Abstract
Introduction: Diabetes mellitus is the leading cause of nontraumatic lower extremity amputation worldwide. Risk of developing a foot ulcer in diabetic patients has increased to 12-25% during their lifetime. Inappropriate sampling technique leading to contaminated samples is a well-known threat among patients of diabetic foot. In this study we compared two microbiological sampling techniques, superficial swabbing and deep tissue biopsy for identification of pathogens.
Material and Methods: It was a prospective observational cross-sectional study. Diabetic foot was defined as per standard guidelines. Careful and meticulous examination of diabetic foot ulcer was done. Two specimens were collected from each wound. The first specimen was a wound swab collected by using Levine technique; another was taken via punch biopsy from the ulcer base. It was a deep tissue sample of 4 mm size.
Results: A total of 120 diabetic patients with diabetic foot infection were included in this study. In swab culture 80.83% patients had mono microbial growth, 15% had polymicrobial and 4.10% had no growth. In deep tissue culture 87.50% had mono microbial growth, 2.50% had poly microbial and 10% were sterile. Staphylococcus aureus was most common isolate followed by E. coli. It was observed that though monomicrobial growth was most common in both the types of samples but was higher in deep tissue biopsy than in superficial swab samples.
Conclusion: The isolation of microorganism via deep tissue sampling in diabetic foot ulcer patients is more reliable compared to superficial swab samples and a better guide to antibiotic therapy.
Keywords: DFU- diabetic foot ulcers; T2DM-Type 2 diabetes mellitus; IWGDF- International Working Group on Diabetic Foot; FBS- fasting blood sugar; PPBS- Post prandial blood sugar; BMIBody mass index
Introduction
Globally, Type 2 Diabetes Mellitus (T2DM) has been associated with increased risk of developing cardiovascular morbidity and mortality. In India 69 million people are suffering from diabetes, which accounts for the second largest diabetic population of the world and this figure is expected to rise to 110 million by 2030 (>90% T2DM) [1]. Diabetes patients have a 12-25% risk of developing a foot ulcer during their lifetime [2]. Diabetes mellitus is the leading cause of non-traumatic lower extremity amputation in the United States. Foot ulcers and infections are also a major source of morbidity in individuals with diabetes mellitus. Risk factors for foot ulcers or amputation include male sex, diabetes for >10 years, peripheral neuropathy, abnormal structure of foot (bony abnormalities, callus, thickened nails), PAD, smoking, history of previous ulcer or amputation, visual impairment and poor glycaemic control [3]. Despite all preventive measures, it is well known that patients with Diabetes Mellitus (DM) complicating with foot ulcerations and infections create potentially a serious problem. Mostly infection in a diabetic foot ulcer are diagnosed clinically, by the presence of wound purulence or at least two classical signs or symptoms of inflammation (erythema, warmth, tenderness, pain, induration). However, some favour quantitative microbiologic assessment and define infection by the growth of ≥105 organisms per gram of tissue [4]. Therefore, it is very important to isolate the causative microorganism for appropriate treatment of the infected Diabetic Foot Ulcers (DFU). In our present study, we compared the superficial swab sample with deep tissue biopsy sample prospectively, for identification of microorganism and antimicrobial sensitivity testing.
Materials and methods
It is a prospective observational cross-sectional study which was conducted in tertiary care hospital from north India. This study was approved by the Institutional ethical committee, and was conducted according to the guidelines in the Helsinki Declaration. Written informed consent was obtained from all patients.
Patients
All known cases of diabetes Meletus of either sex of age> 18 years with history of diabetic foot were included. Cases of nondiabetic neuropathic foot, PVD, and traumatic foot ulcer were excluded. Diabetic foot with dry ulcer and dry gangrene were also excluded. Patients were excluded if they had antibiotic exposure (systemic or local) during preceding 4 weeks, if they were with known immunocompromised state or were on immunosuppressive drugs, if they have history of active malignancy. Patients not willing to participate in the study were also excluded.
Methods
After applying necessary inclusion and exclusion criteria, patient’s demographic, anthropometric and clinical variables were recorded according to standard methods. A detailed personal and family history along with assessment of metabolic risk factor profile was done. Diabetic status including disease duration, treatment type and complication status was recorded. Diabetic foot was defined as per standard guidelines by world health organization and International Working Group on the Diabetic Foot. Careful and meticulous examination of diabetic foot ulcer was done.
Specimen collection and microbiological culturing
Specimens were collected from each wound after the wound had been cleansed (using sterile saline and gauze) and debrided (removal of necrotic tissue, foreign material, calluses, and undermined wound edges) [24]. No antimicrobial agent (e.g., alcohol or iodine) or antiseptic was introduced into the wound before specimen collection. Each wound was swabbed using the Levine technique, involving rotation of a wound swab over a 1 cm2area of the wound for 5 seconds, using sufficient pressure to extract fluid from the inner part of the wound [25]. A deep tissue specimen of approximate 4mm in diameter was obtained from the base of the ulcer via punch biopsy [26].The specimens were placed into sterile transport containers and sent to the microbiology laboratory for aerobic culturing within 20 minutes. Anaerobic culturing was not performed in this study. Cultures were processed following the same standard procedures for the swab and tissue samples.
Results
We prospective analysed data of 120 diabetic patients admitted with diabetic foot. The mean age of study population was 56.9 ± 15.61 years with 2/3rd of patients being males. The mean BMI of patients was 24.86 ± 3.2kg/m² with 17.50%being normal, 22.50% were overweight and 60% were obese. The baseline characteristics of study cohort is given in table 1. More than ½ of patients had diabetes for <10years with a mean HBA1c of 9.94 ± 2.8.Among the microvascular complication, neuropathy was most common and present in 100% of patients followed by nephropathy (30%) and retinopathy (25%) whereas PAD was most common macrovascular complication present in 2/3rd of patients followed by CAD and CVA with frequency of 18.3% and 7.5% respectively. As per Wagner’s grading of DFU done at the time sample collection 47(39.17%) patients had grade 2 ulcer, 39(32.50%) patient had grade 3 ulcer, 32(26.67%) patient had grade 4 ulcer and 2(1.67%) patients had grade 5 ulcer. The males were affected more than females by DFU with a male to female ratio of 1.72, and majority of deep and infected ulcers were seen in males compared to females and between the group difference was statistically significant (p<0.05).
Age
56.9 ± 15.61
Sex ( males )
64.17%
BMI
Normal(<23)
17.50%
Overweight(23-24.9)
22.50%
Obese(=25)
60%
Diabetes duration
<10 yrs
68(56.67%)
>10 yrs
52(43.33%)
Glycaemic indices
FBS(mg/dl)
191.7 ± 61.5
PPBS(mg/dl)
254 ± 77.6
HbA1c (%)
9.94 ± 2.8
Complication status of DM
CAD
18.33%
CVA
7.50%
PAD
66.67%
Retinopathy
25.00%
Nephropathy
30.00%
Neuropathy
100%
Antidiabetic treatment
OHA
74.17%
OHA + Insulin
19.17%
Ayurvedic
5.00%
No treatment
1.67%
Smoking
48.33%
Hypertension
55%
Dyslipidaemia
96.67%
Anaemia
55.83%
Wagner’s grade of Diabetic foot (2-5)
Grade 2
39.17%
Grade 3
32.50%
Grade 4
26.67%
Grade 5
1.67%
Prior antibiotic exposure for Diabetic foot ulcer.
No
66.70%
Yes
33.30%
Table 1: Baseline characteristics of study cohort (n=120).
Number and types of Pathogens Isolated
A total of 115 (95.8%) culture positive samples were observed in superficial swab samples, while in deep tissue biopsy samples, there were 108 (90%) culture positives. We have isolated 131 microorganisms in swab samples versus 111 in biopsy samples. Mono microbial growth was observed in 80.8% of swab sample patients while in deep tissue samples, it was seen in 87.5% patients. Poly microbial growth was seen among 15% patients of swab samples and 2.5% deep tissue sample patients. It was seen that 4.1% superficial swab and 10% deep tissue biopsy samples had no growth (Figure 1).
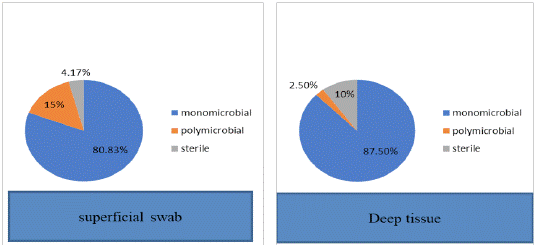
Figure 1: Comparison of culture results.
Gram positive bacteria accounted for 53.4 % in superficial swab and 66.6% in deep tissue biopsy. Among gram positive bacteria, staphylococcus was the most common pathogen. Among the total isolates of S. Aureus, MRSA was found in 17.8% and 18% isolates from superficial swabs and deep biopsy respectively. Gram negative bacteria accounted for 46.5% in swab samples and 33.3% in deep tissue biopsy samples. Overall gram negative bacteria accounted for 40% of total isolates with E. coli the most common isolate (Table 2).
Variables
Superficial swab samples (n=120)
Deep tissue biopsy (n=120)
Culture positive samples
115(95.8%)
108(90%)
Monomicrobial
97(80.83%)
105(87.5%)
Polymicrobial
18(15%)
3(2.5%)
No. of isolates
131
111
Mean number of isolates
1.11
0.93
Gram positive bacteria
70(53.43%)
74(66.66%)
a. Staphylococcus aureus
56(42.74%)
61(54.95%)
b. Coagulase negative staphylococcus (CONS)
7(5.34%)
7(6.30%)
c. Enterococcus species
6(4.58%)
5(4.50%)
d. Streptococcus pneumoniae
1(.76%)
1(0.9%)
Gram negative bacteria
61(46.56%)
37(33.33%)
a. E. coli
25(20.83%)
15(12.5%)
b. Klebsiella pneumoniae
6(4.58%)
3(2.7%)
c. Proteus species
5(3.81%)
5(4.50%)
d. Citrobacter species
13(9.92%)
8(7.20%)
e. Enterobacter species
1(0.76%)
1(0.9%)
f. Pseudomonas aeruginosa
8(6.10%)
4(3.60%)
I. Acinetobacter baumanii
3(2.29%)
1(0.90%)
Table 2: Comparison of culture results among superficial swab and deep tissue samples.
Among the isolates of E.coli 32% were Extended Spectrum B Lactamase (ESBL) producers. Most of the isolates of S. Aureus were sensitive to Linezolid, vancomycin and gentamicin. Most of the Enterobacteriaceae isolates were sensitive to amikacin, gentamicin, imipenem, meropenem and piperacillin-tazobactam (Table 3).
Antibiotic
S aureus
Enterococcus spp
E.coli
Klebsiella pneumoniae
Pseudomonas aeruginosa
Penicllin
26
Ampicillin
28
60
22
18
Amoxycillin clavulanic acid
65
58
Piperacillin-tazobactum
74
94
85
Ceftriaxone
62
60
Ceftazidime
66
59
63
Imipenem
94
88
92
Aztreonam
76
68
57
Gentamicin
39
87
80
58
High level Gentamicin
86
Amikacin
78
90
86
Ciprofloxacin
58
47
42
29
Levofloxacin
42
47
71
Tetracycline
62
68
Erythromycin
67
37
Clindamycin
82
Trimethoprim-sulfamethoxazole
32
39
34
8
Vancomycin
100
91
Linezolid
99
82
Table 3: Antimicrobial susceptibility patterns (% susceptible) of bacterial isolates in diabetic foot infections.
Concordance between Swab and Tissue Cultures: In Wagner grade 2, superficial swab identified 42/47 (89.36%) of microorganisms isolated from the corresponding deep tissue biopsy specimens. The proportion of concordance decreases as the Wagner grading of wound increases. It was 48.71% in grade 3 and further decreased to 40.62% in grade 4 patients (Table 4).
Wagner Grade
Concordance with deep biopsy result
Discordance with deep biopsy result
II
42/47(89.36%)
5/47(10.63%)
III
19/39(48.71%)
20/39(51.28%)
IV
13/32(40.62%)
19/32(59.37%)
V
1/2 (50%)
1/2(50%)
Table 4: Concordance between swab and tissue cultures according to ‘Wagner grading’.
Discussion
A reliable sampling technique, followed by isolation of causative microorganism has been given the utmost importance at the time of institution of definitive antimicrobial therapy in diabetic foot ulcer patients. As per a systemic review on ‘diagnosis of infections in diabetic foot ulcers’ it was stated that there is no definitive clinical evidence as far as optimal sampling technique is concerned [10]. Various studies consider biopsy as a most reliable method of identifying the pathogens as chances of superficial contamination is considered to be very less in such sampling technique [11]. In a study done by Ying Huang et al, in China it was found that swab samples were found to be reliable for culturing diabetic foot wounds until grade 2 severity. However, in grade ≥3 wounds, tissue sampling was advised rather than superficial swab as chances of missing microbial organisms is less in tissue sampling [12]. Monomicrobial growth was most common both from swab and deep tissue culture in our current study which is in accordance with the study conducted by Mutluoglu M et al [13]. In our study, the mean number of isolates per specimen was similar in superficial swab and deep tissue biopsy samples, these findings were in concordance with the study done by Slater R et al [14]. The rate of culture negativity in superficial swab compared to deep tissue biopsy culture in our study was is in accordance with study done by others [13]. The rate of culture negativity is more in deep biopsy results when compared with superficial swab culture results which can be explained by growth of contaminants in superficial swab compared to deep tissue biopsy culture. Our results showed 89.36% concordance between swab cultures and deep tissue biopsies in Wagner grade 2, which dropped to 40.62% in Wagner grade 4. This observation is in accordance with study done by Ying Huang et al [12] in which they observed that for grade 2 wounds, superficial swab culture identified 90% microorganisms isolated from the corresponding deep tissue specimens, whereas this proportion decreased to 41.2% in Wagner grades 4 wounds. In the present study, gram positive bacteria were predominating isolates (60%) from both superficial swab and deep tissue biopsy with Staphylococcus aureus being the most common isolate, while gram negative bacteria accounted for 40% isolates in both superficial swab and deep tissue biopsy samples with E.coli the most common isolate. Our results are consistent with similar studies done in the past [15-17]. In our present study, we could not isolate anaerobes and fungal microbes; this has been proved as a major limitation of present study. Therefore, the study needs to be carried forward so that effectiveness of swab samples and tissue samples could be explored for identification of anaerobes and fungal microbes as well. After incorporating results of various studies with the present study we suggest that tissue sampling is the most reliable technique for isolation of microorganisms in patients presenting with infected diabetic foot ulcers. It may optimize the institution of evidence based antimicrobial therapy in these patients.
Conclusions
It is concluded that deep tissue biopsy is more reliable method for identification of microbial organism in comparison to the superficial swab technique as former has less chances of missing the pathogens. Also, contamination is less in this sampling method. Keeping in view of the results of our study and recommendations of various other studies it has been suggested that in patients of diabetic foot infections, deep tissue sample for culture is the key to optimize the evidence based antimicrobial therapy.
Conflict of interest: None.
Financial disclosure: None.
References
- Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/ or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdiaDIABetes (ICMR-INDIAB) study. Diabetologia. 2011; 54: 3022-3027.
- Andersen C, Roukis T. The Diabetic Foot. Surg Clin North Am. 2007; 87: 1149-1177.
- Alvin C. Powers. Diabetes Mellitus: Complications. Harrison’s Principles of Internal Medicine. Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J (ed): Mc Graw Hill Education, New York; II 2015; 2428-2429.
- Reiber G, Lipsky B, Gibbons G. The burden of diabetic foot ulcers. Am J Surg. 1998; 176: 5S-10S.
- Gardner S, Frantz R. Wound Bioburden and Infection-Related Complications in Diabetic Foot Ulcers. Biol Res Nurs. 2008; 10: 44-53.
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin Infect Dis. 2012; 54:e132-e173.
- Nelson A,Wright Hughes A, Backhouse MR ,Lipsky BA,Nixon J, et al. CODIFI(Concodancein Diabetic Foot Ulcer Infection): a crosssectional study of wound swab versus tissue sampling in infected diabetic foot ulcers in England. BMJ Open. 2018; 8: e019437.
- Levine NS, Lindberg RB, Mason Jr AD, Pruitt Jr BA. The quantitatives wabculture and smear:A quick,simple method for determining the number of viable aerobicbacteriaon open wounds. J Trauma. 1976; 16: 89-94.
- Richard B, Thomson JR. Specimen collection, transport and processing: bacteriology. Manual of Clinical Microbiology. Murray PR, Baron EJ, Jorgensen JH, LandryML, Pfaller MA (ed): ASM Press,Washing ton DC; I: 2007; 291-333.
- O’Meara S, Nelson E, Golder S, Dalton J, Craig D, Iglesias C. Systematic review of methods to diagnose infection in foot ulcers in diabetes. Diabet Med. 2006; 23: 341-347.
- Bill TJ, Ratliff CR, Donovan AM, Knox LK, Morgan RF, Rodeheaver GT. Quantitative swab culture versus tissue biopsy: a comparison in chronic wounds. Ostomy wound manage. 2001; 47: 34-37.
- Huang Y,Cao Y, Zou M, Luo X, Jiang Y, et al. A Comparison of Tissue versus Swab Culturing of Infected Diabetic Foot Wounds. Int J Endocrinol. 2016; 1-6.
- Mutluoglu M, Uzun G, Turhan V, Gorene kL, Ay H, Lipsky BA. How reliable are cultures of specimens from superficial swabs compared with those of deep tissue in patients with diabetic foot ulcers?. J Diabetes Complications. 2012; 26: 225-229.
- Slater R, Lazarovitch T, Boldur I, Ramot Y, Buchs A, et al. Swab cultures accurately identify bacterial pathogens in diabetic foot wounds not involving bone. Diabet Med. 2004; 21: 705-709.
- Mendes J, Marques-Costa A, Vilela C, Neves J, Candeias N, et al. Clinical and bacteriological survey of diabetic foot infections in Lisbon. Diabetes Res Clin Pract. 2012; 95: 153-161.
- Otta S, Debata NK, Swah B. Bacteriological profile of diabetic footulcers. CHRISMEDJ Health Res. 2019; 6: 7-11.
- Sharma VK, Khadka PB, Joshi A, Sharma R. Common pathogens isolated in diabetic foot infection in Birhospital. Kathmandu Univ Med J. 2006; 4: 295-301.
