Abstract
Wellens syndrome is an Electrocardiographic (ECG) pattern that is associated critical stenosis of the Left Anterior Descending (LAD) artery. It manifests as either biphasic T waves in V2-V3 (type A) or deeply negative T waves in V2-V4 (type B). These T-wave abnormalities are persistent and can endure for hours to weeks. Patients with Wellens syndrome often lack chest pain and typically display normal or slightly elevated cardiac enzyme levels. It is critical for clinicians working in both outpatient and inpatient clinical settings to understand and recognize these ECG changes and its association with critical LAD obstruction and significant risk of anterior wall myocardial infarction.
In this case report, we present a unique case of Wellens syndrome type B in an asymptomatic 83-year-old male patient initially assessed and evaluated in an outpatient family practice clinical setting.
Keywords: Wellens syndrome; Left anterior descending artery; LAD Stenosis; Electrocardiography (ecg); Cardiology; ECG abnormalities
Introduction
Wellens syndrome is a pre-infarction stage of coronary artery disease. It is commonly found in patients with total or near total occlusion of the proximal left anterior LAD [1-3]. Patients with Wellens syndrome often present to the Emergency Department (ED) pain-free and usually with normal or slightly elevated cardiac enzymes [1-2]. However, it is important to recognize Wellens syndrome ECG patterns as patients can quickly deteriorate due to their high risk for large anterior wall acute myocardial infarction [1-3]. Wellens syndrome describes a pattern of ECG changes which is highly specific for critical, proximal stenosis of the LAD.
The clinical and ECG criteria for Wellens syndrome are as follows [1-2]:
• Type A Wellens syndrome – biphasic T waves (with initial positivity and terminal negativity) in V2 and V3 [1]
OR
PLUS
• Isoelectric or minimally elevated ST segment, less than 1 mm (in other words, no signs of an acute anterior wall myocardial infarction)
• Preservation of precordial R-wave progression AND no precordial Q waves (in other words, no signs of old anterior wall infarct)
• Recent history of angina
• ECG patterns present in a pain-free state
• Normal or slightly elevated cardiac markers
Drs. De Zwann, Wellens, and colleagues were first to identify the syndrome in the early 1980’s; in their discoveries, it was noted that 75% of patients with Wellens syndrome ECG findings went on to develop an acute anterior wall myocardial infarction within weeks if only treated with medical management [4-5]. Definitive treatment typically involved cardiac catheterization with Percutaneous Coronary Intervention (PCI) to relieve the occlusion [1-3,5-6]. We present a case of Wellens syndrome type B in an asymptomatic 83-year-old male patient who was initially evaluated in an outpatient family practice clinical setting.
Case Presentation
An 83-year-old male presented at an outpatient family practice clinic with a complaint of intermittent dizziness ongoing for about 1 year, with a noticeable worsening in the last 1 to 2 months. The patient has a past medical history of type 2 diabetes mellitus, hypertension, hyperlipidemia, and stage 3A chronic kidney disease. The dizziness appears to only be triggered when transitioning from lying to seated positions. Patient denies chest pain or tightness, palpitations, diaphoresis, shortness of breath, or syncope.
On physical examination, the patient was afebrile with a heart rate of 55, blood pressure of 118/78, respiratory rate of 18, and oxygen saturation of 98% in room air. Physical exam performed in clinic was notable for grade 3/6 systolic murmur and 1+ lower extremity edema bilaterally. Cranial nerves II-XII were intact, with no observable focal neural deficits. The point-of-care ECG revealed sinus bradycardia with a heart rate of 51 and a right bundle branch block as well as T-wave inversion in leads V2-V4 (Figure 1). These findings prompted the decision to transfer the patient to an emergency department for a comprehensive cardiac evaluation.
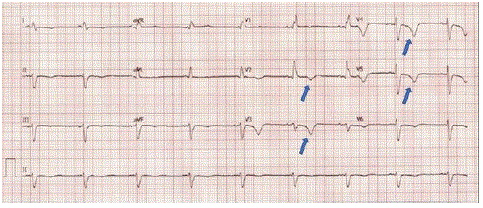
Figure 1: Arrows depict T-wave inversion pattern in V2-V5, but no ST-Elevation Myocardial Infarction (STEMI) pattern.
In the Emergency Department (ED), the patient denied any chest pain or shortness of breath. The ECG performed in the ED was interpreted as sinus bradycardia with bifascicular block. Cardiac enzymes and electrolytes were within normal limits. The patient was discharged home with outpatient follow-up with their primary care physician and referral to cardiology.
Subsequent to the cardiology office visit, the patient underwent monitoring with a Holter monitor for a total duration of 7 days. Over this monitoring period, the patient experienced 4 episodes of ventricular tachycardia and 42 episodes of supraventricular tachycardia. Notably, no significant pauses or instances of high-grade atrioventricular block were observed during this monitoring period.
Following the 7-day Holter monitor evaluation, the patient underwent a pharmacological stress test, which revealed a left ventricular perfusion abnormality. Of note, there were no indications of ECG changes suggestive of ischemia, and the patient did not report any symptoms during the stress test. Given the abnormal findings on both the Holter monitor and the stress test, the patient was scheduled for a heart catheterization procedure with the possibility of angioplasty. During the heart catheterization, a severe stenosis exceeding 99% was identified in the midportion of the LAD. Subsequently, an angioplasty using a standard balloon was performed, and a drug-eluting stent was successfully placed in the midportion of the LAD which resolved the obstruction.
Discussion
The risk factors associated with Wellens syndrome overlap with those of conventional acute coronary syndrome, encompassing conditions such as diabetes mellitus, hypertension, increased age, hyperlipidemia, and smoking [1-3]. In line with other documented cases of Wellens syndrome [1-3], our patient shared similar risk factors.
Wellens syndrome represents a pre-infarction state. However, due to unstable coronary perfusion, these patients face a considerably elevated risk of a substantial anterior wall myocardial infarction or potential death [1-3].
While the precise electrophysiological mechanism underpinning the ECG alterations seen in Wellens syndrome remains uncertain, some hypotheses that coronary artery spasms and myocardial stunning may be account for the observed ECG variations [1-3]. Conversely, other researchers propose that the ECG modifications stem from repetitive transmural ischemia-perfusion episodes that may eventually culminate in myocardial infarction [3]. The possibility of plaque rupture also exists, which could swiftly result in complete obstruction of the left anterior descending coronary artery, precipitating a myocardial infarction.
Wellens syndrome presents itself in two distinct patterns, classified as Type A and Type B. Type A is characterized by biphasic T waves, displaying an initial positive deflection followed by terminal negativity, a pattern observed in about 25% of cases [1-2]. Conversely, Type B is characterized by symmetrically inverted T waves, a presentation seen in approximately 75% of cases [2-3]. In our patient's case, the presence of symmetrically inverted T waves in V2-V5 aligns most closely with the characteristics of Wellens syndrome Type B.
This patient displayed a pain-free presentation during evaluation in the outpatient primary care setting, as well as when further assessed in the emergency department on the same day. This aligns with the common observation that patients with Wellens syndrome frequently present to the emergency department without chest pain and often exhibit normal or only slightly elevated cardiac enzyme levels [1-3]. The absence of pain or clear signs of acute infarction led the emergency medicine team to refrain from immediate and aggressive interventions. This ominous and critical clinical presentation has been documented in several prior cases of Wellens syndrome [6-8]. Consequently, it becomes imperative for clinicians working in both inpatient and outpatient settings to understand and recognize Wellens syndrome's ECG patterns. This knowledge equips clinicians to make quicker and more informed clinical decisions, facilitating the implementation of essential aggressive interventions and ultimately improving patient outcomes and mortality rates.
Early intervention, facilitated by a multidisciplinary management approach encompassing evaluation, diagnosis, and treatment of Wellens Syndrome, has been well-documented to significantly enhance patient outcomes across various clinical settings [6-8]. For non-ST elevation acute coronary syndrome, particularly in patients without chest pain, like the one discussed in our case report, early percutaneous coronary interventions stand as the preferred treatment modality [1-3]. The Wellens pattern on ECG will typically return to baseline after treatment, as was clearly demonstrated in our patient's ECG eight weeks after the angioplasty procedure (Figure 2). Subsequent outcomes following catheterization have proven successful, mirroring our patient's experience, when appropriate interventions were undertaken. Delay in definitive treatment may precipitate an anterior wall infarction and potentially result in a fatal outcome [1- 3,9].
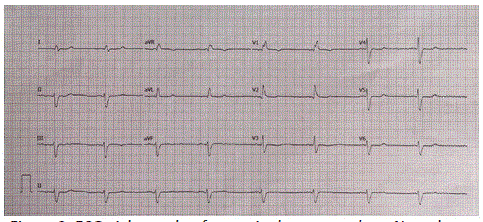
Figure 2: ECG eight weeks after angioplasty procedure. Note the absence of T-wave changes previously demonstrated on the initial ECG prior to the angioplasty procedure.
Conclusion
Wellens syndrome serves as a crucial indicator of the pre-infarction stage in the progression of coronary artery disease. It is imperative for clinicians, including those in primary care, to understand and recognize the distinctive ECG patterns associated with Wellens syndrome. Sole reliance on medical management without intervention poses a substantial risk, as approximately 75% of patients displaying ECG findings consistent with Wellens syndrome face a heightened risk of myocardial infarction within weeks. Timely recognition of Wellens' ECG changes enables the identification of high-risk patients who require evaluation and treatment via cardiac catheterization. In cases where diagnosis remains uncertain and the medical team chooses conservative management, it is essential for the team to remain vigilant to the potential for rapid deterioration of the patient.
References
- Okobi OE, Bakare IO, Evbayekha EO, Olawoye A, Umeh CC, Sowemimo A. Wellens syndrome: A possible precursor. Cureus. 2022; 14: e31963.
- Miner B, Grigg WS, Hart EH. Wellens syndrome. Stat. 2023.
- Ramanathan S, Soaly E, Cherian A, Heidous MA. ”T” twist: Wellens syndrome. Q J Med. 2019; 112: 373-4.
- De Zwaan C, Bär FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982; 103: 730-6.
- De Zwaan C, Bär FW, Janssen JH, Cheriex EC, Dassen WR, Brugada P, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowin of the proximal LAD coronary artery. Am Heart J. 1989; 117: 657-65.
- Singh D, Suliman I, Chyshkevych I, Dabage N. A pathognomonic electrocardiogram that requires urgent percutaneous intervention: A case of Wellens syndrome in a previously healthy 55-year-old male. Am J Case Rep. 2019; 20: 117-20.
- Wang X, Sun J, Feng Z, Gao Y, Sun C, Li G. Two case reports of Wellens’ syndrome. J Int Med Res. 2018; 46: 4845-51.
- Udechukwu N, Shrestha P, Khan MZ, Donato AA. Wellens’ syndrome: a close call. BMJ Case Rep. 2018; 2018: bcr2018225376.
- Stankovic I, Kafedzic S, Janicijevic A, Cvjetan R, Vulovic T, Jankovic M, et al. T-wave changes in patients with Wellens syndrome are associated with increased myocardial mechanical and electrical dispersion. Int J Cardiovasc Imaging. 2017; 33: 1541-9.
