
Editorial
Austin J Environ Toxicol. 2017; 3(1): 1020.
Blood Cholinesterase Activity in Northern Bobwhite (Colinus virginianus) Quail in the Rolling Plains Ecoregion of Texas and Oklahoma, USA
Pappas SA, Turaga U, Kumar N and Kendall RJ*
Wildlife Toxicology Laboratory, The Institute of Environmental and Human Health, Texas Tech University
*Corresponding author: Kendall RJ, Wildlife Toxicology Laboratory, Texas Tech University USA
Received: June 13, 2017; Accepted: October 20, 2017; Published: October 27, 2017
Abstract
In an effort to identify factors contributing to the population decline of Northern bobwhites (Colinus virginianus) in the Rolling Plains ecoregion of Texas and Oklahoma, blood samples were collected from quail during the summer (August) and fall (October) of 2013. The blood cholinesterase activity in the samples was evaluated to investigate the exposure of wild bobwhites to cholinesterase inhibiting chemicals. Baseline blood cholinesterase activity in quail was established by evaluating cholinesterase activity in pen-raised quail. Significant differences in the blood cholinesterase activities of pen-raised and wild bobwhites were observed. Additionally, significantly higher blood cholinesterase activity was observed in wild bobwhites captured during August compared to those captured in October. A significantly lower blood cholinesterase activity in the wild bobwhites, particularly in October, suggests a potential for exposure to cholinesterase inhibiting chemicals in the Rolling Plains ecoregion.
Keywords: Northern Bobwhites, Rolling Plains ecoregion, Cholinesterase, Cholinesterase Inhibiting Chemicals
Abbreviations
Cholinesterase: (ChE); Dried Blood Spot (DBS); The Institute of Environmental and Human Health (TIEHH); Ethylene Diamine tetracartic Acid (EDTA)
Introduction
Data from the US Geological Survey’s Breeding Bird Survey revealed that Northern bobwhite (Colinus virginianus) populations have been on the decline for several decades in the Rolling Plains ecoregion of Texas and Oklahoma, with a more severe decline observed over the last decade [1]. Considering the economic and ecological significance of bobwhites to the Rolling Plains ecoregion [2], it is important to investigate the potential factors causing the decline. The decline in bobwhite populations could be attributed to many different factors such as the quality of habitat [3], weather [4, 5], predation [6, 7], and parasites [8-10]. Another important factor to be considered is the exposure of bobwhites to environmental contaminants.
Previous studies have revealed that bobwhites in the ecoregion are being exposed to neurotoxic chemicals like organochlorines, lead, mercury, and neonicotinoids [11, 12]. The widespread use of chemically treated seeds is a common agricultural practice not only in the Rolling Plains ecoregion, but also across the United States [13]. Because wild bobwhites frequently feed near agricultural fields, there is an increased possibility of ingesting these chemically treated seeds [14]. Considering the many direct and indirect behavioral effects these neurotoxic chemicals exert on avian species [15-17], it is important to monitor the exposure of bobwhites to neurotoxic/Cholinesterase (ChE) inhibiting chemicals.
The objective of the present study is to non-destructively monitor the exposure of wild bobwhites to ChE inhibiting chemicals such as insecticides, herbicides, fungicides, etc. in the ecoregion. A non-destructive assessment of exposure to chemicals necessitates the selection of suitable biomarkers, and an often-used biomarker to assess the exposure of avian species to ChE inhibiting chemicals is blood ChE [18]. Here, we evaluate blood ChE levels in wild bobwhite in the Rolling Plains using a Dried Blood Spot (DBS) technique [19].
Materials and Methods
Trapping of Wild Bobwhites
Northern bobwhites were trapped on 35 ranches spread out over 24 counties in Texas and nine counties in Oklahoma (Figure 1).
Quail were collected under Texas Parks and Wildlife permit SPR-1098-984, Texas Tech University Institutional Animal Care and Use protocol 11049-07, and Texas A&M University Acceptable Use Policy (2011-93). A total of 233 bobwhites were trapped during the months of August and October. Because widespread cotton and wheat planting activity happens across the ecoregion during these months, collecting samples from wild bobwhites during these months provided the best opportunity to assess the exposure of bobwhites to ChE inhibiting chemicals. In all, 109 male and 90 female bobwhites were captured, and the gender of the remaining 34 bobwhites could not be determined. Of the 109 male bobwhites, there were 80 juveniles, 28 adults, and 1 that age could not be determined. Similarly, the 90 female bobwhites consisted of 65 and 23 juveniles and adults, respectively. The age of the remaining two bobwhites could not be determined. Lastly, 33 of the 34 bobwhites with undetermined gender were found to be juveniles, and the age of the remaining bobwhite could not be determined. Additional details about the bobwhites are presented in (Table 1).
August
October
Adult male quail
7
21
Adult female quail
3
19
Juvenile male quail
24
56
Juvenile female quail
25
41
Table 1: Age and sex details of the wild bobwhites captured in the months of August and October.
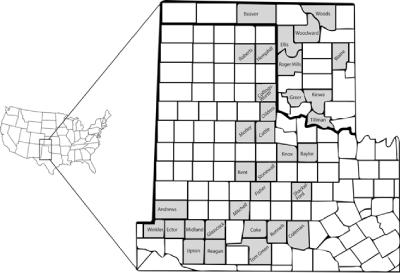
Figure 1: Rolling plains ecoregion of Texas and Oklahoma. Bobwhites were
collected from the ranches in shaded counties.
Collection of Blood from Bobwhites
Approximately 50 μL was collected from each bobwhite in an EthyleneDiamineTetraAceticacid (EDTA) treated vial. The vials were stored in a refrigerated box as soon as they were collected. Within 48 hours from collecting the sample, the samples were transported to The Institute of Environmental and Human Health (TIEHH) Central Receiving Laboratory. Once at TIEHH, 20 μL of blood was removed from each vial and spotted onto a FTA_DMPK C card (GE Healthcare, Buckinghamshire, UK). Blood spots were allowed to air dry for approximately 15 to 20 minutes and were placed into a foil envelope with a desiccators pack. This DBS technique was used because it was less expensive and required less blood. Samples were then stored in a cool, dry place until analysis.
Blood from pen-raised bobwhites was collected to evaluate and establish the baseline blood ChE levels in quail that were not exposed to any chemicals. Eight adult male and 9 adult female bobwhites were used as controls. All blood samples were collected, stored, processed, and analyzed in a similar fashion.
Evaluation of Blood ChE Activity
The blood ChE activities of wild bobwhites were evaluated using a modified Augustinsson method [20, 21] and compared against the blood ChE activity of pen-raised quail. The modified version of Augustinsson’s method was used in order to take into account that the blood samples were stored as dried blood spots rather than as frozen whole blood samples. Propionylthiocholine is used as a substrate for acetylcholine in the Augustinsson’s method. The product of the reaction between propionylthiocholine and acetylcholinesterase from the blood samples is thiocholine. This, in turn, reacts with 4, 4-dithiopyridine (4-PDS) to produce 4-thiopyridine which can be measured at 324 nm [22].
All ChE activities (μmol/min/L) were calculated using the equation below:
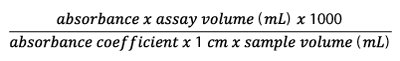
Where the absorbance coefficient for 4-thiopyridone is 1.98 e 4 L/mol/cm [23, 24]. Once calculated, the activity was adjusted by subtracting the value of spontaneous hydrolysis of the substrate. This value was measured by analyzing the absorbance of the reagent blank, samples containing only buffer solution and the substrate.
Statistical Analysis
Three-factor ANOVA was performed (at level α = 0.05) involving gender (two levels: male and female), age (two levels: adult and juvenile), and time of sampling/habitat (three levels: August/wild, October/wild and pen-raised). If sex or age could not be determined, that bobwhite was excluded from the analysis. The lack of juveniles in pen-raised quail/control group meant that any observed differences in blood ChE activity due to age can only be attributed to wild bobwhites. All factors were treated as fixed factors and a two-way interaction between gender/age, and the time of sample collection/habitat was considered. Again, the lack of juvenile pen-raised bobwhites meant that the interaction between age and time of sample collection/habitat could not be included in the analyses. Homogeneity of variance and normality test were performed before the analyses. All statistical analyses were performed using Minitab 17 [25].
Results and Discussion
Turkey’s pairwise comparison indicated that the mean blood ChE activity in pen-raised bobwhites was significantly higher than the blood ChE activity in wild bobwhites (Figure 2). Establishing the baseline levels of blood ChE activity in wild bobwhites is important as it enables the diagnosis of ChE intoxication in birds [26]. Adult pen-raised male and female bobwhites were observed to have a significantly higher blood ChE activity compared to male and female bobwhites in the wild, respectively. Additionally, no significant difference in blood ChE activities was observed between male and female bobwhites (p=0.909), or between adult and juvenile bobwhites (p =0.061).Similar results were observed by Horowitz et al. [26] in other avian species.
Analysis of the blood ChE activities of pen-raised and wild bobwhites has revealed a 74% decrease in the blood ChE activity in wild bobwhites. According to some authors, a reduction in ChE activity as high as 30-50% is an indicator of exposure to Carbamate and organophosphorous pesticides [27]. Hence, it could be inferred that wild bobwhites in the Rolling Plains ecoregion are potentially getting exposed to chemicals with ChE inhibiting activity. However, studies have also suggested that blood ChE activities vary and depend on many factors such as: 1) age; 2) circadian and circannual rhythms; 3) sex; 4) reproductive status; 5) nutritional status; 6) environmental temperature; 7) pathophysiological status and 8) disease status [27,28]. Therefore, additional studies need to be conducted to investigate the low blood ChE activities observed in wild bobwhites. Additionally, Linoettoet al. 2013 have thoroughly reviewed the sensitivity of blood ChE inhibiting activity of heavy metals, polycyclic aromatic hydrocarbons, pyrethroids, triazines, paraquat, detergents, and other contaminants [28].Any physical manifestation of toxicity from exposure to neurotoxic chemicals could not be studied due to the multi-institutional nature of this study.
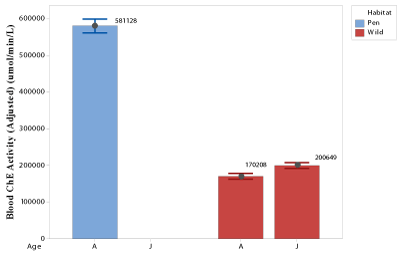
Figure 2: Blood ChE activities of pen-raised and wild bobwhites (A= Adult;
J= Juvenile).
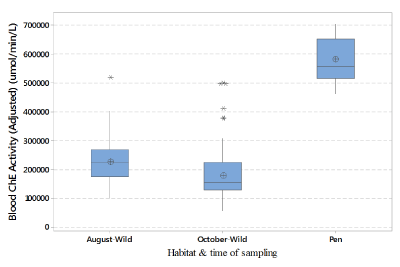
Figure 3: Blood ChE activity of Northern Bobwhites captured during the
months of August and October.
The time of sampling also seems to affect the blood ChE activity in wild bobwhites. Wild bobwhites captured in August were found to have a significantly higher blood ChE activity compared to those captured in October (F (2,205) = 174.62, p = 0.000; (Figure 3). Studies have suggested that most of the economically damaging pest/insect infestations in cotton agriculture in the ecoregion occur during the month of August [29]. This results in a considerable increase in the use of insecticides, defoliants, and other chemicals beginning from the month of August [29]. For this reason, wild bobwhites trapped during the October trapping sessions could have had an increased exposure to ChE inhibiting chemicals. The increased exposure to ChE inhibiting chemicals in the case of wild bobwhites trapped in October could have resulted in a decrease in their blood ChE activity.
Another potentially important factor to take into consideration is the age of dried blood spot samples. It may be important to note that dried blood samples from wild bobwhites were stored for over a period of 16 to18 months, while dried blood samples from pen-raised bobwhites were only stored for a period of 6 months. The nature of the project being multi-institutional and the difficulty in obtaining late season pen-raised bobwhites played a key role in the discrepancies in the time frame. Deterioration of enzyme activity is a possibility over an extended period of time, even when stored on chemically untreated FTA cards to prevent denaturing of proteins. Although, no studies to our knowledge have demonstrated the long-term storage/stability of enzymes on DBS cards, and direct communication with the manufacturer (GE Healthcare, Buckinghamshire, UK) of the cards suggested that they did not have any evidence that extended storage of blood samples on their cards would cause enzyme degradation. However, the manufacturer noted that it still is a possibility (personal communication, 2015).Hence; the possibility of enzyme degradation in the case of dried blood samples stored for an extended period of time cannot be discounted.
However, Batter man and Cher yak (2014) have demonstrated the long-term stability of organic compounds on DBS cards for more than a year under frozen conditions [30].They have also suggested that organic compounds remained stable on DBS cards at room temperature [30]. This suggests that not all of the observed decrease in the ChE activity can be attributed to the phenomenon of enzyme degradation. Furthermore, we would have expected lower ChE levels in the August samples compared to October if the decrease was completely due to enzyme degradation as they would have been stored for a longer period. Because of this, we believe that exposure to ChE inhibiting chemicals also played a role in the observed decrease in blood ChE activity.
Conclusion
Blood ChE activity of wild bobwhites captured in the months of August and October were evaluated in the present study. Literature suggests that quail in the Rolling Plains ecoregion are being exposed to ChE inhibiting chemicals, and wild bobwhites had a significantly lower blood ChE activity compared to pen-raised bobwhites. Additionally, wild bobwhites captured in August had a significantly higher blood ChE activity compared to those captured in October. Despite the mentioned limitations in the study, it appears that bobwhite quail in the Rolling Plains ecoregion are being exposed to ChE inhibiting chemicals.
Acknowledgment
The authors would like to thank Park Cities Quail and the Rolling Plains Quail Research Foundation for their financial support. Wildlife Toxicology Laboratory personnel are acknowledged for their assistance.
References
- Sauer JR, Hines JE, Fallon JE, Pardieck KL, Ziolkowski Jr, DJ, Link, WA, et al. The North American Breeding Bird Survey, results and analysis 1966-2015. USGS Patuxent Wildlife Research Center. 2013.
- Johnson JL, Rollins D, Reyna KS. What’s a quail worth? A longitudinal assessment of quail hunter demographics, attitudes, and spending habits in Texas. Proceedings of the National Quail Symposium. 2012; 7: 294-299.
- Hernández F, Brennan LA, DeMaso SJ, Sands JP, Wester DB. On reversing the northern bobwhite population decline: 20 years later. Wildlife Soc B. 2013; 37: 177-188.
- Rollins D. A pattern to quail irruptions in the Rolling Plains of Texas. Proceedings, Preserving Texas' Quail. 1999; 13-15: 33-36.
- Bridges AS, Peterson MJ, Silvy NJ, Smeins FE, Wu XB. Differential influence of weather on regional quail abundance in Texas. J Wildlife Manage. 2001; 65: 10-18.
- Cox SA, Peoples AD, DeMaso SJ, Lusk JJ, Guthery FS. Survival and cause-specific mortality of northern bobwhites in western Oklahoma. J Wildlife Manage. 2004; 68: 663-671.
- Rader MJ, Teinert TW, Brennan LA, Herandez F, Silvy NJ, Wu XB, et al. Identifying predators and nest fates of bobwhites in southern Texas. J Wildlife Manage. 2007; 71: 1626-1630.
- Moore J, Simberloff D, Freehling M. Relationships between bobwhite quail social-group size and intestinal helminth parasitism. Am Nat. 1988; 131: 22-32.
- Moore J, Simberloff D. Gastrointestinal helminth communities of bobwhite quail. Ecology. 1990; 71: 344-359.
- Dunham NR, Soliz LA, Fedynich AM, Rollins D, Kendall RJ. Evidence of an Oxyspirura petrowi epizootic in northern bobwhites (Colinus virginianus), Texas, USA. J Wildlife Dis. 2014; 50: 552-558.
- Baxter CE, Pappas S, Abel MT, Kendall RJ. Organochlorine pesticides, lead, and mercury in northern bobwhite (Colinus virginianus) and scaled quail (Callipeplasquamata) from the rolling plains ecoregion of Texas and Oklahoma. Environ Toxicol Chem. 2015; 34: 1505-1510.
- Turaga U, Peper ST, Dunham NR, Kumar N, Kistler W, Almas S, et al. 2016. A survey of neonicotinoid use and potential exposure to northern bobwhite (Colinus virginianus) and scaled quail (Callipeplasquamata) in the Rolling Plains of Texas and Oklahoma. Environ Toxicol Chem. 2016; 35: 1511-1515.
- Douglas MR, Tooker JF. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environ Sci Technol. 2015; 49: 5088-5097
- Avery ML, Fischer DL, Primus TM. Assessing the hazard to granivorous birds feeding on chemically treated seeds. Pestic Sci. 1997; 49: 362-366.
- Walker CH. Neurotoxic pesticides and behavioral effects upon birds. Ecotoxicology. 2003; 12: 307-316.
- Galindo JC, Kendall RJ, Driver CJ, Lacher Jr. The effect of methy parathion in susceptibility of bobwhite quail (Colinus virginianus) to domestic cat predation. Behav Neural Biol.1985; 43: 21-36.
- Bussiere JL, Kendall RJ, LacherJr TE, Bennett RS. Effect of methyl parathion in food discrimination in northern bobwhite (Colinus virginianus). Environ Toxicol Chem. 1989; 8: 1125-1131.
- Fildes K, Azabo JK, Hooper MJ, Buttemer WA. Astheimer LB. Plasma cholinesterase characteristics in native Australian birds: significance for monitoring avian species for pesticide exposure. Emu. 2009; 109: 41-47.
- Ostler MW, Porter JH, Buxton OM. Dried blood Spot collection of health biomarkers to maximize participation in population studies. JoVE. 2014; 83: e50973.
- Augustinsson KB, Holmstedt B. Determination of cholinesterase in blood samples dried on filter-paper and its practical application. Scand J Clin Lab Invest.1965; 17: 573-583.
- Augustinsson KB, Eriksson H, Faijersson Y. A new approach to determining cholinesterase activities in samples of whole blood. Clin Chim Acta. 1978; 89: 239-252.
- Eriksson H, Faijersson Y. A reliable way of estimating cholinesterases from whole blood in the presence of anticholinesterases. Clin Chim Acta. 1980; 100: 165-171.
- Bocquené G, Carbamates, Galgani F. ICES techniques in marine environmental science: Biological effects of contaminants: cholinesterase inhibition by organophosphate and carbamate compounds (No. 22). International Council for the Exploration of the Sea. 1998; 1-12.
- Trudeau S, Cartier GS. Biochemical methods to determine cholinesterase activity in wildlife exposed to pesticides. Hull Québec Canada. Canadian Wildlife Service. 2000.
- Minitab 17 Statistical Software. State College PA. Minitab Inc. 2010
- Horowitz IH, Yanco EG, Landau S, Nadler-Valency R, Anglister N, Bueller-RosenzweigA, et al. Whole Blood cholinesterase activity in 20 species of wild birds. J Avian Med Surg. 2016; 30: 122-126.
- Trudeau S, Mineau P, Cartier GS, Fitzgerald G, Wilson, L, Wheler C, et al. Using dried blood spots stored on filter paper to measure cholinesterase activity in wild avian species. Biomarkers. 2007; 12: 145-154.
- . Lionetto MG, Caricato R, Calisi A, Giordano MG, Schettino T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: New insights and future perspectives. BioMed Res Int. 2013; Article ID: 321213.
- Managing cotton insects in the high plains, Rolling plains and Trans Pecos areas of Texas 2008.
- Batterman S, Chernyak S. Performance and storage integrity of dried blood spots for PCB, BFR and pesticide measurements. Sci Total Environ. 2014; 494-495: 252-260.