
Research Article
Austin J Environ Toxicol. 2021; 7(1): 1036.
Stability and Presence of Pesticide Residue Sample Extracts of Soil and Vegetable: Eloor & Edayar Region, Kerala, Industrial Hub Nearer to Arabian Sea
Divya KR1*, Midhun TR3, Moushmi KS1, Cheriyan AS1, Nisari AR1, Ratheeshkumar CS4, Shaiju P1, MartinGD1 and Chandramohanakumar N1,2
1Department of Chemical Oceanography, Cochin University of Science and Technology, India
2Inter University Centre for Development of Marine Biotechnology, Cochin University of Science and Technology, India
3Department of Applied Chemistry, Cochin University of Science and Technology, India
4School of Environmental studies, Cochin University of Science and Technology, India
*Corresponding author: Divya KR, Department of Chemical Oceanography, Cochin University of Science and Technology, Cochin, India
Received: February 19, 2021; Accepted: March 15, 2021; Published: March 22, 2021
Abstract
Eloor-Edayar, (latitude 9° 3´N and 10° 6´N and longitudes 76° 18´E and 76° 30´ and an area of 14.21km2) the landmass situated on the banks of River Periyar, is known as the industrial backbone of Kerala. The previous reports reveal this region is highly contaminated by heavy metal from industrial effluents. The present study focuses on pesticides in vegetables (Ladies finger, Papaya, Kachil- yam, Guava, Tapioca, Ginger) and related soil extracts when kept in cold conditions and their presence after a long period. The samples collected in May 2008 pre-monsoon, the environment when the soil and vegetables were free from volatile organic components, accelerates the adsorption of pesticides and high temperature. The samples shoot vegetable ladies finger, edible root vegetables Kachil-Yum, Tapioca and Ginger, fruits like Papaya and Guava and the sediments nearer land. The sample extracts analyzed in 2019 after keeping in the cold as well as the usual conditions. The Analysis report shows the pesticides present in the vegetables and soil are a, β, γ BHC Aldrin, Dicofol, a, β, Endosulfan, Dieldrin, OPDDT and PPDDT. Aldrin, Dicofol and BHC are the related forms in ladies Finger contains γ BHC, a, β, Endosulfan, Dieldrin, Aldrin, PPDDT. Ginger added a, β, γ BHC Aldrin, Dicofol, a, β, Endosulfan, OPDDT and PPDDT. Tapioca carried a, β, γ BHC, Dicofol and PPDDT. Kachil-Yum absorbed a, β, γ BHC Aldrin, Dicofol, a, β, Endosulfan, OPDDT and PPDDT. Papaya contains a and γ BHC. Guava captivated a, γ BHC Aldrin, Dicofol, a Dieldrin OPDDT and PPDDT.
Keywords: Eloor-edayar; Organochlorine pesticides; Endosulfan; DDT
Introduction
The “Green Revolution” initiated in the 1960s made India No. 1 manufacturer of pesticides in Asia and 12th globally. Due to uncontrolled use, Indian foods and vegetables have the highest residues in the world. At the national level in India, Kerala is the highest [1] among in districts Kasaragod [2] and Palakkad [3].
Environmental bioaccumulation potential and associated health issues, most of the OCL pesticides categorized as environmental hazards and banned by The Stockholm Convention [4-6]. WHO reports that at present, in Developing countries, organochlorine pesticides used for farming [7] even though Developed countries have declared them as primary pollutants [7-11]. Microorganisms, invertebrates, plants, birds like the peregrine falcon, sparrow hawk and bald eagle, fish and blood plasma of agrifarmers affected badly by OCLs [12-18].
Among Humans, effects of pesticide contamination causes Neuromuscular disorders, stimulation of drug and steroid metabolism [19,20], potential risk factor for gallstone disease [21], vitamin D deficiency [22] and also affects endocrine-disruption activity in patients with neonatal thyroid hormone status. Endosulfan residues in humans bioaccumulated through plants and animal foods and Gastrointestinal absorption of it damages CNS-central nerve system causing acute inhalation toxicity [23,24]. Dialdrin is partly responsible for risk increment of Parkinsons disease. Thyroid hormone levels of the newborn are affected by β-HCH, HCB and DDT residues. Heptachlor bring mitochondria-mediated cell death by spoiling electron transport chain complex III and become neurotoxicant and in patients with Parkinson’s disease [25]. In the research on passing from one species to its offspring it seemed Pesticide residue are seen present in eggs of sea birds [26] and humans in Korea, Guerrero, Mexico, China and India transferred via maternal cord sera, blood, and the placenta and breast milk [27].
Chemical compounds that terminate insects, fungi, bacteria, herbs or rodents are generally known as Pesticides. Pesticides are classified based on their nature, application, and targeted pests. By nature, they are organochlorine (DDT, Dieldrin, lindane, Endosulfanetc), organophosphorus (Parathion, Malathion etc.). It is applicable for Agriculture, Public health, and Domestic purposes. It targets pests as insects -insecticide, fungi- fungicide, herbs-herbicide and rodentsrodenticides [28]. At the application of organochlorine pesticide group (DDT, DDD, Dicofol, Eldrin, Dieldrin, Chlorobenziate, Lindane, BHC, Methoxychloro Aldrin, Chlordane, Heptachlor, Endosulfan, Isodrin, Isobenzan, Toxaphene, Chloropropylate) is well known than the rapidly hydrolyzed degradation of the environmental organophosphorous [29,30]. Organochlorine pesticides persist for days to years in the environment, Endosulfan C9H6Cl6O3S has a halflife of 35(a-isomer) to 150(β-isomer) days, and Dichloro Diphenyl Trichloroethane (DDT) C14H9Cl5 has a half-life of 2 to 15 years [4,31].
The study region Eloor-Edayar, in Ernakulam district, is the Industrial Area of Kerala and is a hot spot categorized under Green peace [32]. A partial pesticide manufacturing unit, which was closed by Govt due to the local public’s protest during the period of sample collection. Many studies conducted here showed that pesticides contaminate the area. A series of pesticide studies from 1990 to 1999 on water and sediment from the Periyar River line was done by the Department of Chemical Oceanography, CUSAT. Menon et al. 2000 [33] studied macro Benthic, Benthic fauna, Prawn, Fish, mollusc and Polychaaetens in this region. Further studies on the survived species were carried out and revised by Martin et al. 2011 [34].
Previous studies focused on the water and sediment samples near estuary or estuarine sediments. The presence of organo phosphorous pesticides Malathion and Methyl Parathion and organo chlorine pesticide Endosulfan samples of Periyar side has been reported by Sujatha et al. 1999 and 1994 [35-37].
This study focused on the baseline data of organochlorine pesticide stability, degradation or pesticide residues in the extracts of vegetables from the land area located at the river end.
Materials and Methods
Study area
Eloor is an industrial area-north of Kochi in Ernakulam District in Kerala in India situated between north latitudes 9° 3´and 10° 6´ and east longitudes 76° 18´ and 76° 30´. This island has an area of 14.21km2 formed between two rivers Periyar distributaries and is the largest industrial belt in Kerala. Most of the industries, (70% approximately 250 companies) of Kerala state in this region, including chemical, engineering, food, drug, paper, rayon, rubber, textiles, and plywood industries. At the time of sample collection in 2008, Eloor, a Panchayat, now turned to Municipality. During the sampling period survey of Department of Industries Govt. of Kerala and Eloor Grama Panchayat had 20 wards of population density 2425/Km2. The panchayat is house to 4.2% of industrial company workers. The vegetarian and non-vegetarian comprise 3.9% and 0.1% of the population.14.4% people from Eloor utilized their land for cultivation of food including grains 3.88%, vegetables 1.48%, fruits 1.292%, coconut 16.17% and roots 0.25%. 95.93% of water in wells is contaminated. The sampling was during May 2008 Pre Monsoon period (Figure 1 and Table 1).
Sampling site
Soil colour
Vegetable/Fruit
S1
Eloor panchayat colony (10.084686N; 76.291281E) near to Panachithodu (10.08815N; 76.290820E)
Blackish
Kachil-Yam (root vegetables)
S2
Kanjirakkuzhi (10.086123N; 76.291994E)
Yellow
-
S3
Kuzhikkandamthodu (10.04508N; 76.17301E)
Blackish
-
S4
Periyar, Muttinakamkadavu (10.085290N; 76.283535E)
Reddish-brown
-
S5
Near Periyar (10.084686N; 76.291281E)
Reddish-brown
Guava
S6
Depot road (Agricultural land) (10.085316N; 76.17201E)
Slight blackish
Tapioca and Ginger (root vegetables), ladies finger (shoot vegetables)
S7
Opposite to HIL (10.079591N; 76.301873E)
Blackish
Papaya (Fruit)
Table 1: Sampling site and Sample Description.
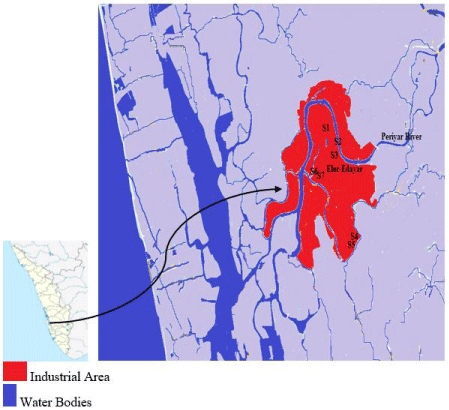
Figure 1: Sampling site Eloor-Edayar region.
The soil samples and vegetables were collected and transferred to the laboratory in glass bottles. pH and Eh of the soil analyzed by APHA methods [38].
Extraction of pesticides
About 2g wet weight of vegetables or 10gm soil/sediment was taken in a 250ml glass-stoppered Erlenmeyer flask and converted to dry weight data. The sample was extracted with 150ml acetone for 24h on a mechanical shaker. The acetone extraction was repeated twice for each sample. The combined supernatant was evaporated to dryness and dissolved into 10ml hexane. The hexane extract was dried by passing it through a layer of anhydrous sodium sulfate and then concentrated to 2ml by using a Kuderna-Danish (KD) concentrator. A 2ml of the KD extracts of soil/sediment was subjected to Florisil column chromatography (Florisil PR: Floridin Co., 10g) cleanup and fractionation. The Florisil gel was activated at 130°C for 12h, before the fractionation with 120ml of 100% hexane and again concentrated to 2ml.
Stability analysis of extract
The extracts were fractionated with 100% hexane and concentrates was kept in different temperature from -200C in a cold stored to room temperature for 10 years (2008 to 2018). It was made up to 2ml and analyzed for pesticides using GC-ECD. The GC conditions are as follows: injection port temperature 2500C, detector temperature 3500C, oven temperature program: 1100C (5min) at 50Cmin-1 to 1900C (2min) at 150Cmin-1 to 2800C (10min).
Result and Discussion
The soil’s pH in this area varied from 5.24 and 8.39 and Eh of soil -288mV to 245mV (Figure 2). pH and Eh of soils affect plant metabolism and catabolism [39], by influencing nutrient and ion toxicity transport [40,41]. The optimum pH and Eh for plants is 6.5 to 7, +400 to +450 mV, and favorable conditions for plant growth are between pH 5.5 and 8. Furthermore, Eh greater +350mV [42]. The soil samples collected from Kuzhikkandamthodu (Eh -197mV & pH 7), Panachithodu (Eh -288 mV & pH 7.15), Muttinakamkadavu (Eh -159mV & pH 7.29), Eloor Panchayat colony (Eh 0.012mV & pH 8.39) under the category of highly reduced soils whereas the soils from the area of Depot road (Eh 245mV & pH 5.24) and Kanjirakkuzhi (Eh 242mV & pH 6.54) were found to be moderately reduced. The soil analyzed categorized four types as followed.
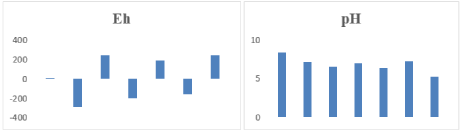
Figure 2: Eh and pH of the soil samples collected Eloor-Edayar region.
Aerated soils over: > +400mV
Moderately reduced soils: +100mV - +400mV
Reduced soils: -100mV - +100mV
Highly reduced soils: -100mV - -300mV [43]
The study report, Eh indicated the soils in these locations are moderately reduced [44,45], the soil in between approved limits of -300mV to +900mV. 1979, Bressy [46] reported the soil-related stress of pH and oxidative stress pesticides acts as oxidants. Fewer distances between sampling sites of Eloor Panchayat shows Eh and pH variations in the soil, which came to an understanding of the other soil scientific reports [42,47].
The residue of (a, β, γ) BHC, Aldrin, Dicofol, (a, β) Endosulfan, Dieldrin OPDDT and PPDDT analyzed as dry basis from Shoot vegetable ladies finger, edible root vegetables Kachil-Yum, Tapioca and Ginger, fruits like Papaya and Guava along with the sediments with different locations (Figure 3a and 3b).
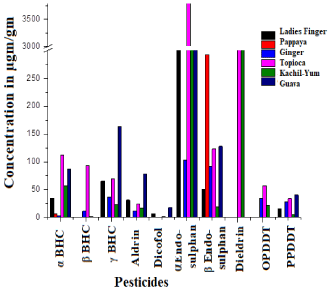
Figure 3a: Concentration of pesticide in vegetables and fruits.
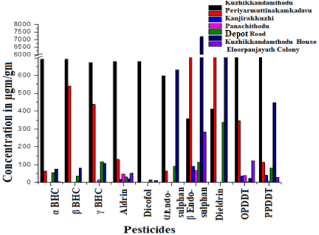
Figure 3b: Concentration of pesticide in the soil samples collected from
Eloor-Edayar region.
a, BHC high concentration in soils 689μgm/gm (Kuzhikkandamthodu) to ND (in Kanjirakkuzhi & Panachithodu). The root vegetables 112μgm/gm (Tapioca) and lower in 2μgm/gm (Ginger).
β, BHC in the soils 690μgm/gm (Kuzhikkandamthodu) to ND (Eloorpanjayath colony, Kanjirakkuzhi & Panachithodu) and only in root vegetables and a high value of 92μgm/gm (Tapioca).
γ, BHC detected high 671μgm/gm (Kuzhikkandamthodu) to ND (Eloor panjayath colony and Kanjirakkuzhi) and the fruits 163μgm/ gm (Guava) to ND (Papaya).
Aldrin residues present 676μgm/gm (Kuzhikkandamthodu) to lowest 17μgm/gm (Kanjirakkuzhi) high 78μgm/gm in (Guava) to ND (Papaya).
Dicofol high 678μgm/gm (Kuzhikkandamthodu) present low concentration in the soil Eloor panjayath colony and Depot road, vegetables 17μgm/gm (Guava) present root vegetables tapioca and ginger and ND.
a, Endosulfan concentration in 631μgm/gm (Kuzhikkandamthodu House) and present Kuzhikkandamthodu, Periyar Muttinakamkadavu and Depot road whereas in vegetable 3.8x103μgm/gm (Tapioca) and ND (Papaya).
β, Endosulfan sensed 861μgm/gm (Periyar Muttinakamkadavu) ND (Eloor panjayath colony Kanjirakkuzhi & Panachithodu) and in vegetable 554μgm/gm (Kachil-Yum) and the other presence in Tapioca.
Dieldrin, the highest concentration of all pesticides 7187μgm/gm (Kuzhikkandamthodu House) and lowest 65μgm/gm (Panachithodu) and the fruit 292μgm/gm (Papaya) to 19μgm/gm (Kachil-Yum).
OPDDT showed high in 805μgm/gm (Kuzhikkandamthodu) and lowest 4μgm/gm (Depot road) and only in root vegetables 554μgm/ gm (Tapioca) and 22μgm/gm (Kachil-Yum).
PPDDT highest in 871μgm/gm (Kuzhikkandamthodu) and lowest 6μgm/gm (Panachithodu) contained as 40μgm/gm (Guava) to ND (Papaya).
The Papaya fruit and root vegetable Ginger had the least presence of pesticides. High metal polluted industrial waste and soil temperature might have led to the decomposition and low residue presence. The water channels which carry industrial effluents and reach the River Periyar, carried these residues. In the seasonal wise studies on DDT in industrial river line area, the pesticide is not observed in the monsoon period36, which revealed the effect of temperature and water runoff.
Correlation between decomposed residues of pesticides in the soil and vegetables is shown in Table 2. The correlations showed that β BHC, OPDDT strong negative, Dialdrin moderate, strong negative and Dicofol week negative. These studies emphasized the earlier monitoring study of pesticide’s effect on organism lipid level by Sujatha et al. 1995 [48]. The soil in the Industrial Area is contaminated with organic and inorganic components, which affect the pH and Eh. Furthermore, Sujatha et al. 1991 [49] studied the equilibrium/ partition model on the fate of pesticides in the soil, which depends on clay materials, hydrous oxides, organic matter, redox stands, pH of soil system, and climate. This study strongly recommends removing the pesticides from soils of agricultural areas.
Pesticides in Soil
Pesticides in Vegetables
a BHC
β, BHC
γ BHC
Aldrin
Dicofol
a endosulphan
β endosulphan
Dieldrin
OPDDT
PPDDT
a BHC
-0.173
-0.313
0.078
0.122
-0.18
-0.266
-0.308
-0.307
-0.492
-0.178
β, BHC
-0.456
-0.417
-0.244
-0.192
-0.295
-0.489
0.314
-0.479
-0.681
-0.541
γ BHC
-0.398
-0.446
-0.196
-0.128
-0.28
-0.462
0.17
-0.394
-0.697
-0.534
Aldrin
-0.227
-0.242
-0.04
-0.011
-0.296
-0.259
-0.204
-0.329
-0.44
-0.246
Dicofol
-0.171
-0.24
0.042
0.071
-0.245
-0.224
-0.353
-0.281
-0.394
-0.151
a endosulphan
0.183
-0.438
0.723
0.787
0.61
-0.17
-0.217
-0.421
-0.685
0.293
β endosulphan
0.368
-0.275
0.863(*)
0.896(*)
0.983(**)
0.001
0.124
-0.344
-0.457
0.547
Dieldrin
-0.18
-0.612
0.143
0.253
0.391
-0.447
0.57
-0.354
-0.871(*)
-0.358
OPDDT
-0.367
-0.308
-0.158
-0.128
-0.337
-0.38
0.041
-0.44
-0.536
-0.38
PPDDT
-0.037
-0.421
0.421
0.475
0.219
-0.271
-0.264
-0.444
-0.65
0.06
*Correlation is significant at the 0.05 level; **Correlation is significant at the 0.01 level (2-tailed).
Table 2: Correlation between the decomposition of pesticides in Vegetables and Soil.
Conclusion
The current study is the Baseline for the pesticide pollutants in the industrial area and their persistence after years in the extraction of sediments and vegetables. The samples collected from the Eloor -Edayar region, the industrial zone of Kerala, indicated significant pesticide levels in the soil and vegetables. The presence of pesticide residues in vegetable extract showed the transport of pollutants from sediment to primary producers, which raises the question of the health of the people lives in that region.
Acknowledgement
The authors gratefully acknowledge the Dean and Director, School of Marine Sciences, CUSAT for facilities and support. Dr. Salas PM and Dr. Deepulal PM, Department of Chemical Oceanography, CUSAT.
References
- Indira Devi P. Pesticide use in the rice bowl of Kerala: Health costs and policy options. SANDEE working paper/South Asian Network for Development and Environmental Economics. 2007.
- Akhil PS, Sujatha CH. Prevalence of organochlorine pesticide residues in groundwaters of Kasargod District, India. Toxicological & Environmental Chemistry. 2012; 94: 1718-1725.
- Gopalan NK, Chenicherry S. Fate and distribution of Organochlorine Insecticides (OCIs) in Palakkad soil, India. Sustainable Environment Research. 2018; 28: 179-185.
- Quijano RF. Endosulfan Poisoning.
- Mancini F, Van Bruggen AH, Jiggins JL, Ambatipudi AC, Murphy H. Acute pesticide poisoning among female and male cotton growers in India. International journal of occupational and environmental health. 2005; 11: 221-232.
- Remor AP, Totti CC, Moreira DA, Dutra GP, Heuser VD, Boeira JM. Occupational exposure of farm workers to pesticides: biochemical parameters and evaluation of genotoxicity. Environment international. 2009; 35: 273-278.
- Townson H. Public health impact of pesticides used in agriculture. Geneva: World Health Organization. 1990: 128.
- Wesseling C, Aragón A, Castillo L, Corriols M, Chaverri F, Cruz ED, et al. Hazardous pesticides in central America. International journal of occupational and environmental health. 2001; 7: 287-294.
- Konradsen F, van der Hoek W, Cole DC, Hutchinson G, Daisley H, Singh S, et al. Reducing acute poisoning in developing countries—options for restricting the availability of pesticides. Toxicology. 2003; 192: 249-261.
- Coronado GD, Thompson B, Strong L, Griffith WC, Islas I. Agricultural task and exposure to organophosphate pesticides among farmworkers. Environmental health perspectives. 2004; 112: 142-147.
- Aktar W, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdisciplinary toxicology. 2009; 2: 1-2.
- Monirith I, Ueno D, Takahashi S, Nakata H, Sudaryanto A, Subramanian A, et al. Asia-Pacific mussel watch: monitoring contamination of persistent organochlorine compounds in coastal waters of Asian countries. Marine Pollution Bulletin. 2003; 46: 281-300.
- Liess M, Brown C, Dohmen P, Duquesne S, Heimbach F, Kreuger J, et al. Effects of Pesticides in the Field–EPIF. Brussels. 2005.
- Grande M, Andersen S, Berge D. Effects of pesticides on fish. Norwegian Journal of Agricultural Sciences. 1994; 13: 195-209.
- Castillo LE, Martínez E, Ruepert C, Savage C, Gilek M, Pinnock M, et al. Water quality and macroinvertebrate community response following pesticide applications in a banana plantation, Limon, Costa Rica. Science of the total environment. 2006; 367: 418-432.
- DeLorenzo ME, Scott GI, Ross PE. Toxicity of pesticides to aquatic microorganisms: a review. Environmental Toxicology and Chemistry: An International Journal. 2001; 20: 84-98.
- Frankart C, Eullaffroy P, Vernet G. Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor. Environmental and Experimental Botany. 2003; 49: 159-168.
- Mitra A, Chatterjee C, Mandal FB. Synthetic chemical pesticides and their effects on birds. Res J Environ Toxicol. 2011; 5: 81-96.
- Subramaniam K, Solomon J. Organochlorine pesticides BHC and DDE in human blood in and around Madurai, India. Indian Journal of Clinical Biochemistry. 2006; 21: 169.
- Forns J, Lertxundi N, Aranbarri A, Murcia M, Gascon M, Martinez D, et al. Prenatal exposure to organochlorine compounds and neuropsychological development up to two years of life. Environment international. 2012; 45: 72- 77.
- Su Y, Dai Y, Lin Y, Gao X, Han Y, Zhao B. Serum organochlorine pesticide residues and risk of gallstone disease: a case-control study in Xiamen. Annals of epidemiology. 2012; 22: 592-597.
- Yang JH, Lee YM, Bae SG, Jacobs Jr DR, Lee DH. Associations between organochlorine pesticides and vitamin D deficiency in the US population. PLoS One. 2012; 7: e30093.
- Briz V, Molina-Molina JM, Sánchez-Redondo S, Fernández MF, Grimalt JO, Olea N, et al. Differential estrogenic effects of the persistent organochlorine pesticides dieldrin, endosulfan and lindane in primary neuronal cultures. Toxicological Sciences. 2011; 120: 413-427.
- USEPA. Endosulfan. The Health Effects Divion’s Human Health Risk Assessment. EPA DP Barcode: D372569. 2010.
- Hong S, Hwang J, Kim JY, Shin KS, Kang SJ. Heptachlor induced nigral dopaminergic neuronal loss and Parkinsonism-like movement deficits in mice. Experimental & molecular medicine. 2014; 46: e80.
- Pearce PA, Elliott JE, Peakall DB, Norstrom RJ. Organochlorine contaminants in eggs of seabirds in the Northwest Atlantic, 1968–1984. Environmental Pollution. 1989; 56: 217-235.
- Li C, Cheng Y, Tang Q, Lin S, Li Y, Hu X, Nian J, Gu H, Lu Y, Tang H, Dai S. The association between prenatal exposure to organochlorine pesticides and thyroid hormone levels in newborns in Yancheng, China. Environmental research. 2014; 129: 47-51.
- Jayaraj R, Megha P, Sreedev P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary toxicology. 2016; 9: 90-100.
- Gupta PK. Pesticide exposure-Indian scene. Toxicology. 2004; 198: 83-90.
- FAO. Proceedings of the Asia Regional Workshop, Regional Office for Asia and the Pacific, Bangkok. 2005.
- Augustijn-Beckers PW, Hornsby AG, Wauchope RD. The SCS/ARS/ CES pesticide properties database for environmental decision-making. II: Additional compounds. Reviews of environmental contamination and toxicology. 1994; 137: 1-82.
- Kumar R, Joseph MM, Gireesh Kumar TR, Renjith KR, Manju MN, Chandramohanakumar N. Spatial variability and contamination of heavy metals in the inter-tidal systems of a tropical environment. International journal of environmental research. 2010; 4: 691-700.
- Menon NN, Balchand AN, Menon NR. Hydrobiology of the Cochin backwater system: A review. Hydrobiologia. 2000; 430: 149-183.
- Martin GD, Nisha PA, Balachandran KK, Madhu NV, Nair M, Shaiju P, et al. Eutrophication induced changes in benthic community structure of a flow-restricted tropical estuary (Cochin backwaters), India. Environmental monitoring and assessment. 2011; 176: 427-438.
- Sujatha CH, Nair SM, Chacko J. Determination and distribution of endosulfan and malathion in an Indian estuary. Water research. 1999; 33: 109-114.
- Sujatha CH, Nair SM, Kumar NC, Chacko J. Distribution of dichlorodiphenyltrichloroethane (DDT) and its metabolites in an Indian waterway. Environmental Toxicology and Water Quality. 1994; 9: 155-160.
- Sujatha CH, Nair SM, Chacko J. Sorption of malathion and methylparathion by tropical aquatic sediments: influence of pH. Toxicological & Environmental Chemistry. 1994; 41: 47-55.
- American Public Health Association, American Water Works Association, Water Pollution Control Federation, Water Environment Federation. Standard methods for the examination of water and wastewater. American Public Health Association. 1912.
- Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, et al. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant physiology. 2002; 129: 1807- 1819.
- Brady NC, Weil RR, Brady NC. Elements of the nature and properties of soils. Upper Saddle River, NJ: Pearson educational international. 2010.
- Marschner H. Mechanisms of adaptation of plants to acid soils. Plant and soil. 1991; 134: 1-20.
- Husson O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant and Soil. 2013; 362: 389-417.
- Kaurichev IS, Shishova VS. Oxidation-Reduction Conditions of Coarse- Textured Soils of Meshchera Lowland. Soviet Soil Science-Ussr. 1967: 636.
- Pearsall WH, Mortimer CH. Oxidation-reduction potentials in waterlogged soils, natural waters and muds. The Journal of Ecology. 1939 Aug 1:483-501.
- Pezeshki SR. Wetland plant responses to soil flooding. Environmental and Experimental Botany. 2001; 46: 299-312.
- Hinsinger P, Bengough AG, Vetterlein D, Young IM. Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant and soil. 2009; 321: 117- 152.
- Yang J, Hu Y, Bu R. Microscale spatial variability of redox potential in surface soil. Soil science. 2006; 171: 747-753.
- Sujatha CH, Nair SM, Chacko J. Tissue lipid levels of the clam, Villorita cyprenoides var. cochinensis, following exposure to endosulfan, malathion, and methyl parathion. Environmental Toxicology and Water Quality. 1995; 10: 231-235.
- Sujatha CH, Chacko J. Malathion sorption by sediments from a tropical estuary. Chemosphere. 1991; 23: 167-180.