Abstract
Background: Acute eosinophilic pneumonia as a paraneoplastic syndrome is an infrequent finding, produces Acute Respiratory Distress Syndrome (ARDS) with a fatal outcome, and is associated with a poor oncological prognosis.
Case Summary: 27-year-old male patient with a history of weight loss three months before admission, asthenia, and night sweats; a month before lymphadenopathy in cervical, inguinal, and axillary lymph node chains, associated with a dry cough that progresses to moderate hemoptysis, with worsening of the symptoms, a requirement for home oxygen (3L), persistence of hemoptysis, and constitutional symptoms, went to the hospital.
The patient was admitted to the ICU with ARDS. After ruling out other pathologies, based on the clinical and tomographic findings, it is considered eosinophilic pneumonia.
Under treatment with corticosteroid therapy, the patient experiences an improvement in his clinical condition.
Conclusion: Acute respiratory failure associated with eosinophilic pneumonia and peripheral eosinophilia constitutes a complex clinical condition that requires diagnostic expertise to guarantee timely treatment. Although they respond favorably to treatment, the presence of these elements in the context of cancer is associated with an unfavorable prognosis.
Keywords: Acute eosinophilic pneumonia; Paraneoplastic syndrome; Paraneoplastic disorder; Respiratory failure; Pulmonary eosinophilia; Seminoma
Core Tip
Acute Respiratory Distress Syndrome (ARDS) caused by paraneoplastic eosinophilic pneumonia in the context of secondary immune response to cancer, especially in the case of seminoma, is an infrequent phenomenon. It manifests itself in both solid and hematological tumors, and its presence is linked to a dismal oncological prognosis. Diagnosing this condition presents significant challenges, requiring rapid and accurate identification to guarantee the prompt implementation of timely treatment. We present the first case of metastatic seminoma in a 23-year-old man admitted to the Intensive Care Unit (ICU) for respiratory failure-type severe ARDS due to eosinophilic pneumonia as the main symptom.
Introduction
Critical oncology patients have increased in recent years [1], and acute respiratory failure is one of the leading causes of admission to the Intensive Care Unit (ICU). It occurs less frequently in solid tumors at 15% [2]. Acute respiratory illness due to eosinophilic pneumonia is rare and of varying severity; it has been described as idiopathic or secondary depending on the presence or absence of a known underlying cause [3]. Identifiable causes include medications, infections, and tobacco exposure [3]. The pathogenesis is poorly known and is characterized by the infiltration of eosinophils into the alveoli and pulmonary interstitium, preserving the architecture. It was described for the first time by Allen et al. in 1989 as a febrile illness with diffuse pulmonary infiltrates and pulmonary eosinophilia [4].
Paraneoplastic syndromes are rare disorders that occur without a direct tumor invasion. Acute eosinophilic pneumonia as a paraneoplastic syndrome is an infrequent finding, produces Acute Respiratory Distress Syndrome (ARDS) with a fatal outcome, and is associated with a poor oncological prognosis [3]. It has been described in both solid and hematological tumors. We present the first case of metastatic seminoma in a 27-year-old man who was admitted to the ICU due to severe acute respiratory distress syndrome-type respiratory failure. The greatest challenge in cancer scenarios is timely diagnosis, which is of vital importance for early corticosteroid-based treatment.
This report aims to publicize a rare pathology in the field of cancer, following the CARE (Consensus-based clinical case report guideline development) writing guide for case reporting [5].
Case Presentation
Chief Complaints
A 27-year-old Ecuadorian male presented to the oncology clinic with persistent respiratory symptoms and constitutional symptoms.
History of the Present Illness
The persistence of hemoptysis and constitutional symptoms led him to the hospital, where he was additionally diagnosed with non-Hodgkin's lymphoma and received corticosteroid oral therapy for a month. He was referred to our health center for comprehensive management of his oncological pathology. On bronchoscopy, he presented acute hypoxemic respiratory failure, the need for invasive mechanical ventilation, and admission to the ICU.
History of Past Illness
Three months ago, with a history of weight loss, asthenia, and night sweats, a month before lymphadenopathy in cervical, inguinal, and axillary lymph node chains associated with a dry cough that progressed to moderate hemoptysis, he went to a doctor who considered starting antibiotic therapy (Ampicillin + Clarithromycin and later Levofloxacin) for 10 days, with worsening of the symptoms and a requirement for home oxygen (3L).
Personal and Family History
The patient denied any family history of malignant tumors.
Physical Examination
On physical examination, the vital signs were as follows: Body temperature 37°C; blood pressure 43/22 mmHg; mean arterial pressure 36 mmHg; heart rate: sinus tachycardia 110 beats per minute; 35 breaths per minute; hypoxemia PO2 40 mmHg; oxygen saturation 60%; crackles scattered in both lung fields. Initial mechanical ventilation parameters were FiO2 0.8, PEEP 8, PaO2/FiO2 50 mmHg.
Laboratory Examination
White blood cells are 36,420/μL, neutrophils 28,210/μL (77.6%), lymphocytes 3,330 /μL (9.1%), monocytes 1,510/μL (4.1%), eosinophils 2630/μL (7.3%), and basophils 180/μL (0.5%). Bronchoalveolar lavage (BAL) through the right middle lobe showed no eosinophils; we believe it is due to his previous corticosteroid treatment. The cytology of BAL fluid did not show malignant cells.
Imaging Examination
The chest x-ray showed disseminated reticulum-alveolar infiltrate in the two lung fields, basal (Figure 1). In the ultrasound hemodynamic assessment, ventricular interdependence is observed with a positive McConnell sign; dilated right ventricle, PSAP of 52 mmHg, and right heart failure was considered. In angiotomography of the chest (Figure 2), pulmonary thromboembolism was ruled out, as was an imaging pattern of micro-nodular lesions in the ground glass in both lung fields, mediastinal lymphadenopathy greater than 2 cm in regions 2R, 4R, 10R, and 7, conglomerates of mediastinal and hilar lymph nodes right, bilateral axillary, pulmonary micronodules, and bone blast lesions.
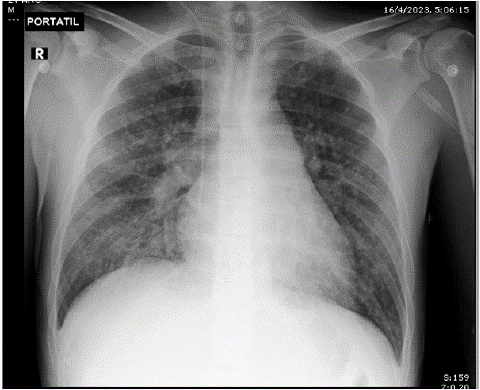
Figure 1: Chest x-ray upon admission to the ICU.
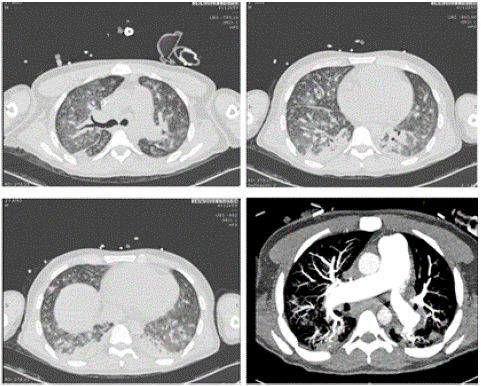
Figure 2: Chest angiotomography on admission to the ICU.
Further Diagnostic Work-Up
The puncture biopsy reports groups of neoplastic cells in background with eosinophilic cytoplasm and polymorphonuclear cells; the cytology of the mediastinal adenopathy regions 7 and 10 R is positive for germ cell tumor type seminoma (Figure 3). Complementary studies are requested to rule out other causes of eosinophilic pneumonia (ANCA, ANA, ANTI CCP, ANTI HTLV 1/2, Rheumatoid Factor, IgG, IgM, IgE, parasite investigation: Echinococcus, Toxocara, Strongyloides IgG/IgM) with negative results.
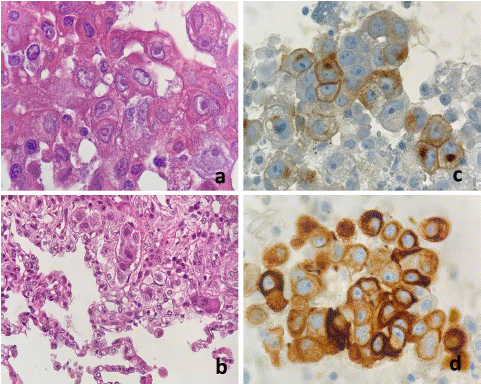
Figure 3: Mediastinal seminoma (a) Large cell nests of polygonal form with defined cell edges, abundant cytoplasm, and central nuclei with prominent nucleoli (H&E staining 60x) (b) Its morphology was like neoplastic infiltration in the lung biopsy specimens (H&E staining 60x) (c,d) immunohistochemically positive for PLAP and Cytokeratin AE1 / AE3.
Final Diagnosis
Since it is an eosinophilic pneumonia as a paraneoplastic syndrome with a known primary, clinical stage III seminoma-type germ cell tumor with lung, brain, and bone metastasis.
Treatment
Given the suspicion of NE, methylprednisolone 500 mg intravenously per day was started. Then first-line oncological treatment for advanced disease is started based on BEP chemotherapy (bleomycin, etoposide, and cisplatin) and continues with corticosteroids (prednisone 60 mg orally for 1 month).
Outcome and Follow-Up
Adequate clinical and radiographic evolution; the control tomography showed decreased alveolar infiltrates (Fig. 4), being discharged to hospitalization.
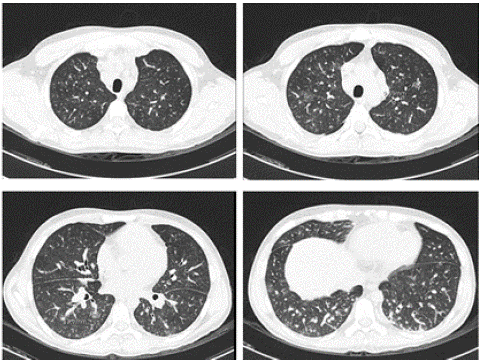
Figure 4: Chest computed tomography on day four of treatment.
The patient was readmitted to the ICU with severe hypoxemia three days after discharge and died.
Discussion
This is the first described case of respiratory failure with ARDS due to eosinophilic pneumonia accompanied by peripheral eosinophilia as a paraneoplastic syndrome in a patient with seminoma. The presence of peripheral eosinophilia in cancer is an atypical manifestation of metastatic disease and is associated with a poor prognosis once other causes of eosinophilia have been ruled out [6]. Peripheral eosinophilia has been described in association with other solid tumors such as thyroid [7,8], breast, lung, gastrointestinal [9,10], hepatocellular [11,12], pancreatic [13], and genitourinary tumors [14,15] and colon [6]. Peripheral eosinophilia and eosinophilic pneumonia as a paraneoplastic syndrome, a rare condition, have been reported in gastric cancer [16], lymphoma [17], prostate carcinoma [18], pulmonary [19,20], colon [6], and angiosarcoma [21] (Table 1).
Author / Characteristics
Horie S (16)
Hirshberg B (17)
Ishiguro T (18)
Kalra A (19)
Verstraeten A (20)
Araujo D (6)
Paraandavaji E (21)
Garcia F
Year
1966
1999
2008
2010
2016 2011
2015
2023
2024
Sex
Male
Female
Man
Female
Male
Male
Male
Male
Age
49
55
80
46
65
53
47
27
Type of malignancy
Gastric cancer
Cutaneous T-cell lymphomas
Prostatic adenocarcinoma
Lung adenocarcinoma
Non-small-cell lung carcinoma
Colon adenocarcinoma
Angiosarcoma
Seminoma
Diagnostics of cancer prior to EN
No
Yes
No
No
No
No
Yes
No
Clinical presentation
Dyspnea, fever, and dry cough (for one month)
Cough and dyspnea (for three months)
Shortness of breath, cough, and fever
Shortness of breath, productive cough, and pleuritic chest pain of 3 weeks duration.
Progressive dyspnea
Dry cough, dyspnea with moderate exercise and general symptoms (5 kg weight loss) lasting one month
Hemoptysis
Dry cough, hemoptysis, asthenia, and night sweats of one month long
Peripheral eosinophils
White blood cells: 12.3 x 109/L, 42% eosinophils (5.12 x 109 cells/L)
White blood cells: 15.26 x 109/L, 38% eosinophils (5.8 x 109 cells/L)
White blood cells: 6.7 x 109/L, 28.8% eosinophils (1.93 x 109 cells/L)
Six percent eosinophils
White blood cells: 16.0 x 109/L, 86.88% eosinophils (13.9 x 109/L)
White blood cells: 13.11 x 109/L, 47.3% eosinophils (6.20 × 109 cells/L)
Fifteen percent eosinophils
White blood cells: 31.71 x 109/L, 7.8% eosinophils (2.47 × 109 cells/L)
Bronchoalveolar lavage fluid
8.35 x 105 cells/mL containing 77% eosinophils
N/R
4.0 x 105 cells/mL containing 73.3% eosinophils
The patient denied
N/R
Severe eosinophilic alveolitis (39%)
Twenty percent eosinophils
N/R
Metastasis
Ribs, sternum, spinal column, and pelvis.
N/R
Thoracic Metastases
N/R
bone metastasis
Liver
No
Lung, brain, and bone
ICU
NR
No
No
Yes
Yes
N/R
No
Yes
Survival
Died 4 months after symptoms began, 3 months after diagnosis.
Follow up for more than 6 months and the patient is alive.
Follow up 4 months, the patient is alive.
Follow up for 6 months and she is alive.
The patient died due to severe hypoxemia.
Died 2 months after diagnosis.
Follow up 3 years, the patient is completely cured.
Died in hospitalization due to severe hypoxemia.
ICU: Intensive Care Unit; N/R: Not Reported, EN: Eosinophilic Pneumonia.
Table 1: Case reports of paraneoplastic eosinophilic pneumonia.
The pathophysiology is poorly understood, but it has been proposed to be related to the stimulation of the bone marrow by IL-5, IL-3, and the colony-stimulating factors G-CSF and GM-CSF produced by the tumor [20].
The clinical presentation is acute and of a short duration of four weeks and, in most cases, less than seven days. In the initial stages, the symptoms may resemble the common flu, with myalgia, headache, nasal congestion, and odynophagia; however, as the disease progresses, respiratory symptoms become predominant. Among the most common are non-productive cough (95%), dyspnea (92%), and fever (88%) [22,23]. Other symptoms and signs may include malaise, night sweats, chills, and pleuritic pain [22,23]. On physical examination, fever, tachypnea, and bibasal inspiratory rales may be found, as well as occasional rhonchi during forced expiration [22,23]. Acute Eosinophilic Pneumonia (AEN) can often lead to hypoxemic respiratory failure and the need for mechanical ventilation in severe cases [22,23]. Rare cases of hyperdynamic shock have been reported [22,23]. No changes in the shape of the fingers (digital clubbing) or signs of cor pulmonale have been reported in this disease [24].
The diagnosis of eosinophilic pneumonia is complex; a good history must be obtained, which excludes a history of asthma, smoking, parasitic infections, HIV, tuberculosis, fungal infections, and hypersensitivity to medications [7,8]. In biometry, there may or may not be an elevated eosinophil count [7,8]. The tomography demonstrates reticular opacities and ground glass patches; pleural effusions, which exudate with 10 to 50% eosinophils, are also described [7,8]. The diagnosis is made with BAL, which demonstrates the presence of eosinophils > 25% and the absence of infection or other causes of pneumonia [7,8].
In this case, no eosinophils were evident due to previous corticosteroid treatment; the diagnosis was made based on the clinical history and tomographic findings, peripheral eosinophilia, and other pathologies.
Eosinophilic pneumonia is a paraneoplastic syndrome; like any eosinophilic pneumonia, treatment with corticoids is essential, but a specific treatment has not been established [6]. Supportive measures should be given with oxygen, antibiotics, and invasive mechanical ventilation [25].
The resolution of symptoms occurs within 12 to 48 hours after treatment with corticosteroids, with rapid improvement and complete resolution of the infiltrates. [9], as happened with our patient. The severity of the clinical picture determines the dosage and duration of treatment [25]. The duration of therapy has not been established. In a retrospective study, Rhee et al. compared the duration of treatment between 2 and 4 weeks, with no significant difference [22]. In patients with severe symptoms who present severe hypoxemia or acute respiratory failure and need mechanical ventilation, as in the case of our patient, high doses of corticosteroids are suggested [6]. It could be managed with corticosteroid pulses or intravenous methylprednisolone, doses between 60 and 125 mg every 6 hours until respiratory failure is overcome, usually in 1 or 3 days [25]. If the condition is milder, prednisone can be administered orally at a dose of 40 – 60 mg each day.
Conclusion
The case described involves a particularly complex clinical context. The discovery of seminoma is complicated by a paraneoplastic syndrome manifested by eosinophilic pneumonia, a clinical condition rarely reported in the scientific literature that responds to prompt treatment with corticosteroids; however, it is a sign of metastatic disease and is associated with a poor prognosis.
Author Statements
Acknowledgements
ANID - MILLENNIUM - NCS2021_013
Informed Consent
Informed written consent was obtained from the patient representative for publication of this report and any accompanying images.
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
CARE Checklist (2016) Statement
The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Author Contributions
EB, HA, WB, DA and MFG contributed to manuscript writing, editing, and data collection; LU, FC contributed to data analysis; NG contributed to conceptualization and supervision; all authors have read and approved the final manuscript.
References
- Sauer CM, Dong J, Celi LA, Ramazzotti D. Improved Survival of Cancer Patients Admitted to the Intensive Care Unit between 2002 and 2011 at a US Teaching Hospital. Cancer Res Treat. 2019; 51: 973–81.
- Azoulay E, Mokart D, Kouatchet A, Demoule A, Lemiale V. Acute respiratory failure in immunocompromised adults. Lancet Respira Med. 2019; 7: 173–86.
- De Giacomi F, Vassallo R, Yi ES, Ryu JH. Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management. Am J Respir Crit Care Med. 2018; 197: 728–36.
- Allen JN, Pacht ER, Gadek JE, Davis WB. Acute eosinophilic pneumonia as a reversible cause of noninfectious respiratory failure. N Engl J Med. 1989; 321: 569–74.
- Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013; 7: 223.
- Araújo D, Meira L, Moreira C, Morais A. Eosinophilic pneumonia as a paraneoplastic manifestation of colon adenocarcinoma. Archives of bronchopneumology. 2016; 52: 224–5.
- Amy D. Treatise on internal medicine. Eosinophilic syndromes. 26th ed. Goldman L, Ausiello DA, Schafer AI, editors. Spain: Elsevier. 2021. 1117–1120.
- Kaul B, Farrand E. Ferri's Clinical Advisor 2024. Eosinophilic Pneumonia. 1st ed. Ferri FF, editor. 2023; 545: E7-545.E10.
- Fridlender ZG, Simon HU, Shalit M. Metastatic carcinoma presenting with concomitant eosinophilia and thromboembolism. Am J Med Sci. 2003; 326: 98–101.
- Uemura K, Nakajima M, Yamauchi N, Fukayama M, Yoshida K. Sudden death of a patient with primary hypereosinophilia, colon tumors, and pulmonary embolism. J Clin Pathol. 2004; 57: 541–3.
- Chang WC, Liaw CC, Wang PN, Tsai YH, Hsueh S. Tumor- associated hypereosinophilia: report of four cases. Changgeng Yi Xue Za Zhi. 1996; 19: 66–70.
- Yuen BH, Reyes CV, Rawal PA, Sosman J, Jensen J. Severe eosinophilia, and hepatocellular carcinoma: an unusual association. Diagnose Cytopathol. 1995; 13: 151–4.
- Hirata J, Koga T, Nishimura J, Ibayashi H. Pancreatic carcinoma associated with marked Eosinophilia: a case report. Eur J Haematol. 1987; 39: 462–6.
- Reddy SS, Hyland RH, Alison RE, Sturgeon JF, Hutcheon MA. Tumor- associated peripheral eosinophilia: two unusual cases. J Clin Oncol. 1984; 2: 1165–9.
- Scherer TA. Tumor Associated Blood Eosinophilia and Eosinophilic Pleural Effusion: Case Report and Review of the Literature. The Internet Journal of Emergency and Intensive Care Medicine. 1996; 1.
- Horie S, Okubo Y, Suzuki J, Isobe M. An emaciated man with eosinophilic pneumonia. Lancet. 1996; 348: 166.
- Hirshberg B, Kramer MR, Lotem M, Barak V, Shustin L, Amir G, et al. Chronicle eosinophilic pneumonia associated with cutaneous T- cell lymphoma. Am J Hematol. 1999; 60: 143–7.
- Ishiguro T, Kimura H, Araya T, Minato H, Katayama N, Yasui M, et al. Eosinophilic pneumonia and thoracic metastases as yet initial manifestation of prostatic carcinoma. Intern Med. 2008; 47: 1419–23.
- Kalra A, Fabius D, Gajera M, Palaniswamy C. Triad of Interstitial Pneumonia, Eosinophilia, and Eosinophilic Pleural Effusion: A Rare Paraneoplastic Manifestation of Lung Adenocarcinoma. Chest. 2010; 138: 1–2.
- Verstraeten AS, De Weerdt A, van Den Eynden G, Van Marck E, Snoeckx A, Jorens PG. excessive eosinophilia as paraneoplastic syndrome in a patient with non - small - cell lung carcinoma: a case report and review of the literature. Clin Belg Act. 2011; 66: 293–7.
- Paraandavaji E, Hadidi H, Norouzi M, Azaddehghan M, Khodaparasti M, Shafiei S, et al. Paraneoplastic acute eosinophilic pneumonia due to carotid angiosarcoma: A rare case. Vol. 11, Clinical case reports. England. 2023; 11: e7348.
- Rhee CK, Min KH, Yim NY, Lee JE, Lee NR, Chung MP, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013; 41: 402–9.
- Simon HU, Plötz SG, Dummer R, Blaser K. Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med. 1999; 341: 1112–20.
- Shorr A, Myers J, Huang D, Nathanson B, Emons M, Kollef M. A risk score for identifying patients with eosinophilic pneumonia at high risks for relapse. Chest. 2013; 144: 1917–23.
- Pahal P, Penmetsa GK, Modi P, Sharma S. Eosinophilic Pneumonia. In Treasure Island (FL). 2023.
