Abstract
Type 2 diabetes (T2D) is no longer considered solely a glucose-centric condition, but rather a chronic cardiometabolic disease that is linked to premature cardiovascular and renal complications and early death. Whereas previously, the level of glycemic control drove management decisions regarding treatment intensification, healthcare providers now have newer classes of agents that not only effectively lower glucose levels but also reduce the long-term risk of cardiovascular and renal complications. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) address several of the progressive multiorgan dysfunctions associated with T2D and a number of GLP-1RAs have been shown to reduce the risk of major adverse cardiovascular events in people with established cardiovascular disease or in those at high cardiovascular risk; GLP-1RAs (or a cardioprotective sodium-glucose cotransporter-2 inhibitor) should be considered in these high-risk patients regardless of their glycated hemoglobin goal attainment status. GLP-1RAs also facilitate substantial weight loss and there is some evidence that they may help to restore β-cell function and slow the decline of kidney function, although further studies are needed to confirm this.
Keywords: β-cell Preservation; Cardiovascular Disease Risk; Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA); Renal Function/Dysfunction; Type 2 Diabetes (T2D); Weight Control/Management
Abbreviations: CI: Confidence Interval; CV: Cardiovascular;DPP4i: Dipeptidyl Peptidase-4 Inhibitor; eGFR: estimated Glomerular Filtration Rate; ER: Extended-Release; GLP-1: Glucagon-Like Peptide-1; GLP-1RA: Glucagon-Like Peptide-1 Receptor Agonist; HR: Hazard Ratio; LV: Left Ventricular; MACE: Major Cardiovascular Adverse Events; s.c.: subcutaneous; SGLT2i: Sodium-Glucose Cotransporter-2 Inhibitor; SU: Sulfonylurea; T2D: Type 2 Diabetes; TZD: Thiazolidinedione
Introduction
Historically, the focus for patients with type 2 diabetes (T2D) has been the prevention of chronic hyperglycemia using lifestyle and dietary changes, plus glucose-lowering medications [1]. However, these patients are at risk of long-term complications owing to the effects of dysfunctions covering multiple organs and systems [2-6].
The concept of T2D as a multiorgan disease is summarized by the “ominous octet” of eight main dysfunctions (Figure 1) [2], which typically manifest as an interrelated association between glucose dysregulation, weight gain, dyslipidemia, and blood pressure abnormalities, with progressively increasing cardiovascular risk and renal impairment. Newer glucose-lowering therapies, including glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs), can influence a multitude of these dysfunctions.
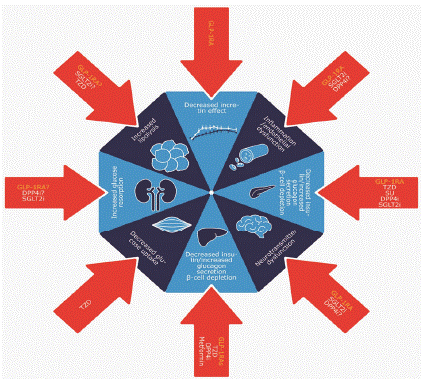
Figure 1: The ominous octet of type 2 diabetes, and glucose-lowering
drugs that have a direct or indirect positive effect on each aspect [2,7-9].
DPP4i: dipeptidyl peptidase-4 inhibitor; GLP-1RA: glucagon-like
peptide-1 receptor agonist; SGLT2i: sodium-glucose cotransporter-
2 inhibitor; SU: sulfonylurea; TZD: thiazolidinedione.
This manuscript reviews recent evidence for the benefits of GLP-1RAs beyond blood glucose regulation, focusing on key aspects of concern to primary care providers caring for patients with T2D.
Literature Search Method
The PubMed database was searched non-systematically for randomized controlled trials of GLP-1RAs in humans with T2D, published in English in the previous 5 years, using the generic names of approved GLP-1RAs (semaglutide, liraglutide, albiglutide, dulaglutide, exenatide, lixisenatide, taspoglutide, and efpeglenatide) in separate searches with the following terms: (cardiovascular OR cardiorenal OR renal OR kidney) OR (ASCVD or atherosclerotic); (beta cell preservation OR protection) OR (glycemic durability); and (weight loss OR reduction). Titles and abstracts of the 230 retrieved articles were scrutinized to remove irrelevant and duplicate results. The bibliographies of the remaining articles were checked to capture additional relevant references. Further references were added based on supplementary searches and the authors’ own subject knowledge.
Beyond Glucose Lowering with GLP-1RAs in T2D
GLP-1RAs target at least six of the eight dysfunctions that make up the ominous octet (Figure 1) [2,7,8]. One underlying dysfunction is the reduced sensitivity to the effect of incretin hormones, including GLP-1 [9].GLP-1 exerts postprandial effects that lead to a reduction in appetite and increased feelings of fullness [10,11]; in addition, GLP-1 also stimulates glucose-dependent insulin secretion and suppresses glucagon secretion. GLP-1 is also thought to increase excretion of sodium via the kidneys and promote glycogen synthesis and glucose oxidation in the muscles [10,12]. Preclinical data suggest that GLP-1 is associated with enhanced β-cell proliferation, as well as uptake of glucose in cells [10,13,14]. GLP-1 also exerts various cardio protective effects [15,16].
Exogenously administered GLP-1RAs circulate at considerably higher concentrations than can be achieved by endogenous GLP-1 to overcome GLP-1 resistance. This explains why their glucose lowering and other effects are greater than those seen with dipeptidyl peptidase-4 inhibitors (DPP4is), which inhibit the enzyme responsible for deactivating GLP-1 but cannot increase GLP-1 levels much beyond that which is physiologically available [17].
GLP-1RAs Reduce Cardiovascular Risk
Atherosclerotic cardiovascular disease (coronary heart disease, cerebrovascular disease, and peripheral artery disease caused by atherosclerosis) is the leading cause of morbidity and mortality in people with diabetes, and studies have shown that controlling individual cardiovascular risk factors prevents or delays development of cardiovascular disease [18]. GLP-1RAs are potentially disease-modifying in the setting of T2D and cardiovascular disease [19-26]; dulaglutide is approved in the US for both primary and secondary cardiovascular risk reduction in people with T2D [27], while liraglutide and subcutaneous semaglutide are indicated for cardiovascular risk reduction in adults with T2D and known heart disease [28,29].
All GLP-1RAs have been evaluated in large cardiovascular outcomes trials in patients with T2D who either had established cardiovascular disease or were at elevated risk for a first cardiovascular event [19-26] (Figure 2). Once-daily liraglutide, once-weekly dulaglutide, and once-weekly subcutaneous semaglutide showed a significant reduction in major adverse cardiovascular events when added to standard care, as well as benefits regarding markers of kidney function [20,21,23]. Albiglutide (no longer marketed) [24] and efpeglenatide (not yet approved) [26] also reduced cardiovascular risk. Lixisenatide, exenatide, and ITCA 650 (continuous subcutaneous infusion of exenatide) were non-inferior to placebo for the risk of cardiovascular events [19,22,30]. A pooled analysis of subcutaneous and oral semaglutide data suggested a cardiovascular benefit regardless of administration route [32]. A long-term, larger, post-approval cardiovascular outcomes study for oral semaglutide (SOUL; NCT03914326) is underway to confirm these findings.
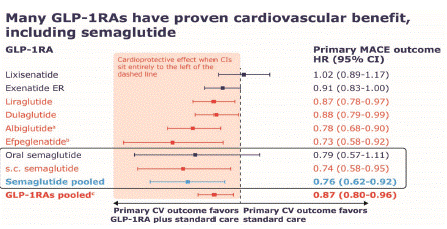
Figure 2: Summary of primary outcomes of cardiovascular outcomes
trials of GLP-1RAs and combined meta-analyses [19-26,30,31].
aAlbiglutide is no longer marketed in the US.
bEfpeglenatide is not currently approved for use in the US.
cAnalysis did not include efpeglenatide.
Note: Red bars that fall entirely within the shaded area left of the
dotted line indicate that a significant reduction in MACE (a composite
of first events of cardiovascular death, nonfatal myocardial
infarction, or nonfatal stroke) was demonstrated with GLP-1RA
therapy. The light blue bar represents the primary outcome of a
post-hoc pooled analysis of the prospective oral semaglutide and
s.c.semaglutide trials.
CI: confidence interval; CV: cardiovascular; ER: extended-release;
GLP-1RA: glucagon-like peptide-1 receptor agonist; HR: hazard ratio;
MACE: major cardiovascular adverse event; s.c.: subcutaneous.
A meta-analysis of GLP-1RA cardiovascular outcomes trials (not including efpeglenatide) found a 13% reduction in major adverse cardiovascular events compared with standard care [33]. GLP-1RAs also reduced the risk of all-cause death by 11% [33]. These outcomes were supported by a more recent analysis [34]. Another meta-analysis found similar results when data were stratified by baseline factors, such as body mass index, glycated hemoglobin, and age [35]. Of note, cardioprotective effects of GLP-1RAs appear to become evident over a period of ~12-24 months, similar to statins [20,21,36]. Notably, there were differences between these trials in terms of design and populations, although patients all had T2D and were at elevated cardiovascular risk [37]. All trials tested cardiovascular safety, and all GLP-1RAs were shown not to increase cardiovascular risk. Two trials are currently recruiting patients to assess the effect of once-weekly subcutaneous semaglutide 2.4 mg in patients with obesity and heart failure with preserved ejection fraction, with and without T2D (NCT04788511 and NCT04916470).
The action of GLP-1 on the heart and blood vessels may be direct or indirect, and involves protective effects, including reducing inflammation, increasing blood flow, and improving endothelial function (Figure 3) [16]. GLP-1-mediated reductions in pro-inflammatory markers and oxidative stress appear likely to be involved [38]. Among the other newer classes of glucose-lowering medications, sodium-glucose cotransporter-2 inhibitors (SGLT2is) also have a positive impact on the cardiovascular system (Figure 3) [8] and have been shown to improve cardiovascular and renal outcomes in people with T2D [39-43].
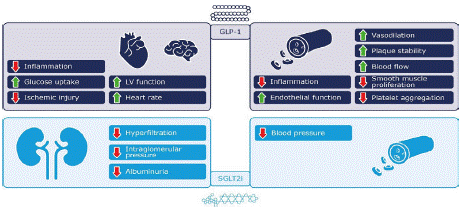
Figure 3: Proposed cardioprotective mechanisms of GLP-1RAs and
SGLT2is [8,16].
GLP-1: glucagon-like peptide-1; GLP-1RA: glucagon-like peptide-1
receptor agonist; LV: left ventricular; SGLT2i: sodium-glucose
cotransporter-2 inhibitor.
Signals for Renal Protection with GLP-1RAs
Diabetic kidney disease is a common and serious comorbidity in patients with T2D [44], and renal dysfunction often coincides with cardiovascular risk. GLP-1RAs are generally well tolerated in patients with renal impairment without the need for dose adjustment (except for exenatide and lixisenatide), and subgroup analyses of cardiovascular outcomes trials – albeit mostly exploratory – suggest that some GLP-1RAs may have a positive effect on renal function [21,45-50]. A large randomized, placebo-controlled study (FLOW, NCT03819153) is underway to further characterize the profile of once-weekly subcutaneous semaglutide in people with T2D and chronic kidney disease. The primary composite outcome measure is time to any one of the following events: decline in kidney function of ≥50% reaching end-stage renal disease, death from kidney disease, or death from cardiovascular disease, with results expected in August 2024. Similarly, the ongoing SOUL trial (NCT03914326) includes the confirmatory secondary endpoint of time to first occurrence of a composite chronic kidney disease endpoint, including the following events: cardiovascular death, renal death, onset of persistent ≥50% reduction in estimated glomerular filtration rate (eGFR) compared with baseline, onset of persistent eGFR <15 mL/min/1.73 m2, or initiation of chronic renal replacement therapy (defined as dialysis or kidney transplant). The results of this trial are expected in July 2024.
GLP-1RAs Mediate Weight Loss
Obesity is intrinsically linked to T2D, cardiovascular disease, and adverse outcomes [18]. Primary care physicians often address weight loss in their patients with diet and lifestyle modification. However, combining behavioral intervention with appropriate pharmacotherapy improves outcomes [51]. As a general principle, the most severe disease often requires pharmacotherapies of different classes and mechanisms of action to work together to achieve optimal outcomes. However, incretin-based treatment offers the potential of a single drug class that works via multiple mechanisms to address obesity.
GLP-1 acts centrally via anorexigenic and orexigenic pathways and slows gastric emptying, thus modifying the physiological and behavioral responses to hunger and satiety and reducing calorie consumption (Figure 4) [52]. In Phase III randomized clinical trials in people with T2D treated with GLP-1RA therapy plus metformin, patients lost an average of 3-5 kg while on treatment for up to 1 year, varying by drug and trial (Table 1). Patients continued to lose weight whilst on therapy, whereas weight loss was found to plateau with some other glucose-lowering medications, such as the SGLT2i empagliflozin. Weight loss was approximately 4 kg with empagliflozin after both 26 and 52 weeks’ treatment but improved with oral semaglutide from ~4 kg at 26 weeks to approaching 5 kg after 52 weeks’ continuous treatment [62]. Achieving a reduction equivalent to 5% of baseline body weight is considered clinically meaningful [63], and more patients with T2D receiving GLP-1RAs in clinical studies achieved this target than with other classes of comparator glucose-lowering medications [54,56,60,63].
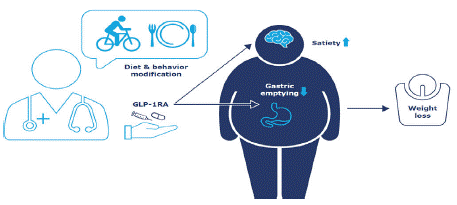
Figure 4: Incorporating GLP-1RAs into weight-management interventions.
GLP-1RA: glucagon-like peptide-1 receptor agonist.
In Phase III trials, semaglutide- and dulaglutide-induced weight loss was consistently greater than for comparators, regardless of baseline body mass index [65,66]. When compared head-to-head in patients with T2D receiving background metformin, once-weekly subcutaneous semaglutide 0.5 mg and 1.0 mg led to significantly greater weight loss (and glycated hemoglobin reduction) than once-weekly dulaglutide 0.75 mg and 1.5 mg after 40 weeks of treatment [67]. Importantly, in patients without overweight/obesity treated with exenatide, there was no excess weight loss [68].
Liraglutide became the first GLP-1RA indicated at a dose of 3.0 mg once daily (higher than the 1.8 mg diabetes dose) for weight loss in patients with overweight/obesity, regardless of their diabetes status [69]. Studies indicate that liraglutide reduces body weight in people with body mass index 27-<30 kg/m² (overweight) and ≥30 kg/m² (obesity) and may decrease the long-term risk of developing T2D in people with pre-diabetes [70,71]. Gradual body weight reductions in patients with obesity treated with liraglutide 3.0 mg were not associated with changes in serum creatinine [72]. Subcutaneous semaglutide 2.4 mg has also recently been approved for chronic weight management in the US [73] after demonstrating efficacy for weight loss in patients with overweight/obesity in the STEP 1-4 trials, which included patients with and without T2D [74,77]. In STEP 2, in which patients had diabetes and a body mass index ≥27 kg/m², once-weekly injections of semaglutide 2.4 mg plus behavioral intervention resulted in over two-thirds of patients experiencing at least 5% weight loss from baseline and more than one in eight patients losing over 20% of their pre-treatment weight [75]. Patients with overweight/obesity but without diabetes lost more weight on semaglutide than those with both conditions; 80% of patients in STEP 1, 3, and 4 lost at least 5% of their baseline weight [74,76,77]. Subcutaneous semaglutide 2.4 mg is now being tested to determine its effects on cardiovascular outcomes in patients with overweight/obesity in the SELECT trial, the first such study in this population (NCT03574597).
GLP-1RAs May Help to Preserve β-cell Function
β cells in the pancreas secrete insulin to manage blood glucose levels following the ingestion of nutrients [9]. In people with normal β-cell function, orally administered glucose prompts a greater insulin response than when glucose is infused intravenously, a phenomenon referred to as the incretin effect because of the potentiating effect of incretin hormones on the release of insulin [78]. However, in people with T2D, β cells become stressed and their insulin-secreting ability progressively declines. Glucose-lowering medications are then needed, and eventually exogenous insulin [1], as β-cell function is progressively lost. However, if the incretin response can be stabilized before critical and irreversible loss of β cells, the use of replacement insulin can be delayed.
Recent analyses have provided indirect evidence that GLP-1RAs may preserve β-cell function. Exenatide and dulaglutide have both shown anti-inflammatory and antioxidant effects that are potentially protective of β cells [79,80]. Among the GLP-1RAs, both liraglutide and semaglutide have demonstrated the ability to restore β-cell function in people with T2D (Figure 5) [81,82]. Logically, the earlier GLP-1RAs are started, the greater the amount of residual β-cell function may be protected. Although β-cell function would be still expected to decline over time, a duration of response of at least 2 years with GLP-1RAs has been demonstrated in clinical studies [83,84]. Although early use of GLP-1RAs is preferred, there is still a benefit to adding them later in the disease course (improving glycemic control, reducing cardiovascular risk, etc.), including in patients already receiving insulin, those with advanced age (≥65 years), and people with longer duration of disease (≥10 years) [85].
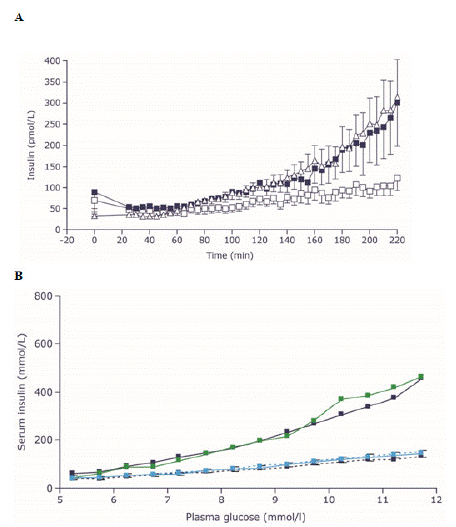
Figure 5: Liraglutide and semaglutide can restore Β-cell function in
people with T2D.
Panel A shows profiles of plasma insulin concentrations during
graded glucose infusion studies after a single injection of liraglutide
(dark blue squares) or placebo (white squares) in patients with
T2D. Results are compared with those of healthy control participants
(triangles) who did not receive the drug. Data are means ±
standard error, n=10 for each group [80]. Panel B shows insulin response
to a graded glucose infusion in participants with T2D before
and after receiving 12 weeks of treatment with semaglutide (n=37,
dark blue) or placebo (n=38, light blue), and in healthy participants
(n=12, green). Dotted lines represent baseline values and solid lines
represent end-of-treatment values [81].
T2D: type 2 diabetes.
Figure 5A Adapted from American Diabetes Association ‘The GLP-1
derivative NN2211 restores beta-cell sensitivity to glucose in type
2 diabetic patients after a single dose’, American Diabetes Association,
2003. Copyright and all rights reserved. Material from this
publication has been used with the permission of American Diabetes
Association. Figure 5B adapted from Kapitza C, et al. Diabetologia
2017;60:1390-99. CC-BY 4.0 (http://creativecommons.org/
licenses/by/4.0/).
Other Potential Benefits of GLP-1RA Therapy
An important aspect of any therapy is the effect on the patient’s health-related quality of life. When added to metformin with or without sulfonylurea, once-weekly subcutaneous semaglutide improved overall treatment satisfaction compared with daily basal insulin – possibly partly because of less frequent injections [60]. Generally, patients noticed differences in outcomes when they were receiving once-daily oral semaglutide rather than placebo [86]. However, there were also significant improvements in craving control, as well as some positive effects on general health and social functioning, with oral semaglutide over the daily oral SGLT2i empagliflozin [63,86]. Exploratory analyses and other studies of GLP-1RAs have hinted at further beneficial physiological effects on peripheral artery disease, cognitive impairment, and memory function [87-89]. The placebo-controlled STRIDE trial (NCT04560998) is currently investigating the use of semaglutide in people with T2D and peripheral artery disease, with results expected in 2023. In addition, the EVOKE (NCT04777396) and EVOKE Plus trials (NCT04777409) are investigating the potential use of oral semaglutide in people with early Alzheimer’s disease.
Practical Considerations for Managing GLP-1RA Therapy in the Primary Care Setting
Despite having been available for nearly 15 years, GLP-1RAs have often been considered the preserve of the diabetes specialist and primary care providers may have been hesitant to prescribe them. However, this is slowly changing because of increasing evidence for the benefits of earlier initiation of GLP-1RAs in people with T2D [1], and potentially now the availability of an oral GLP-1RA.
Which Patients Could/Should Receive GLP-1RAs?
The latest American Diabetes Association guidelines suggest that GLP-1RAs should be considered before basal insulin as the first injectable medication for patients whose T2D is uncontrolled on metformin with or without other oral glucose-lowering medications [1]. Because of their potential to modify cardiovascular risk, GLP-1RAs with proven cardiovascular benefit are recommended for all patients with T2D who have established cardiovascular disease or who are at high risk of cardiovascular events, regardless of their glycated hemoglobin target [1,89]. SGLT2is with proven cardiovascular benefit are preferred to GLP-1RAs in patients with T2D and heart failure and/or chronic kidney disease, but with GLP-1RAs as second choice if a SGLT2i is not appropriate [1,90]. The 2021 American Heart Association/American Stroke Association guidelines note that SGLT2is, unlike some GLP-1RAs, do not show a specific effect on the secondary prevention of stroke [91]. Of note, DPP4is are neutral for cardiovascular outcomes and are not generally recommended to be combined with GLP-1RAs [1,90]. The GRADE trial compared outcomes with a sulfonylurea (glimepiride), DPP4i (sitagliptin), GLP-1RA (liraglutide), and insulin glargine, each given as add-on to metformin. Preliminary results showed that over the 5-year observation period, liraglutide and insulin were the most effective of the four treatments for glycemic control and cardiovascular disease prevention, and liraglutide and sitagliptin provided greatest weight loss [92].
Choosing a GLP-1RA – Mode of Administration and Associated Considerations
Most GLP-1RAs are injected, either daily or weekly. One GLP-1RA – semaglutide – is now available as a daily oral tablet [61], which may facilitate earlier GLP-1RA initiation by helping to overcome barriers in injection-naïve patients. Nevertheless, some patients may prefer a once-weekly injection to a daily tablet and innovations in injectable delivery devices are improving the ease of self-injection. This should be communicated to patients to help them feel more comfortable if an injectable GLP-1RA is under consideration. Dosing instructions for each available GLP-1RA are shown in Table 1.
Generic name
Administration route and frequency
Dose and dosing conditions (adults only unless stated)
Representative effect on glycated hemoglobin (% reduction from baseline) and body weight (ab- solute reduction from baseline) when added to metformin)a
Most common adverse events (% of patients with at least one event)
Exenatide
Subcutaneous injection twice daily [53]
- Start at 5.0 μg
- Increase to 10.0 μg after 1 month based on clinical response
- Dose =1 hour before morning and evening meals (or the two main meals of the day, =6 hours apart) [53]
Glycated hemoglobin (week 24) [54]:
- -1.0% (10.0 μg)
Body weight (week 24) [54]:
- -3.8 kg (10.0 μg)
10.0 μg [54]:
- Nausea: 35%
- Vomiting: 13%
- Diarrhea: 13%
Exenatide extended-release
Subcutaneous injection once weekly [55]
- Start/continue at 2.0 mg
- Dose at any time of day, with or without food [55]
Glycated hemoglobin (week 26) [56]:
- -1.5% (2.0 mg)
Body weight (week 26) [56]:
- -2.3 kg (2.0 mg)
2.0 mg [56]:
- Nausea: 24%
- Diarrhea: 18%
- Vomiting: 11%
Lixisenatide
Subcutaneous injection once daily [57]
- Start at 10.0 μg for the first 14 days
- Increase to 20.0 μg
- Dose at same time each day, =1 hour before the first meal of the day [57]
Glycated hemoglobin (week 24) [54]:
- -0.8% (20.0 μg)
Body weight (week 24) [54]:
- -3.0 kg (20.0 μg)
20.0 μg [54]:
- Nausea: 25%
- Vomiting: 10%
- Diarrhea: 10%
Liraglutide
Subcutaneous injection once daily [29]
Adults [29]:
- Start at 0.6 mg for 1 week
- Increase to 1.2 mg. If additional glycemic control is required, increase the dose to 1.8 mg after 1 further week
- Dose at any time of day, independent of meals
Children =10 years [29]:
- Start at 0.6 mg for =1 week
- Only increase to 1.2 or 1.8 mg if required
- Dose at any time of day, independent of meals
Glycated hemoglobin (week 26) [58]:
- -1.0% (1.2 mg)
- -1.0% (1.8 mg)
Body weight (week 26) [58]:
- -2.6 kg (1.2 mg)
- -2.8 kg (1.8 mg)
1.2 mg/1.8 mg [58]:
- Nausea: 16/19%
- Diarrhea: 8/15%
- Vomiting:
5-7% across the groups
Dulaglutide
Subcutaneous injection once weekly [27]
- Start at 0.75 mg
- Increase to 1.5 mg, and subsequently up to
3.0 mg or 4.5 mg, after at least 4 weeks on each dose, if needed
- Dose at any time of day with or without food [27]
Glycated hemoglobin (week 36) [60]:
- -1.5% (1.5 mg)
- -1.6% (3.0 mg)
- -1.8% (4.5 mg)
Body weight (week 36) [59]:
- -3.0 kg (1.5 mg)
- -3.8 kg (3.0 mg)
- -4.6 kg (4.5 mg)
1.5/3.0/4.5 mg [59]:
- Nausea: 14/16/17%
- Vomiting: 6/9/10%
- Diarrhea: 8/12/12%
Semaglutide
Subcutaneous injection once weekly [28]
- Start at 0.25 mg
- Increase to 0.5 mg after 4 weeks. If after at least 4 weeks additional glycemic control is needed, increase to 1.0 mg . If additional glyc- emic control is needed after at least 4 weeks on the 1.0 mg dose, increase to 2.0 mg.
- Dose at any time of day, with or without food [28]
Glycated hemoglobin (week 30) [60]:
- -1.2% (0.5 mg)
- -1.6% (1.0 mg)
(week 40) [61]:
- -2.2% (2.0 mg)
Body weight (week 30) [60]:
- -3.5 kg (0.5 mg)
- -5.2 kg (1.0 mg)
(week 40) [61]:
- -6.9 kg (2.0 mg)
0.5/1.0 [60]/2.0 mg [61]:
- Nausea: 21/22/14%
- Vomiting: 7/10/8%
- Diarrhea: 16/19/9%
Oral semaglutide
Oral tablet once daily [62]
- Start at 3.0 mg
- Increase to 7.0 mg after 30 days. Dose may be increased to 14.0 mg if additional glycemic con- trol is needed after at least 30 days on 7.0 mg
- Dose tablet on an empty stomach with =4 fl oz (120 mL) of plain water >30 minutes before any further liquid and the first food and/or other oral medication of the day [62]
Glycated hemoglobin (week 26) [63]:
- -1.3% (14.0 mg)
Body weight (week 26) [62]:
- -3.8 kg (14.0 mg)
14.0 mg [63]:
- Nausea: 20%
- Diarrhea: 9%
- Vomiting: 7%
Table 1: Key features of currently available glucagon-like peptide-1 receptor agonists.
The once-weekly injectable GLP-1RAs can be dosed at any time of day regardless of mealtimes (Table 1). This is also true of once-daily liraglutide, but time of day and mealtimes must be considered with other once-daily GLP-1RAs. For oral semaglutide, to ensure optimal absorption, it is advised that the patient takes the tablet in the morning on an empty stomach [62].
Choosing a GLP-1RA – Efficacy and Tolerability
When selecting a GLP-1RA, patient preference (daily vs weekly injections or daily oral therapy) and payer coverage are potentially the most important short-term considerations. However, between the longer-acting GLP-1RAs, small differences in glucose control and weight loss, as well as proven cardiovascular benefit, tend to favor once-weekly subcutaneous semaglutide and dulaglutide, and once-daily liraglutide (Table 1) [54,56,58,59,60,63,93]. When added to metformin in clinical trials, GLP-1RAs at their currently approved doses reduced glycated hemoglobin by 0.8-1.8%, depending on the treatment and dose, baseline glycated hemoglobin, and time point of the primary outcome measurement [54,56,58,59,60,63,93]. Semaglutide appears to be the most effective option for weight loss, with the 1.0 mg subcutaneous dose providing an average 5 kg reduction after 30 weeks [60], but all GLP-1RAs help patients to lose weight (Table 1). Glycated hemoglobin and body weight reductions are generally maintained with ongoing treatment [54,56,58,59,60,63,93]. Semaglutide 2.0 mg was superior to 1.0 mg in reducing glycated hemoglobin, with additional body weight loss and a similar safety profile [61], and dulaglutide 4.5 mg provided superior glycated hemoglobin reductions compared with 1.5 mg, with a similar safety profile [61]. Dulaglutide is now approved at additional doses of 3.0 mg and 4.5 mg if additional glycemic control is needed [27]. Although once-weekly subcutaneous semaglutide 2.4 mg is now indicated for weight loss in people with overweight/obesity, this dose should not be used for management of T2D in patients with body mass index <27 kg/m² [73].
Tolerability profiles are generally consistent between the GLP-1RAs; all agents are associated with a low risk of hypoglycemia when used in combination with other anti-diabetes agents besides insulin and/or a sulfonylurea [54,56,58,59,60,63,93]. This is because GLP-1RAs, like endogenous GLP-1, only act on elevated levels of blood glucose. Gastrointestinal side effects, primarily nausea, vomiting, and diarrhea [54,56,58,59,60,63,93], are the most common adverse events, but their nature and intensity can differ between drugs in the class. Generally, the more potent GLP-1RAs are associated with a greater incidence of gastrointestinal side effects, although most events are mild or moderate, occur at the start or intensification of treatment, and resolve with ongoing use [54,56,58,59,60,63,93].
A gradual increase in the intensity of therapy can mitigate gastrointestinal events, and dose-escalation schedules are recommended in the prescribing information for GLP-1RAs (Table 1). Dose flexibility also provides the option to reduce the dose or pause treatment if an adverse event occurs. Patients should be made aware that gastrointestinal side effects are not unexpected. Suggesting dietary modifications, such as reducing portion sizes and avoiding fatty foods, can help patients manage gastrointestinal issues [94]. Patients should report any serious or ongoing side effects to their physician. Renal function should be monitored in patients with renal impairment reporting severe adverse gastrointestinal reactions [27-29,53,55,57,62].
An appreciable proportion of patients may experience fatigue and/or asthenia when starting GLP-1RA treatment [55,69,73]. This may be due to adjustments in caloric intake rather than a direct effect of the medication. As with gastrointestinal events, this phenomenon generally stabilizes within a few weeks, and counseling patients to expect fluctuations in their energy levels could help encourage treatment persistence.
Contraindications, Warnings, and Precautions
Most GLP-1RAs can be used in patients with renal impairment, including end-stage kidney disease [27-29,62] without dose adjustment. However, exenatide is not recommended in patients with creatinine clearance <30 mL/min (twice-daily formulation) [53] or eGFR <45 mL/min/1.73 m² (weekly extended-release formulation) [55], and lixisenatide is not recommended in patients with end-stage renal disease (creatinine clearance <15 mL/min) [57].
GLP-1RAs have been associated with the development of thyroid C-cell tumors in rodents, but the clinical relevance of this in humans is unknown. There are post-marketing reports of medullary thyroid carcinoma in patients with T2D treated with liraglutide, but in insufficient numbers to establish or exclude a causal association [29]. Nevertheless, a boxed warning in the prescribing information for most GLP-1RAs mandates contraindication in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2 [27-29,55,62]. Routine monitoring of serum calcitonin or using thyroid ultrasound is not advocated. Patients should be counseled regarding the potential risk of medullary thyroid carcinoma and symptoms of thyroid tumors [27-29,55,57,62].
The risk of hypoglycemia increases when GLP-1RAs are combined with insulin or sulfonylurea, both of which are associated with increased rates of death in the setting of severe hypoglycemia [94]. Therefore, consideration should be given to lowering the dose of insulin or sulfonylurea if combined therapy is used [27-29,53,55,57,62].
There was a low risk of acute pancreatitis in clinical trials of GLP-1RAs; patients should be counseled on the symptoms of pancreatitis and be advised to discontinue their GLP-1RA if pancreatitis is suspected. If confirmed, the GLP-1RA should not be restarted [27-29,53,55,57,62].
Diabetic retinopathy complications have been reported in a clinical trial with semaglutide [21,28]. Retinopathy status should be assessed in patients receiving GLP-1RAs [96,97], and patients with a history of diabetic retinopathy should be monitored [27,28,62].
Conclusion
GLP-1RAs address multiple aspects of the physiological dysfunction associated with T2D, potentially slowing disease progression and modifying its course, in particular with relation to cardiovascular outcomes. Earlier initiation of GLP-1RAs in the treatment paradigm of T2D should be considered, particularly in patients at increased cardiovascular risk regardless of glycated hemoglobin target.
Acknowledgments
Medical writing and editorial support were provided by Stephen Purver of Axis, a division of Spirit Medical Communications Group Limited (and were funded by Novo Nordisk Inc.), under the direction of the authors, in accordance with Good Publications Practice Guidelines (www.ismpp.org/gpp3). Novo Nordisk Inc. also performed a medical accuracy review.
Conflicting and Competing Interests
JL is an advisor and speaker for Novo Nordisk.
TMB holds stock in TransMedics Group and has previously held stock in Novavax.
KU reports advisory board participation for Esperion and Novo Nordisk and is a consultant/speaker for Amarin, AstraZeneca, Esperion, Medicure, and Novo Nordisk.
SPM received consulting fees from Novo Nordisk; speaker fees from Abbott and Boston Scientific; research support from Bristol-Myers Squibb and Novo Nordisk; and participated in steering committees, medical education, or clinical trial activities for Abbott, Asahi, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, and Novo Nordisk.
KMP reports, in the previous 12 months, being a member of the speakers’ bureau of AstraZeneca, Corcept Therapeutics, Merck, and Novo Nordisk; a consultant for AstraZeneca, Bayer, Corcept Therapeutics, Diasome, Merck, Novo Nordisk, and Sanofi; and receiving research support from Bayer, Merck, Novo Nordisk, and Twin Health.
Contributors
JL, KU, and KMP were responsible for the conceptualization of the manuscript. The original draft was created by the medical writer, under the direction of the authors. All authors reviewed and edited the drafts and approved the final version for submission.
Funding Statement
Funding for the preparation of this manuscript was provided by Novo Nordisk Inc.
References
- American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, et al. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022; 45: S125-S143.
- DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58: 773-795.
- Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018; 17: 83.
- Yau JWY, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35: 556-564.
- Russell JW, Zilliox LA. Diabetic neuropathies. Continuum (Minneap Minn). 2014; 20: 1226-1240.
- Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016; 118: 1771-1785.
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, et al. Type 2 diabetes mellitus. Nature Rev Dis Primers. 2015; 1: 15019.
- DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes ObesMetab. 2017; 19: 1353-1362.
- Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. 2018; 14: 390-403.
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007; 87: 1409-1439.
- Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001; 86: 4382-4389.
- Muskiet MHA, Tonneijck L, Smits MM, van Baar MJB, Kramer MHH, et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nature Rev Nephrol. 2017; 13: 605-628.
- Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003; 52: 380-386.
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, et al. Glucagonlike peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003; 278: 471-478.
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagonlike peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005; 54: 146-151.
- Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016; 24: 15-30.
- Gilbert MP, Pratley RE. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front Endocrinol (Lausanne). 2020; 11: 178.
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022; 45: S144-S174.
- Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015; 373: 2247-2257.
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375: 311-322.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375: 1834-1844.
- Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017; 377: 1228- 1239.
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019; 394: 121-130.
- Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo controlled trial. Lancet. 2018; 392: 1519-1529.
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019; 381:841-851.
- Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021; 385: 896-907.
- Food and Drug Administration. TRULICITY® prescribing information.
- Food and Drug Administration. OZEMPIC® prescribing information.
- Food and Drug Administration. VICTOZA® prescribing information.
- Ruff CT, Baron M, Im K, O’Donoghue ML, Fiedorek FT, et al. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: a non-inferiority randomized controlled trial. Nat Med. 2022; 28: 89-95.
- Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, et al. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. 2019; 21: 2576-2580.
- Husain M, Bain SC, Jeppesen OK, Lingvay I, Sørrig R, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020; 22: 442-451.
- Lee MMY, Kristensen SL, Gerstein HC, McMurray JJV, Sattar N. Cardiovascular and mortality outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a meta-analysis with the FREEDOM cardiovascular outcomes trial. Diabetes Metab Syndr. 2022; 16: 102382.
- Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021; 9: 653-662.
- Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019; 7: 776-785.
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM Jr, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med .2008; 359: 2195-2207.
- Pantalone KM, Munir K, Hasenour CM, Atisso CM, Varnado OJ, et al. Cardiovascular outcomes trials with glucagon- like peptide-1 receptor agonists: a comparison of study designs, populations and results. Diabetes Obes Metab. 2020; 22: 2209-2226.
- Bray JJH, Foster-Davies H, Salem A, Hoole AL, Obaid DR, et al. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomised controlled trials. Diabetes Obes Metab. 2021; 23: 1806-1822.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373: 2117-2128.
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377: 644-657.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380: 2295-2306.
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380: 347-357.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383: 1436-1446.
- Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2015; 5: 49-56.
- Mann JFE, ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017; 377: 839-848.
- Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, et al. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation. 2018; 138: 2908-2918.
- Mann JFE, Hansen T, Idorn T, Leiter LA, Marso SP, et al. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020; 8: 880-893.
- von Scholten BJ, Persson F, Rosenlund S, Hovind P, Faber J, et al. The effect of liraglutide on renal function: a randomized clinical trial. Diabetes Obesity Metab. 2017; 19: 239-247.
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019; 394: 131-138.
- Tuttle KR, Lakshmanan MC, Rayner B, Zimmermann AG, Woodward B, et al. Body weight and eGFR during dulaglutide treatment in type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7). Diabetes Obes Metab. 2019; 21: 1493-1497.
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018; 320: 1163-1171.
- Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil. 2019; 31: e13546.
- Food and Drug Administration. BYETTA® prescribing information.
- Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013; 36: 2945-2951.
- Food and Drug Administration. BYDUREON® prescribing information.
- Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010; 376: 431-439.
- Food and Drug Administration. ADLYXIN® prescribing information.
- Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009; 32: 84-90.
- Frias JP, Bonora E, Nevarez Ruiz L, Li YG, Yu Z, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (AWARD-11). Diabetes Care. 2021; 44: 765-773.
- Aroda VR, Bain SC, Cariou B, Piletic M, Rose L, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017; 5: 355-366.
- Frias, JP, Auerbach P, Bajaj HS, Fukushima Y, Lingvay I et al. Efficacy and Safety of Once Weekly Semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021; 9: 563-574.
- Food and Drug Administration. RYBELSUS® prescribing information.
- Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019; 42: 2272-2281.
- Food and Drug Administration. Guidance for industry developing products for weight management: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER).
- Ahrén B, Atkin SL, Charpentier G, Warren ML, Wilding JPH, et al. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes Metab. 2018; 20: 2210-2219.
- Fuechtenbusch M, Aberle J, Heitmann E, Nicolay C, Jung H. Weight loss in patients with type 2 diabetes receiving onceweekly dulaglutide plus insulin lispro or insulin glargine plus insulin lispro: a post-hoc analysis of the AWARD-4 study across baseline body mass index subgroups. Diabetes Obes Metab. 2019; 21: 1340-1348.
- Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, openlabel, phase 3b trial. Lancet Diabetes Endocrinol. 2018; 6: 275-286.
- Deng H, Lin S, Yang X, Lv J, Luo S, Zeng L, et al. Effect of baseline body mass index on glycemic control and weight change with exenatide monotherapy in Chinese drug-naïve type 2 diabetic patients. J Diabetes. 2019; 11: 509-518.
- Food and Drug Administration. SAXENDA® prescribing information.
- le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017; 389: 1399-1409.
- le Roux C, Aroda V, Hemmingsson J, Cancino AP, Christensen R. Comparison of efficacy and safety of liraglutide 3.0mg in individuals with BMI above and below 35 kg/m²: a posthoc analysis. Obes Facts. 2017; 10: 531-544.
- von Scholten BJ, Davies MJ, Persson F, Hansen TW, Madsbad S, et al. Effect of weight reductions on estimated kidney function: post-hoc analysis of two randomized trials. J Diabetes Complications. 2017; 31: 1164-1168.
- Food and Drug Administration. WEGOVYTM prescribing information.
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021; 384: 989-1002.
- Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021; 397: 971-984.
- Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP , et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021; 325: 1403-1413.
- Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021; 325: 1414-1425.
- Nauck M, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986; 63: 492-498.
- Dandona P, Ghanim H, Abuaysheh S, Green K, Dhindsa S, et al. Exenatide increases IL-1RA concentration and induces Nrf-2-Keap-1-regulated antioxidant enzymes: relevance to β-cell function. J Clin Endocrinol Metab. 2018; 103: 1180-1187.
- Wang J, Li HQ, Xu XH, Kong XC, Sun R, et al. The effects of once-weekly dulaglutide and insulin glargine on glucose fluctuation in poorly oral-antidiabetic controlled patients with type 2 diabetes mellitus. Biomed Res Int. 2019; 2019: 2682657.
- Chang AM, Jakobsen G, Sturis J, Smith MJ, Bloem CJ, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003; 52: 1786-1791.81.
- Kapitza C, Dahl K, Jacobsen JB, Axelsen MB, Flint A. Effects ofc semaglutide on beta cell function and glycaemic control in participants with type 2 diabetes: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2017; 60: 1390-1399.
- Diamant M, Van Gaal L, Guerci B, Stranks S, Han J, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomisedtrial. Lancet Diabetes Endocrinol. 2014; 2: 464-473.
- Buse JB, Bode BW, Mertens A, Cho YM, Christiansen E, et al. Long-term efficacy and safety of oral semaglutide and the effect of switching from sitagliptin to oral semaglutide in patients with type 2 diabetes: a 52-week, randomized, open-label extension of the PIONEER 7 trial. BMJ Open Diabetes Res Care. 2020; 8: e001649.
- Pantalone KM, Patel H, Yu M, Fernández Landó L. Dulaglutide 1.5 mg as an add-on option for patients uncontrolled on insulin: subgroup analysis by age, duration of diabetes and baseline glycated haemoglobin concentration. Diabetes Obes Metab. 2018; 20: 1461-1469.
- Schneider D, Taddei-Allen P, Dougherty T. The importance of patient-reported outcomes in type 2 diabetes: insight from the PIONEER program with oral semaglutide. Am J Manag Care. 2020; 26: S356-S367.
- Dhatariya K, Bain SC, Buse JB, Simpson R, Tarnow L, et al. The impact of liraglutide on diabetes-related foot ulceration and associated complications in patients with type 2 diabetes at high risk for cardiovascular events: results from the LEADER trial. Diabetes Care. 2018; 41: 2229-2235.
- Cukierman-Yaffe T, Gerstein HC, Colhoun HM, Diaz R, García-Pérez LE, et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 2020; 19: 582-590.
- Vadini F, Simeone PG, Boccatonda A, Guagnano MT, Liani R, et al. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes. 2020; 44: 1254-1263.
- Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, et al. 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43: 487-493.
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021; 52: e364-e467.
- Nathan DM. Results of the glycemia reduction approaches in diabetes–a comparative effectiveness (GRADE) study. Oral presentation at the American Diabetes Association 81st Scientific Sessions, 2021 June 25-29, virtual.
- Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, et al. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014; 37: 2149-2158.
- Reid TS. Practical use of glucagon-like peptide-1 receptor agonist therapy in primary care. Clin Diabetes. 2013; 31: 148-157.
- Zaccardi F, Ling S, Lawson C, Davies MJ, Khunti K. Severe hypoglycaemia and absolute risk of cause-specific mortality in individuals with type 2 diabetes: a UK primary care observational study. Diabetologia. 2020; 63: 2129-2139.
- American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 12. Retinopathy, neuropathy, and foot care: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022; 45: S185-S194.
- Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, et al. HbA1c change and diabetic retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: a meta-analysis and meta-regression. Diabetes Care. 2021; 44: 290-296.
