM study in the laboratory condition. Degenerative changes in columnar epithelial cells and microridge structures and severe mucus as observed under present study were also reported by Ghosh and Chakrabarti [39] in intestine of N. notopterus after arsenic exposure. Senapati, et al. [32] in their study also reported similar findings in intestine of Channa punctatus due to toxicity of glyphosate herbicide. Swelling in mitochondria, deformation and fragmentation of endoplasmic reticulum and dilation of nuclear envelope observed in the present study under TEM study was also reported by Sastry and Siddiqui [45] in C. punctatus after endosulfan and quinalphos exposure. Therefore, the pathogenicity induced by glyphosate-based herbicide, Excel Mera 71 in alimentary canal of concerned test fish species namely in stomach and intestine may be due to formation of organochloride acid in stomach in the presence of secreted HCl from cardiac stomach which ultimately interfering with the oxidative metabolism in stomach and subsequent disturbance in absorption process by the intestinal part.
Conclusion
The present study revealed that glyphosate-based herbicide, Excel Mera 71 is toxic to fish and causes histopathological and ultrastructural changes in alimentary canal namely stomach and intestine under laboratory condition. The results presented herein demonstrated that glyphosate at rice-paddy field concentration caused lesions in the respective fish organs but the cytological responses were comparatively less than laboratory condition. Therefore, these responses could be considered as potential biomarkers for risk assessment of these agrochemicals in aquatic ecosystem, especially nearby agricultural fields where integrated paddy-cum-fish-culture system has gained much importance.
References
- Haylor GS. The Case of the African Catfish, Clarias gariepinus burchell clariidae. A Comparison of the Relative Merit of Tilapia Fishes Especially Oreochromis niloticus L and C. gariepinus Burchell 1822, for African Aquaculture. Aquacult Fish Manage. 1993; 20: 285-297.
- Fagbenro OA, Adedire CO, Owoseni EA, Ayotunde EO. Studies on the Biology and Aquacultural Potential of Feral Catfish. Heterobranchus bidorsalis. Trop Zool. 1993; 6: 67-79.
- Ramah K. Histopathological Study on the Effect of Rice Herbicides on Grass Carp (Ctenopharyngodan idella). Afr J Biotechnol. 2011; 10: 1112-1116.
- Ghosh BR, Ghosh C, Mitra A, Jana MK, Mitra BN. Techniques of Rice-Cum-Fish Culture for Increasing Productivity in Lowlands. Indian Fmg. 1994; 44: 23-26.
- Rand GM. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment. Washington DC: Taylor & Francis. 1995.
- Bose A, Pandey MC. New Formulation of Glyphosate and Recent Developments on the Uses of Glyphosate in India. Pak J Weed Sci Res. 2012; 18: 55-60.
- Reuters, Roundup: cancer cause or crucial for food production? ‘‘The Huffington Post’’, 2011.
- Giesy JP, Dobson S, Solomon KR. Ecotoxicological Risk Assessment for Roundup Herbicide. Rev Env Contam Toxicol 16. 2000; 7: 35-120.
- Wester PW, Canton JH. The Usefulness of Histopathology in Auatictoxicity Studies. Comp Biochem Physiol. 1991; 100: 115-117.
- Thophon S, Kruatrachue M, Upatham ES, Pokethitiyook P, Sahaphong S, Jaritkhuan S. Histopathological Alterations of White Seabass, Lates calcarifer, in Acute and Subchronic Cadmium Exposure. Environ Poll. 2003; 121: 307-320.
- Hinton DE, Baumann PC, Gardner GR, Hawkins WE, Hendricks JD, Murchelano RA, et al. Histopathological biomarkers. Huggett RJ, Kimerle RA, Mehrle PM, Bergman Jr HL, editors. In: Biomarkers: Biochemical, Physiological and Histological Markers of Anthropogenic Stress. Lewis, USA. 1992; 155-196.
- Schwaiger J, Wanke R, Adam S, Pawert M, Honnen W, Triebskorn R. The Use of Histopathological Indicators to Evaluate Contaminant-Related Stress in Fish. J Aquat Ecosyst Stress Recovery. 1997; 6: 75-86.
- The SJ, Adams SM, Hinton DE. Histopathological Biomarkers in Feral Freshwater Fish Populations Exposed to Different Types of Contaminant Stress. Aquat Toxicol. 1997; 37: 51-70.
- Stebbing ARD. A possible synthesis. Bayne BL, editors. In: The Effects of Stress and Pollution on Marine Animals. Praeger, New York. 1985.
- Fanta E, Rios FS, Romao S, Vianna ACC, Freiberger S. Histopathology of the Fish Corydoras paleatus Contaminated With Sublethal Levels of Organophosphorus in Water and Food. Ecotoxicol Environ Saf. 2003; 54: 119-130.
- Hinton DE, Lauren DJ. Liver structural alterations accompanying chronic toxicity in fishes: potential biomarkers of exposure. McCarthy JF, editors. In: Biomarkers of Environmental Contamination. Boca Raton, Lewis. 1990; 17-57.
- Oloruntuyi OO, Mulero O, Odukale B. The Effects of Two Pesticides on Clarias giriepinus. Proceeding of the 10th Annual Conference of the Fisheries Society of Nigeria. 1992.
- Neskovic NK, Poleksic V, Elezovic I, Karan V, Budimir M. Biochemical and Histopathological Effects of Glyphosate on Carp, Cyprinus carpio L. Bull Environ Contam Toxicol. 1996; 56: 295-302.
- Koviznych JA, Urbancikova M. Acute Toxicity of Acetachlor Pollution for Zebra Fish (Danio rerio) and Guppy (Paccilia reticulate). Ecology. 1998; 17: 449-456.
- Abd El-Gawad AM. Histopathological Studies on the Liver and Gills of Tilapia Nilotica (Oreochromis nilticus) Exposed to Different Concentrations of Lead Acetate and Zinc Sulphate. J Egypt German Soc Zool. 1999; 30: 13-22.
- Visoottiviseth P, Thamamaruitkum T, Sahaphong S, Reingroipitak S, Kruatrachua M. Histological Effects of Triphemyltin Hydroxide on Liver, Kidney and Gill of Nile Tilapia (Oreochromin niloticus). Appl Organomet Chem. 1999; 13: 749- 763.
- Babatunde MM, Oludimiji AA, Balogun JK. Acute Toxicity of Gamaxone to Oreochromis niloticus (Treweva) in Nigeria. Water Air Soil Pollut. 2001; 13: 1-10.
- Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Gram S, Pokethitiyook P. Histopathologocal Effects of Roungup, a Glyphosate Herbicide, on Nile Tilapia (Oreochromis niloticus). Sci Asia. 2002; 28: 121-127.
- Ramirez-Duarte WF, Rondon-Barragan LS, Eslava-Mocha PR. Acute Toxicity and Histopathological Alterations of Roundup® Herbicide on “Cachama Blanca” (Piaractus brachypomus). Pesquisa Veterinaria Brasileira. 2008; 28: 547-554.
- Samanta P, Mukherjee AK, Pal S, Kole D, Ghosh AR. Toxic Effects of Glyphosate-Based Herbicide, Excel Mera 71 on Gill, Liver and Kidney of Heteropneustes fossilis under Laboratory and Field Conditions. J Microscopy Ultrastructure. 2016; 4: 147-155.
- Samanta P, Pal S, Mukherjee AK, Senapati T, Ghosh AR. Histopathological and Ultrastructural Alterations in Anabas testudineus Exposed to Glyphosate-Based Herbicide, Excel Mera 71 under Field and Laboratory Conditions. J Aquac Res Development 7: 436.
- Jan MR, Shah J, Muhammad M, Ara B. Glyphosate herbicide residue determination in samples of environmental importance using spectrophotometric method. J Hazard Mater. 2009; 169: 742-745.
- Chattopadhyay DN, Mohapatra BC, Adhikari S, Pani PC, Jena JK, Eknath AE. Effects of Stocking Density of Labeo rohita on Survival, Growth and Production in Cages. Aquacult Int. 2013; 21: 19-29.
- Samanta P, Pal S, Mukherjee AK, Ghosh AR. Evaluation of Metabolic Enzymes in Response to Excel Mera 71, a Glyphosate-Based Herbicide and Recovery Pattern in Freshwater Teleostean Fishes. BioMed Research Interanational. 2014.
- Samanta P, Pal S, Mukherjee AK, Ghosh AR. Biochemical Effects of Glyphosate Based Herbicide, Excel Mera 71 on Enzyme Activities of Acetylcholinesterase (AChE), Lipid Peroxidation (LPO), Catalase (CAT), Glutathione-S-Transferase (GST) and Protein Content on Teleostean Fishes. Ecotoxicol Environ Saf. 2014; 107: 120-125.
- APHA, AWWA, WPCF. Standard Methods for the Examination of Water and Wastewater. Washington DC. 2005.
- Senapati T, Mukherjee AK, Ghosh AR. Observations on the Effect of Glyphosate Based Herbicide on Ultrastructure (SEM) and Enzymatic Activity in Different Regions of Alimentary Canal and Gill of Channa punctatus (Bloch). J Crop Weed. 2009; 5: 236-245.
- Mohamed FAS. Histopathological Studies on Tilapia zilli and Solea vulgaris from Lake Qarun, Egypt. World J Fish Marine Sci. 2009; 1: 29-39.
- Haloi K, Kalita M, Nath R. The Study on the Histopathological Changes of Stomach of Channa punctatus (Bloch). By Used Pesticide Endosulfan. Global J Sci Front Res Biol Sci. 2013; 13.
- Hanna M, Shaheed I, Elias N. A Contribution on Chromium and Lead Toxicity in Cultured Oreochromis niloticus. Egypt J Aquat Biol Fish. 2005; 9: 177-209.
- Cengiz EI, Unlu E. Histopathology of Gills in Mosquitofish, Gambusia affinis after Long-Term Exposure to Sublethal Concentrations of Malathion. J Environ Sci Health Part B. 2003; 38: 581-589.
- Soufy H, Soliman M, El-Manakhy E, Gaafa A. Some Biochemical and Pathological Investigations on Monosex Tilapia Following Chronic Exposure to Carbofuran Pesticides. Global Veterinaria. 2007; 1: 45-52.
- Ghanbahadur A, Ghanbahadur G. Histopathological Effect of Organochloride Endosulfan on Intestine and Stomach of Larvivorous Fish Rasbora daniconius. DAV Int J Sci. 2012; 1: 126-127.
- Ghosh AR, Chakrabarti P. Impact of Inorganic Arsenic on Mucosal Surface of Stomach, Intestine and Intestinal Caeca of Notopterus Notopterus (Pallas). ICIPACT. 2001; 469-473.
- Bose R. Effects of Lead and Cadmium on the Digestive System and Kidney of Indian Fresh Water Perch, Anabas testudineus Cuvier and Subsequent Recovery by EDTA. The University of Burdwan, West Bengal, India, 2005.
- Rebolledo IM, Vial JD. Fine Structure of the Oxynticopeptic Cell in the Gastric Glands of Elasmobranch Species (Halaelurus chilensis). Anat Record. 1979; 193: 805-822.
- Carrassón M, Grau A, Dopazo LR, Crespo S. A Histological, Histochemical and Ultrastructural Study of the Digestive Tract of Dentex Dentex (Pisces, Sparidae). Histol Histopathol. 2006; 21: 579-593.
- Mandal PK, Kulshrestha H. Histopathological Changes Induced by the Sublethal Sumithion in Clarias batrachus (Linn.). Indian J Exp Biol. 1980; 18: 547-552.
- Sharma RR, Pandey AK, Shukla GR. Histopathological Alterations in Fish Tissues Induced by Pesticides Toxicity. Aquaculture. 2001; 2: 31-43.
- Sastry KV, Siddiqui AA. Effect of Endosulfan and Quinalphos on Intestinal Absorption of Glucose in the Freshwater Murrel, Channa punctatus. Toxicol Lett. 1982; 12: 289-293.