
Research Article
Austin J Environ Toxicol. 2021; 7(1): 1033.
Histopathology of the Foot, Gill and Digestive Gland Tissues of Freshwater Mussel, Lamellidens marginalis Exposed to Oil Effluent
Balamurugan S¹* and Subramanian P²
¹Department of Zoology, Arignar Anna Government Arts College, India
²Department of Animal Science, Bharathidasan University, India
*Corresponding author: Balamuirugan S, Department of Zoology, Arignar Anna Government Arts College, Musiri-621 211, Tiruchirappalli-District, Tamilnadu, India
Received: December 04, 2020; Accepted: December 30, 2020; Published: January 06, 2021
Abstract
We investigated the histopathological alterations in the tissues of freshwater mussel, Lamellidens marginalis in response to oil effluent. Based on the previous acute toxicity, two sub lethal [1/4th (11.88ppt) and 1/10th (8.55ppt)] concentrations of oil effluent (hydrocarbon) were prepared and exposed to mussels. In a first series of experiment, animals were exposed/accumulated for 30 days [Ist, 8th, 15th, 22nd and 30th days] by two sub lethal concentrations of oil. In a second series of experiment, oil exposed animals were thereafter transferred to clean water and kept in it up to 30 days [Ist, 8th, 15th, 22nd and 30th days] to assess the recovery pattern (depuration). At seven-day intervals, histopathological alterations were analyzed in foot, gill and digestive gland tissues of mussel. First series of experimental observation showed remarkable damages in foot (disorganized outer epithelium, necrosis of the cell, the formation of lumina, disorganized muscle bundle); in gill (disruption of gill filaments, odema formation, necrosis, dis-aggregated cilia) and in digestive gland (stoma, detached glandular epithelium, vertical clefts, presence of leucocytes, dense accumulation of luminal material) and also oil effluent inducement are confirmed with the aforementioned results. At second series of experiment, it was found that oil effluent tended to accumulate in tissues in a duration-dose-dependent manner. Tissue burden by oil effluent of mussels completely were restored at 30th day. The present experimental findings may be of early warning signals of oil effluent pollution. In conclusion oil effluent are highly toxic to the Lamellidens marginalis.
Keywords: Oil effluent; Accumulation and depuration; Histopathology of foot; Gill; Digestive gland; Lamellidens marginalis
Abbreviations
EP: Epithelium; MU: Muscle Tissue; CI: Cilia; NE: Nucleus; BS: Blood Sinus; MU: Muscle Tissue; NE: Necrosis of Epithelial Tissue; GF-Gill Filaments: CR: Chitinus Rod; FL: Frontal Lateral Cilia; FC: Frontal Cilia; IS: Interlamellar Space; IJ: Interlamellar Junction; WC: Water Chamber; MU: Muscle Tissue; SC: Supra Brachial Chamber; GF: Gill Filaments; FL: Frontal Lateral Cilia; DD: Digestive Diverticula; LU: Lumen; ST: Stomach
Introduction
Researchers with different expertise have converged towards a common interest for understanding and solving the problems associated with the occurrence of toxic level of contaminants in the environment, giving raise to the spectacular development of research in the field of Environmental Contamination and Toxicology, which has emerged as a multidisciplinary science resulting from the integration of classical disciplines such as toxicology, cell and molecular biology, physiology, ecology, chemistry, etc [1]. Uptake and accumulation of xenobiotics in the tissues of aquatic organisms occur from the sediment, contaminated water column and food chain [2] that cause deleterious effects. Incorporation of even very low levels of toxicants in the body of aquatic organisms causes various biochemical, physiological and hematological alterations in vital tissues [3]. Most common usage of the term biomarker has been for biochemical, physiological or histological indicators of their exposure to or the effects of xenobiotic chemicals at the sub-organismal or organismal level [4]. Most of the monitoring programmes are confined to the chemical analysis of accumulated substances, but sometimes include toxic responses, for instance histopathological effects [5,6] or physiological/biochemical responses [7]. When Polycyclic Aromatic Hydrocarbon (PAHs) exposed animals, several deleterious effects such as DNA damage [8]. As an indicator of exposure to contaminant, histology represents a useful tool to assess the degree of pollution, particularly for sub lethal and chronic effects [9]. Histological changes appear as a medium-term response to sub-lethal stressors, and histology provides a rapid method to detect effects tissues and organs [10]. Summary of some relevant earlier literature on marine as well as freshwater mussels histopathological observations with various toxicant exposure results are compiled (Table 1). In molluscs, especially in Lamellidens marginalis histological injuries, in response to the exposure to oil effluent, remains unexplored. Gills are the vital organs, which come into direct contact with water and are indicative of any environmental stress and also in fishes gills are the major vital respiratory organs [11]. The numerous lamellae along the double row of filament attached to the gill arch are affected by toxicants [12]. Molluscs are widely used in different biomonitoring projects and their histopathological analysis provides information about the general health of the animals and contaminant-specific changes in the tissues. Although laboratory as well as field studies suggest that pollutants cause toxic effects to molluscs, the histopathological effects of chemical contaminants have not generally been measured [13]. Blue mussels can retain on their gills, including oil particles have observed [14]. Like numerous bivalves, they concentrate many xenobiotics in their tissues and have been used extensively for biomonitoring of pollutants [15] but there is inadequate contribution on freshwater mussels. Gills [16] are suitable organs for histological examination in order to determine the effect of pollution. Histological changes occurs in the bivalves especially in the hepatopancreas (digestive gland) as they are the metabolically active sites and are responsible for food collection, absorption, digestion, enzymatic activity as well as accumulation and biotransformation (detoxification) of various organic and inorganic toxic substances upon exposure to the organic and inorganic contaminants in the water. Pathological changes in the vital tissues of bivalves have been reported after pollutant exposure [17,6]. Owing to their poor existence and meagre information about the histopathology in invertebrates remarkably in freshwater mussels. This present study attempts to understand the pathological injuries in mussels. Therefore, present investigation were examined during acumulation (30 days) and depuration (recovery) period (30 days) in response to sub lethal concentration of 1/4th (11.88ppt) and 1/10th (8.55ppt) of oil effluent in freshwater mussel tissues of foot, gill and digestive gland. The aims of present study were to observe (1) histopathological damages in foot, gill and digestive gland tissues of mussels during accumulation period of both sublethal concentrations of oil effluent in comparison to control (2) whether these histopathological damages in various tissues of mussels recoverd/ restored in the depuration period in comparison to control mussels and (3) whether these alteration/damages would serve as a biomarker to detect the accumulated oil effluent (hydrocarbon) in this species.
Marine Mussels
Pollutants
Tissues
Responses/Effect
Year
Reference
Mytilus galloprovincialis
Metals
Gill, digestive gland
brown cells, metal burden in tissues
1997
[42]
Perna indica
Heavy metals
Gill, digestive galnd
Clumping of ciliary, damage of gill filaments, dislodged epithelial cells, disintegration of digestive tubules
2005
[46]
Crenomytilus grayanus
Heavy metals and pesticides
Digestive gland
Heavy vacuolization of digestive cells, desquamation of digestive cells of tubules, necrosis, Edemata, lysis of vesicular cells and of muscle fifibers
2006
[17]
Perna viridis
Heavy metals
Gill
Loss of cilia, epithelial cell damage, swollen lumen, elongation of gill filaments.
2008
[40]
Mytilus edulis
Heavy metal
Gill, Digestive gland and adductor muscle
Inflammation and necrosis.
2010
[44]
Gafrarium divaricatum (clams)
xylene, benzene and gear oil-WSF
Hepatopancreas
Cell debris, fusion of Nuclei, interruption of lumen line, disintegration of epithelial cells, necrosis, iated epithelial layer, detachment of epithelial cells.
2011
[53]
Perna viridis
Heavy metal
Gill, Digestive gland and adductor muscle
digestive epithelium, hemocytic infiltration in the gills and myodegeneration in the muscle tissue, necrosis and digestive tubule thickness
2012
[45]
Mytilus galloprovincialis
PAH
Digestive gland
Altered diverticula, damages in digestive tubule
2013
[47]
Ruditapes decussatus
Anthropogenic activities
Gill ,Digestive gland
intertubular tissue necrosis, lesions such as digestive tubule (diverticula)
2013
[52]
Mytilus galloprovincialis
Industry Effluent (Iron, paper Harbour, cement etc.,)
Gill, Hepatopancreas
dejeneration, cell loss and necrose, lumen enlargement, Cilia loss and fusion, haemocytic infiltration, vacuolar degeneration
2016
[48]
Mytilus galloprovincialis
Cadmium
Digestive gland
lumen of digestive tubules, increase of the atrophic tubule
2016
[51]
Mytilus spp.
Mixture of Microplastics
Gill, Digestive gland
Necrosis, atrophies, lumen enlargement
2019
[39]
Mytilus galloprovincialis
Insecticide
Gill, Digestive gland
Vacuolation, epithelial alterations, lipofuscin aggregates, presence of brown cells, digestive tubule alterations, hypertrophy, hyperplasia.
2020
[36]
Freshwater Mussel
Lamellidens marginalis
Oil effluent (hydrocarbon)
Foot, Gill, Digestive gland
Foot-disorganization ofouter epithelium, necrosis of the cell, formation of lumina, disorganisation of muscle bundle, Gill-disruption of the epithelium, oedima formation and necrosis, cilia appeared disaggregated Digestive gland- stroma, detached glandular epithelium, dense accumulation leucocytes, integrity of the epithelium, vertical clefts.
2020
*Our Results
Lamellidens marginalis
Heavy metals
Foot, Hepatopancreas
splitting of muscle bundles, loss of connective tissue, oedema ,atrophy of muscle bundles, Cell necrosis, damage to the intertubular connective tissue
2008
[26]
Lamellidens marginalis
Insecticide
Gill
The bulging of primary filament gill tips, curling of secondary filament, fusion of gill lamellae, hyperplasia, necrotic and clavate globate lamellae of the gills
2011
[33]
Lamellidens marginalis
Dimethoate
Hepatopancreas
Distruption in digestive tubules, disrupted epithelial lining and necrotic tissue in the lumen, Hypertrophic nucleus, Necrotic tissue
2011
[54]
Lamellidens marginalis
Dimethoate
Gill
Disruption in epithelium, damage in epithelial lining, nuclear hypertrophy etc.
2012
[34]
Lamellidens marginalis
Pesticide
Gill, Digestive gland
gill exhibiting reduced space between water channel and interlamellar junction ,intense infiltration of hyper chromatic anaplastic cells , tissue swelling ,irregular shaped branchial filaments, digestive gland exhibiting hepatic tubules with disintegrated epithelial cells and infiltrated basophilic cells
2012
[35]
Dreissena polymorpha
Fluoride
Gill, Digestive gland
scattered pyknotic nuclei, condensed nuclei, altered morphology of the cells
2012
[50]
Lamellidens marginalis
Monocrotophos
Foot
Hyperplasia of marginal pedal glands, distruption of nuclei of the epithelial glands.
2015
[27]
Lamellidens marginalis
Mercury choloride
Gill
Hypoplasia of epithelial cells, gill filaments altered, oedematic, necrotic and vacuolated epithelium
2016
[41]
Anodonta cygnea
Heavy metals
Gill, digestive gland and gonads
Gills-lamellar fusion, dilated hemolymphatic sinus, clumping, and generation of cilia and hemocytic infiltration digestive gland-inflammation, hydropic vacuolation, and lipofuscin pigments, and gonads-atresia, necrosis, granulocytoma, hemocytic infiltration
2018
[49]
Unio Pictorum
Pesticide
Gill
damaged cilia, epithelium rupture, damaged epithelium
2019
[37]
Lamellidens marginalis
Pesticide
Gill
Damaged ciliated epithelium, Elongated gill filament, Delaminated ciliated epithelium, gill epithelium ruptured with damaged ciliary lining
2019
[38]
Table 1: Summary of Relevant Earlier Literatures on Freshwater and Marine Mussels Histolagical Changes with Various Xenobiotic Exposures and Comparison with Our Results.
Materials and Methods
Animals
Almost uniform size fresh water mussel Lamellidens marginalis (total length 6-7 cm and weight 25-27 g) were collected from the River Cauvery (Tiruchirappalli, India) and maintained in the laboratory.
Acute toxicity experiment
The aqueous oil effluent originated from the coal conversion plant, turbine section of boiler units in the Boiler plants of Bharat Heavy Electricals Limited (BHEL) situated 14 km away from Tiruchirappalli, are collectively released into a drainage canal. It consisted mainly of hydrocarbons. Initial experiments were conducted to assess the minimum concentration of oil effluent to obtain maximum mortality, for freshwater mussel, Lamellidens marginalis, over a 96-hr exposure. After confirming the minimum concentration, 10 animals in 5L of tubs (each) and exposed to various concentrations of oil effluent, ranging from 4ppt to 16ppt for a period of 96-hr to ascertain LC50 concentration. In addition, a control was also maintained. The 96- hr LC50 values with 95% confidence limits were calculated using National Crop Production Centre Technical Bulletin [18].
Exposure experiment
Based on the 96-hr LC50 value of oil effluent, sublethal concentrations of 1/4th (11.88ppt) and 1/10th (8.55ppt ) of LC50 were prepared and used for histopathology. In this study, two sets of 10-l plastic tubs were used. In each tub, mussels were exposed to 11.88ppt or 8.55ppt of oil effluent. A control was also run simultaneously without the addition of oil effluent. At seven days interval, mussels were sacrificed for histological analysis. After 30 days, the treated mussels were released into freshwater 30-day depuration (recovery) study was conducted. Four mussels were randomly chosen and removed from each of the two tubs (n=8) for dissection.
Preparations of tissue samples
Control set of animals tissues were sacrificed for Ist, 8th, 15th, 22nd and 30th days of foot, gill and digestive gland tissues of mussels. At every seven days intervals, accumulation and depuration (recovery) period of Ist, 8th, 15th, 22nd and 30th days of foot, gill and digestive gland tissues of mussels from both exposures were sacrificed for the evaluation of histological analysis.
Paraffin method: For the paraffin method, the above specified tissues were fixed in Bouin’s fluid, and embedded in paraffin. Serial sections were obtained at 3-5 μm thickness using a Leica (Germany) microtome with provision of disposable blade. Serial sections stained in haematoxylin and eosin [19] and mounted in DPX mountant for microscopical observations.
Microscopic analysis: For light microscopic observation Carl- Zeiss (Germany) Axioskop 2-research microscope was used, and the images were captured in a computer using Carl-Zeiss (Germany) Axiovision Software and the images processed using the same software. The histopathological changes in the tissues of experimental as well as control mussels were recorded and compared.
Results
Histology of foot
Foot is the locomotory organ chiefly employed for burrowing and is formed of an outer dense epithelium, which is grown into tall folds (villous); the epithelium lies top of the cells the musculature, variety of protractor, retractor muscles. Blood sinuses are found between the fine muscles. The muscular wall of the foot surrounds the coelomic phase which itself is lined by coelomic epithelium. In the outer epithelium, the cells differ in height and length of the nucleus. In some of the cells the nucleus is extremely elongated. The outer borders of the epithelium have dense cilia (Figures 1-4).
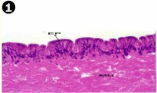
Figure 1: Haematoxylin and eosin stained paraffin sections of control mussel
Lamellidens marginalis foot tissue. (X100, 400).
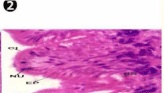
Figure 2: Haematoxylin and eosin stained paraffin sections of control mussel
Lamellidens marginalis foot tissue. (X100, 400).

Figure 3: Haematoxylin and eosin stained paraffin sections of treated mussel
(1/4th accumulation-Ist, 8th and 15th days) foot tissue. (X400).

Figure 4: Haematoxylin and eosin stained paraffin sections of treated mussel
(1/4th accumulation-Ist, 8th and 15th days) foot tissue. (X400).
Histopathology of foot
During the exposure of both sublethal concentrations (1/4th and 1/10th) of oil effluent little changes were observed in the foot tissues of mussel at Ist day. However, from the 8th day onwards the outer epithelium was thoroughly disorganized resulted in necrosis of the cell and formation of lumina both on top of the musculature, with exposure for longer durations the muscle bundle themselves were disorganized in both sublethal concentration of oil effluent exposure (Figures 5-11). During the depuration (recovery) process, the fresh water mussel brought about almost complete restorations of the histo-architecture of the foot tissues (Figures 12-21).
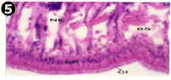
Figure 5: Haematoxylin and eosin stained paraffin sections of treated mussel
(1/4th accumulation-Ist, 8th and 15th days) foot tissue. (X400).
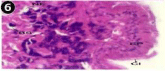
Figure 6: Haematoxylin and eosin stained paraffin sections of treated mussel
(1/4th accumulation-Ist, 8th and 15th days) foot tissue. (X400).

Figure 7: Haematoxylin and eosin stained paraffin sections of treated mussel
(1/4th accumulation-22nd and 30th days) foot tissue. (X400).

Figure 8: Haematoxylin and eosin stained paraffin sections of treated mussel
(1/4th accumulation-22nd and 30th days) foot tissue. (X400).

Figure 9: Haematoxylin and eosin stained paraffin sections of treated mussel
(1/10th accumulation- 8th, 15th and 22nd days) foot tissue. (X400, X400, X100).

Figure 10: Haematoxylin and eosin stained paraffin sections of treated
mussel (1/10th accumulation- 8th, 15th and 22nd days) foot tissue. (X400, X400,
X100).

Figure 11: Haematoxylin and eosin stained paraffin sections of treated
mussel (1/10th accumulation-8th, 15 and 22nd days) foot tissue. (X400, X400,
X100).
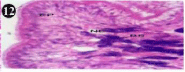
Figure 12: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/4th depuration-Ist day) foot tissue. (X400).
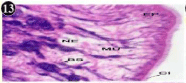
Figure 13: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/4th depuration-8th, 15th, 22nd and 30th days) foot
tissues. (X400, X1000).
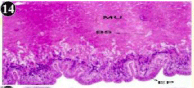
Figure 14: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/4th depuration-8th, 15th, 22nd and 30th days) foot
tissues. (X400, X1000).

Figure 15: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/4th depuration-8th, 15th, 22nd and 30th days) foot
tissues. (X400, X1000).
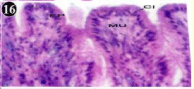
Figure 16: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/4th depuration-8th, 15th, 22nd and 30th days) foot
tissues. (X400, X1000).
Figure 17: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/10th depuration-Ist and 8th days) foot tissues.
(X400).
Figure 18: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/10th depuration-Ist and 8th days) foot tissues.
(X400).
Figure 19: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/10th depuration-Ist and 8th days) foot tissues.
(X100, X400).
Figure 20: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/10th depuration-15th, 22nd and 30th days) foot
tissues. (X100, X400).
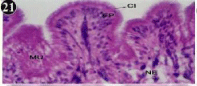
Figure 21: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel (1/10th depuration-15th, 22nd and 30th days) foot
tissues. (X100, X400).
Histology of gill
The gill of the mussel is formed of an outer and inner lamellae called ctenidium, each folded to form the outer and inner lamellae. The lamellae are connected by inter-lamellar junctions, which contain blood vessels. The gill filament constitutes a longitudinal array and adjacent filaments are connected by inter-filamentor junctions. The gill filament is lined by an epithelium formed of a single row cells, short cuboidal adiphase and tall columnar towards the tip. Three types of cilia are associated with gill filament, they are, tall lateral-cilia, tall latero-frontal cilia and short frontal -cilia at the tip. The stroma underlying the epithelium bridges of connective tissue containing connecting rods for support and also blood vessels (Figures 22,23).
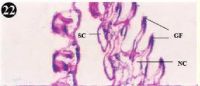
Figure 22: Haematoxylin and eosin stained paraffin sections of control
mussel gill tissues (X400, X1000).
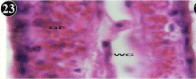
Figure 23: Haematoxylin and eosin stained paraffin sections of control
mussel gill tissues (X400, X1000).
Histopathology of gill
When exposed to both sublethal concentrations of oil effluent caused disruption of the epithelium of the gill filaments and all the versions of the cilia. The epithelium indicated severe pathological changes of the oedima formation and necrosis. Disaggregated cilia were appeared (Figures 24-32). During the recovery period, brought about partial to almost complete restoration of the histo-architecture of the gill filaments. The cilia appeared normal. The epithelium shows almost free from oedima formation and necrosis (Figures 33-42).
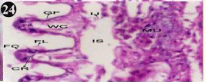
Figure 24: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/4th accumulation-1st day) (X400).
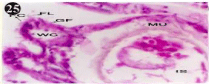
Figure 25: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/4th accumulation-8th, 15th, 22nd and 30th days and 1/10th
accumulation-Ist day and 8th day). (X400).
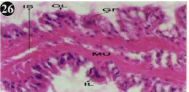
Figure 26: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/4th accumulation-8th, 15th, 22nd and 30th days and 1/10th
accumulation-Ist day and 8th day). (X400).
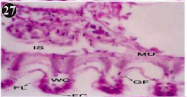
Figure 27: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/4th accumulation-8th, 15th, 22nd and 30th days and 1/10th
accumulation-Ist day and 8th day). (X400).
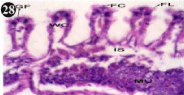
Figure 28: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/4th accumulation-8th, 15th, 22nd and 30th days and 1/10th
accumulation-Ist day and 8th day). (X400).
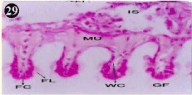
Figure 29: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/4th accumulation-8th, 15th, 22nd and 30th days and 1/10th
accumulation-Ist day and 8th day). (X400).
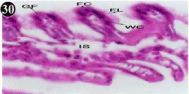
Figure 30: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/4th accumulation-8th, 15th, 22nd and 30th days and 1/10th
accumulation-Ist day and 8th day). (X400).
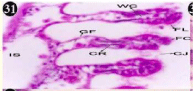
Figure 31: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/10th accumulation-22nd and 30th days). (X400).
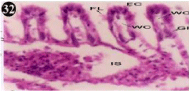
Figure 32: Haematoxylin and eosin stained paraffin sections of treated
mussel gill tissues (1/10th accumulation-22nd and 30th days). (X400).
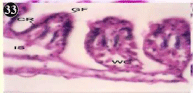
Figure 33: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/4th depuration-Ist, 8th, 15th, 22nd
days). (X400).
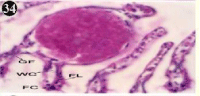
Figure 34: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/4th depuration-Ist, 8th, 15th, 22nd
days). (X400).
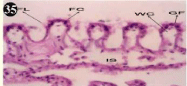
Figure 35: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/4th depuration-Ist, 8th, 15th, 22nd
days). (X400).
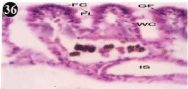
Figure 36: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/4th depuration-Ist, 8th, 15th, 22nd
days). (X400).
Figure 37: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/4th depuration-30th days). (X400).
Figure 38: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/10th depuration-Ist, 8th, 15th, 22nd
and 30th days). (X400).
Figure 39: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/10th depuration-Ist, 8th, 15th, 22nd
and 30th days). (X400).
Figure 40: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/10th depuration-Ist, 8th, 15th, 22nd
and 30th days). (X400).
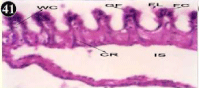
Figure 41: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/10th depuration-Ist, 8th, 15th, 22nd
and 30th days). (X400).
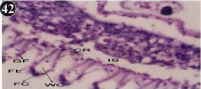
Figure 42: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel gill tissues (1/10th depuration-Ist, 8th, 15th, 22nd
and 30th days). (X400).
Histology of digestive gland
The digestive gland consists of diverticula and their ducts, which connected to be, and inter diverticula tissue (Figure 43). The glandular epithelium is formed of different types, most of which are tall columnar. The different heights of the cell, render the epithelium appears live villous folds, lamellae propitious underlies the epithelium. The lumen of diverticulate contains a few materials, which are likely the ingested food (Figure 44). The nucleus of epithelium is elongated, spindle shaped and darkly staining, it is located at different heights of the cells. The folds of epithelium produce pockets in the profile of the lumen (Figure 45).

Figure 43: Haematoxylin and eosin stained paraffin sections of control
mussel Lamellidens marginalis digestive gland tissue. (X100, X400, X100).
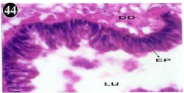
Figure 44: Haematoxylin and eosin stained paraffin sections of control
mussel Lamellidens marginalis digestive gland tissue. (X100, X400, X100).

Figure 45: Haematoxylin and eosin stained paraffin sections of control
mussel Lamellidens marginalis digestive gland tissue. (X100, X400, X100).
Histopathology of digestive gland
When exposed to both subletahal concentrations of oil effluent, produced gross changes in the epithelium of the glands as well as the stroma. The glandular epithelium was invariably detached from the stroma (Figure 46-65). This stroma adds dense accumulation leucocytes from Ist day of both sublethal concentrations. The integrity of the epithelium was thoroughly disrupted and a major change was consisted of vertical clefts. Another important feature was noticed towards the dense accumulation of the luminal material, which also contain leucocytes, which were otherwise confined to stroma. During the recovery (depuration) period, the fresh water mussel brought about a gradual restoration of the organization of the digestive gland and nature of the epithelium. The day 15th onwards the glandular architecture was comparable to that of the control mussels, though the dense accumulation of leucocytes between the stroma and epithelium continued till the 30th day, but the stroma was absent i.e. completely restored (Figure 66-75).
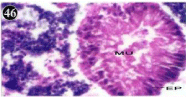
Figure 46: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-Ist, 8th and 15th days)
(X400).
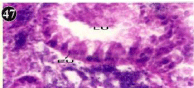
Figure 47: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue (1/4th accumulation-Ist, 8th and 15th days) (X400).
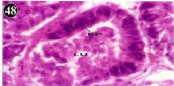
Figure 48: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-Ist, 8th and 15th days)
(X400).
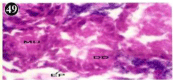
Figure 49: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-22nd and 30th days) (X400).

Figure 50: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-22nd and 30th days) (X400).
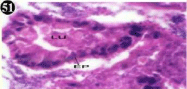
Figure 51: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th and 22nd days)
(X400).
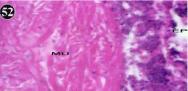
Figure 52: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th and 22nd days)
(X400).
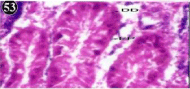
Figure 53: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th and 22nd days)
(X400).
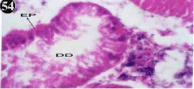
Figure 54: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th and 22nd days)
(X400).
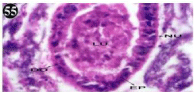
Figure 55: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissues (1/10th accumulation-30th days). (X400).

Figure 56: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-Ist, 8th, 15th, 22nd and 30th
days) (X1000).
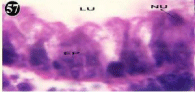
Figure 57: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-Ist, 8th, 15th, 22nd and 30th
days) (X1000).
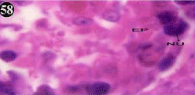
Figure 58: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-Ist, 8th, 15th, 22nd and 30th
days) (X1000).

Figure 59: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-Ist, 8th, 15th, 22nd and 30th
days) (X1000).
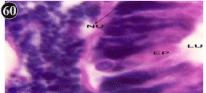
Figure 60: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/4th accumulation-Ist, 8th, 15th, 22nd and 30th
days) (X1000).

Figure 61: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th, 22nd and 30th
days). (X1000).

Figure 62: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th, 22nd and 30th
days). (X1000).
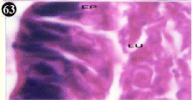
Figure 63: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th, 22nd and 30th
days). (X1000).
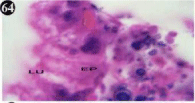
Figure 64: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th, 22nd and 30th
days). (X1000).
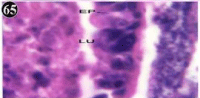
Figure 65: Haematoxylin and eosin stained paraffin sections of treated
mussel digestive gland tissue. (1/10th accumulation-Ist, 8th, 15th, 22nd and 30th
days). (X1000).
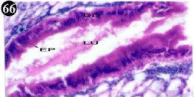
Figure 66: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/4th depuration-Ist day)
(X1000).
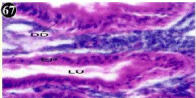
Figure 67: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/4th depuration-8th, 15th,
22nd and 30th days; 1/10th depuration-1st and 8th days). (X1000).

Figure 68: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/4th depuration-8th, 15th,
22nd and 30th days; 1/10th depuration-1st and 8th days). (X1000).

Figure 69: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/4th depuration-8th, 15th,
22nd and 30th days; 1/10th depuration-1st and 8th days). (X1000).
Figure 70: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/4th depuration-8th, 15th,
22nd and 30th days; 1/10th depuration-1st and 8th days). (X1000).
Figure 71: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/4th depuration-8th, 15th,
22nd and 30th days; 1/10th depuration-1st and 8th days). (X1000).
Figure 72: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/4th depuration-8th, 15th,
22nd and 30th days; 1/10th depuration-1st and 8th days). (X1000).
Figure 73: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/10th depuration-15th
22nd and 30th days) (X1000).
Figure 74: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/10th depuration-15th
22nd and 30th days) (X1000).
Figure 75: Haematoxylin and eosin stained paraffin sections during
depuration period of mussel digestive gland tissue. (1/10th depuration-15th
22nd and 30th days) (X1000).
Discussion
Biological, physiology and morphological structure of molluscan systems were described [20-22]. The normal structure of mussels gill, foot, digestive gland has been well-described [23,24]. In some publications, lesions have only been described morphologically [25]. Summary of some relevant earlier literature on marine as well as freshwater mussels histological observations with various toxicants and comparison with our present results are compiled (Table 1). In the present study, when exposed to both sublethal concentrations of oil effluent little changes were observed in mussels foot tissue on Ist day. From 8th day onwards, outer epithelium of the mussels’ foot tissue were disorgnaised and resulted in necrosis of the cell. When exposed to the sublethal concentrations of oil effluent, for longer duration’s muscle bundle themselves were also disorgnaised in mussel foot tissue. This could lead to the failure of a number of biochemical activities as well as osmo and iono-regulatory functions of the foot. Present histopathological findings may explain a defensive reaction from the mussels under investigation as similar results were observed by molluscan researchers. Thus, the present results are in agreement with [26] who exposed to heavy metals splitting of muscle bundles in foot tissue were observed in the fresh water mussel, Lamellidens marginali. Similar findings [27] were observed disruption of nuclei of the epithelial glands in foot tissue when exposed to pesticide. The histopathology of foot indicated that concentration and duration of exposure period resulted in massive destruction in normal architecture of foot tissue of mussel. Similarly, the disruption of the epithelium of the gill filaments and all the versions of the cilia were observed when exposed of both sublethal concentrations of oil effluent. The epithelium indicated severe pathological changes of the oedima formation and necrosis. The cilia appeared disaggregated during accumulation of both sublethal concentrations of oil effluent. The gills (ctenidia) of lamellibranch bivalves play a dominant role in controlling the interaction between the individual and its environment. A great deal of literature is available on the mechanisms of food particle retention, as well as on the nature and activities of the cilary systems of such organs [28]. Histological alterations and biochemical changes of gill tissues produced by the chemical stress causes disturbed metabolism, enzyme inhibition, retardation of growth, fecundity reduction and longevity of the organism, which will affect balance in the ecosystem [29]. An exhaustive review of toxicant/ irritant induced changes in the gill, stated that the inflammatory changes tend to be largely non-specific, and seen to reflect physiological adaptation to stresses were made [16]. Tributyltin (TBT) treated mussel Mytilus galloprovincialis the structure of gill was destroyed, interfilament junctions and cilia disappeared and lateral and endothelial cells were changed [30]. A wide variety of histopathological and physiological responses to naphthalene exposure were described in the mummichog Fundulus heteroclitus [31]. Structural changes and proliferate lesions of gills in Salmo trutta, Oncorhychus mykiss, was observed [32] when exposed to sewage plant effluents. When exposed to pesticides, fusion of gill lamellae in Lamellidens marginalis [33], gill epithelium lining and disruption in Lamellidens marginalis [34], gill exibits reducing space between channels, tissue swelling in Lamellidens marginalis [35], vacuolation, epithelial alterations in Mytilus galloprovincialis [36], damaged epithelium, cilia, epithelium ruptured in gill of Unio pictorum [37], elongated gill filament, gill epithelium ruptured with damaged ciliary lining in Lamellidens marginalis [38] were observed and when exposed to microplastics, epithelial alterations, necrosis were observed in Mytilus spp. [39]. When exposed to heavy metals loss of cilia, epithelial damage, swollen lumen, elongation of gill filaments in Perna viridis [40]; gill filaments altered, oedematic, necrotic and vacuolated epithelium in Lamellidens marginalis [41] were observed. Hence, the changes in the histopathological structure of the gill can be use as biomarkers of exposure in the aquatic environment and the freshwater bivalve Lamellidens marginalis can be considered as a bioindicator organism to assess the water quality. Similarly, the digestive gland produced gross changes in the epithelium of the glands as well as the stroma. The glandular epithelium invariably detached from the stroma were observed in mussel, during accumulation period of oil effluent. This stroma adds dense accumulation of leukocytes from Ist day exposure of both concentrations. Thoroughly disrupted integrity of the epithelium was observed during the accumulation of both sublethal concentrations of oil effluent and a major change was consisted of vertical clefts. Another important feature were observed towards the dense accumulation of the luminal material, which also contain leukocytes, which were otherwise confined to stroma. The responses of digestive gland of mussels to various pollutants exposure of previous results and our present study are in agreement with the following findings of works. Disruption in the epithelial of glands, tissue burden, digestive tube thickness, lumen enlargement, necrotic tissues by heavy metals in Mytilus galloprovincialis [42,43], in mytilus edulis [44], in Perna viridis, Perna indica [45,46], in Crenomytilus grayanus [17]; PAH induced altered diverticula, damages in digestive tubules in Mytilus galloprovincialis [47]; industrial effluents alters vacuolar degeneration in Mytilus galloprovincialis [48]; mixture of micro-plastics altered atrophies, lumen enlargement in Mytilus spp [39]; insecticide damages digestive tubule alterations, hypertrophy, hyperplasia in Mytilus galloprovincialis [36]; heavy metals damages hemocytic infiltration digestive gland, inflammation in Anodonta cygnea [49], condensed nuclei, altered morphology of cell in Dreissena polymorpha [50], increase of atrophic tubule, damages lumen of digestive tubule in Mytilus galloprovincialis [51]; anthropogenic contaminant induces diverticula, inter-tubular tissue necrosis in Ruditapes decussatus [52]; xylene, benzene and gear oil-WSF damages cell debris, fusion of nuclei, disintegration of epithelial cells necrosis in Gafrarium divaricatum (calm) [53]; pesticide alters disruption in digestive tubules, epithelial lining, necrotic tissue in the lumen in Lamellidens marginalis [54] were observed. Disruption of normal lysosomal function is indicated by the increased fragility of lysosomes in digestive cells throughout the experiment and this is reflected in a reduced physiological scope for growth [55]. Formation of neoplasams in the digestive gland in Unio pictorum by subjecting 200-400 ppm diethyl- and dimethylnitrosamines were observed [56]. Number of gill mucous cells and the heights of digestive diverticula tubule cells were different, compared with the control, at 2 or 4 weeks, when exposed to cadmium were observed [57]. During depuration (recovery) period, the mussel brought about almost complete restorations of the histo-architecture of the foot tissue. Similarly, brought about partial to almost complete restoration of the histoarchitecture of the gill filaments. The cilia appeared normal. The epithelium is almost free from oedima formation and necrosis. Likewise, the mussel brought about a gradual restoration of the organization of the digestive gland and nature of the epithelium. The day 15th onwards the granular architecture was comparable to that of the control mussels, though the dense accumulation of leukocytes between the stroma and epithelium continued till the 30th day, but the stroma was absent, completely restored. Such a high level of oil effluent toxicity (concentration and duration) in digestive gland might be responsible of histological alterations. The digestive gland is also helpful for metabolism of xenobiotics. The histological damage to digestive gland tissue of Lamellidens marginalis due to exposure of oil effluent, definitely disturbs its normal functions like secretion, absorption and storage of nutrient materials and this gland is also helpful for metabolism of oil effluent. Present histopathology investigation on the freshwater mussel is sparse, to fill up the gap, a preliminary study has been carried out on the mussel Lamellidens marginalis.
Conclusion
The results of this study are well under the aims and objective of the study. However, results of our research enabled us to understand that histopathological changes in the foot, gill and digestive gland tissues of freshwater mussel, Lamellidens marginalis and is noted accumulator organism of oil pollution. The observed damage to foot, gill and digestive gland tissues due to oil effluent and definitely disturbs its normal functions and storage of nutrient materials. Particularly, the digestive gland is also helpful for metabolism of xenobiotics in freshwater mussel Lamellidens marginalis. These histopathology might be due to the possible utilization for metabolic purpose and may be of early warning signals of environmental pollution.
Acknowledgement
Authors wish to thank, Department of Animal Science, Bharathidasan University, Tiruchirappalli, Tamilnadu, India, for providing necessary facilities for completion of research work.
References
- Cajaraville MP. Towards an integrative approach in environmental contamination and toxicology. The Sci Total Environ. 2000; 247: 95-97.
- Livingstone DR, Forlin L, George SG. Molecular biomarkers and toxic consequences of impact by organic pollution in aquatic organisms. In: Sutcliffe, D.N. (Ed.), Water Quality and Stress Indicators in Marine and Freshwater Systems: Linking Levels of Organization. Freshwater Biological Association, Ambleside. 1994; 154-171.
- Mathur DS, Agarwal MD, Rane PD. Histopathological changes in liver and intestine of Rana cyanoplyctis induced by aldrin. J Environ Biol. 1981; 2: 105- 107.
- Huggett RJ, Kimerle RA, Mehrle PM. In Biomarkers, Biochemical, Physiological, and Histlogical Markers of Anthropogenic Stress. Boca Raton, Florida: Lewis Publishers. 1992.
- Bowmer CT, Van der Meer M, Scholten MC. A histopathological analysis of wild and transplanted Dreissena polymorpha from the Dutch sector of the River Maas. Comp Bioche Physiol. 1991; 100: 225-229.
- Balamurugan S, Subramanian P. Oil effluent toxicity and detoxification mechanism in the freshwater mussel, Lamellidens marginalis. Bharathidasan University, Tiruchirappalli, Tamilnadu. India. 2003.
- Moore MN, Livingstone DR, Widdows J. Molecular, cellular and physiological effects of oil-derived hydrocarbons in molluscs and their use in impact assessment. Phil Trans R Soc Lon B.1987; 316: 603-623.
- Bolognesi C, Rabboni R, Roggieri P. Genotoxicity biomarkers in Mytilus galloprovincialis as indicators of marine pollution. Comp Biochem Physiol. 1996; 113: 319-323.
- Bernet D, Schmidt H, Meier W. Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis. 1999; 22: 25-34.
- Johnson LL, Stehr CM, Olson OP. Chemical contaminants and hepatic lesions in water flounder (Pleuronectus americanus) from the Northeast Coast of the United States. Environ Sci Tech. 1993; 27: 2759-2771.
- Hughes GM. Measurement of gill area in fishes: Practices and problems. J Mar Biol Assoc UK. 1984; 65: 637-655.
- Haniffa MA, Sundaravadananm S. Effect of distillery effluents on food utilization of fresh water fish, Barbus stigma. Life Sci Adv. 1983; 2: 146.
- Hemelraad J, Herwig HJ, Van Donselaar EG. Effects of cadmium in fresh water calms II Ultra structural alterations in the renal system of Anodonta cygnea. Arch Environ Contam. Toxicol. 1990; 19: 691-698.
- Baussant T, Sanni S, Jonsson G. Bioaccumulation of ploycyclic aromaic compounds: 1.Bioconcentration in two marine species and in semipermeable devices during chronic exposure to dispersed crude oil. Environ Toxicol Chem. 2001; 20: 1175-1184.
- Sabaliunas D, Lazutka J, Sabaliuniene L. Use of semipermiable membrane devices for studying effects of organic pollutants: Comparision of pesticide uptake by semipermeable membrane devices and mussels. Environ Toxicol Chem.1998; 17: 1815-1824.
- Mallatt J. Fish gill structural changes induced by toxicants and other irritants: a statistical review. Canadian J Fish Aquatic Sci.1985; 42: 630-648.
- Usheva LN, Vaschenko MA, Durkina VB. Histopathology of the Digestive Gland of the Bivalve Mollusk Crenomytilus grayanus (Dunker, 1853) from Southwestern Peter the Great Bay, Sea of Japan. Russian J Mar Biol. 2016; 32: 166-172.
- National Crop Production Centre (NCPC). NCPC Technical Bulletin No: 1. Basic computer programmes for the study of populations and other applications (E Benigo Ed.). 1986.
- Humason GL. Animal Tissues Techniques. San Francisco: Freeman WH and Co. 1979.
- Johnson LL, Stehr CM, Olson OP, Myers MS, Pierce SM, Wigren CA, et al. Hyman. The Invertebrates: Mollusca I (Vol. VI). McGraw-Hill, New York. 1967.
- Purchon RD. The biology of mollusca. 2nd edn. Pergamon, Oxford. 1977.
- Barnes RD. Invertebrate Zoology. WB Saunders Co Phila. 1963; 632.
- Owen G. Lysosomes, peroxisomes and bivalves. Sci Prog Oxf. 1972; 60: 299-318.
- Langton RW. Synchrony in the digestive diverticula of the Mytilus edulis L. J Mar Biol Assoc UK. 1975; 55: 221-229.
- Hinton DE, Lantz RC, Hampton JA. Normal versus abnormal structure: considerations in morphologic responses of teleosts to pollutants. Environ Health Perspect. 1987; 71: 139-146.
- Venkata Chandrudu M, Radhakrishnaiah K. Effect of Cadmium on the Histology of Hepatopancreas and Foot of the Freshwater Mussel Lamellidens marginalis (Lam.) Nature Environment and Pollution Technology. 2008; 7: 397-402.
- Teeni Janet Raj TG, Shyla Suganthi A. Toxic effect of Monocrotophos on the Foot of the Freshwater Mussel, Lamellidens marginalis. ISSN 0976-5417. Cross Res. 2015; 6.
- Morton B. Feeding and digestion in bivalvia. In: The molusca, vol.5, Physiology, Part, 2 PP-65-187.Ed. by Saleuddin, ASM and Wilbur K. London Academic Press.1983.
- Sole M, Rivera-Ingraham G, Freitas R. The use of carboxylesterases as biomarkers of pesticide exposure in bivalves: A methodological approach. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2018; 212: 18-24.
- Micic M, Bihari N, Labura Z. Induction of apoptosis in the blue mussel Mytilus galloprovincialis by tri-n-butyltin chloride. Aquat Toxicol. 2001; 55: 61-73.
- Di Michele L, Taylor MH. Histopathological and physiological response of Fundulus heteroclitus to naphthalene exposure. J Fish Res Bd Canada. 1978; 35: 1060-1066.
- Schmidt H, Bernet D, Wahli T. Active biomonitering with brown and rainbow trout in diluted sewage plant effluents. J Fish Biol. 1999; 54: 585-596.
- Stalin A, Saiyad musthafa M, Amanullah B. Histological alterations in the gills of freshwater mussel Lamellidens marginalis exposed to sub-lethal concentration of an organophosphorus insecticide, chlorpyrifos. Asian Journal of Microbiology, Biotechnology and Environmental Science. 2011; 13: 139-142.
- Kumar S, Pandey RK, Das S. Dimehoate alters respiratory rate and gill histopathology in freshwater mussel lamellidens marginalis (lamarck). J App Biosci. 2012; 38: 154-158.
- Krishnendu D, Mitali R, Sajal R. Structural impairment of gill and digestive gland of Lamellidens marginalis exposed to Cypermethrin. J Ani Biol. 2012; 3: 117-126.
- Stara A, Pagano M, Capillo G. Assessing the effects of neonicotinoid insecticide on the bivalve mollusc Mytilus galloprovincialis. The Sci Total Environt. 2020; 700: 134914.
- Khudhur SM, Shekha YA. Histopathological and Biochemical Biomarker Response of Mussel, Unio Pictorum, to Carbamate Pesticide Carbaryl: A Laboratory Study. Indian J Ani Res. 2019; B-1157: 1-5.
- Rane MS, Kolhe BG. Histopathological alterations in gills of freshwater bivalve, Lamellidens marginalis (Lamarck) after acute exposure to Thiamethoxam and Triazophos. Int J Life Sci. 2019; A13: 24-28.
- Revel M, Lagarde F, Perrein-Ettajani H, Bruneau M, Ackha F, Sussarellu R, et al. Tissue-Specifific Biomarker Responses in the Blue Mussel Mytilus spp. Exposed to a Mixture of Microplastics at Environmentally Relevant Concentrations. Frontiers in Environ. Sci. 2019; 7: 1-14.
- Bhargavan B. Haematological responses of green mussel Perna viridis (Linnaeus) to heavy metals copper and mercury. Thesis, Cochin University OF Science and Technology, Kochi-682016, India. 2008.
- Pandey A, Shanthanagouda AH. Devendra Pathak. Histopathological effects in gills of freshwater mussels Lamellidens marginalis exposed to mercury chloride. The Bioscan. 2016; 11: 2277-2280.
- Soto M, Ireland MP. The contribution of metal/shell-weight index in targettissues to metal body burden in sentinel marine molluscs. 2. Mytilus galloprovincialis. The Sci Total Environ. 1997; 198: 149-160.
- Domouhtsidou GP, Dimitriadis VK. Ultrastrucural localization on heavy metals (Hg, Ag, Pb and Cu) in gills and digestive gland of mussels, Mytilus galloprovincialis (L). Arch Environ Contam Toxicol. 2000; 38: 472-478.
- Sheir SK, Handy RG. Tissue injury and cellular immune response to cadmium chloride exposure in the common mussel, Mytilus edulis: Modulation by lipopolysacchararide. Arch Environ Contam Toxicol. 2010: 59: 602-613.
- Vasanthi LA, Revathi P, Arulvasu C. Biomarkers of metal toxicity and histology of Perna viridis from Ennore estuary, Chennai, south east coast of India Ecotoxicol. Environ Saf. 2012; 84: 92-98.
- Philip Mathew, Menon NR. Histological aberrations accompanying chronic metal toxicity in the mussel Perna indica. J Mar Biol Ass India. 2005; 47: 144-149.
- Cappello T, Maisano M, D’Agata A, Natalaotto A, Mauceri A, Fasulo S. Effects of environmental pollution in caged mussels (Mytilus galloprovincialis). Marine Environ Res. 2013; 91: 52-60.
- Katalay S, Yavasoglu A, Yigittürk G. Histological effects of pollution on gill and hepatopancreas tissues of black mussels (Mytilus galloprovincialis) from izmir bay of turkey. Fresenius. Environ. Bull. 2016; 25: 1460-1466.
- Khan MI, Khisroon M, Khan A. Bioaccumulation of Heavy Metals in Water, Sediments, and Tissues and Their Histopathological Effects on Anodonta cygnea (Linea, 1876) in Kabul River, Khyber Pakhtunkhwa, Pakistan. Bio Med Research International. 2018; 2018: 1-10.
- Casellato S, Masiero L, Ballarin L. Toxicity of fluoride to the freshwater mollusc, Dreissena polymorpha: Effects on survival, histology, and antioxidant enzyme activity. Research Report Fluoride. 2012; 45: 35-46.
- Rocha TL, Morais SMTS, Bebianno MJ. Histopathological assessment and inflammatory response in the digestive gland of marine mussel Mytilus galloprovincialis exposed to cadmium-based quantum dots. Aquatic Toxicol. 2016; 177: 306-315.
- Pedro M, Carreira S, Maria H. Development of histopathological indices in a commercial marine bivalve (Ruditapes decussatus) to determine environmental quality. Aquatic Toxicol. 2012; 126: 442-454.
- Agwuocha S, Kulkarni BG, Pandey AK. Histopathological alterations in hepatopancreas of Gafrarium divaricatum exposed to xylene, benzene and gear oil-WSF. J Environ Biol. 2011; 32: 35-38.
- Kumar S, Pandey RK, Das S. Pathological changes in hepatopancreas of freshwater mussel Lamellidens marginalis (Lamarck) exposed to sub-lethal concentration of Dimethoate. GERF Bull. Biosci. 2011; 2: 18-23.
- Widdows J, Moore SL, Clarke KR. Uptake, tissue distribution and elimination of [1-14C] naphthalene in the mussel Mytilus edulis. Mar Biol. 1983; 76: 109- 114.
- Khudolei VV, Sirenko OA. Tumor development in the bivalve mollusk Unio pictorum induced by N-nitroso compounds. Bull Expre Biol Med. 1997; 83: 684-686.
- Giraud AS, Webster LK, Fabris JG. Absence of histopathological responses to cadmium in gill and digestive diverticula of the mussel, Mytilus edulis. Bull Environ Contam Toxicol. 1986; 36: 146-149.