Abstract
Background: Duchenne Muscular Dystrophy (DMD) poses significant challenges, especially in Low- and Middle-Income Countries (LMIC) with limited access to Non-Invasive Mechanical Ventilation (NIV), which prolongs life expectancy. Two years after Stage 1 of the successfull “1500 years of survival” NIV programme commenced with 8 DMD boys in Ukraine in 2021, this study further explores the Stage 2 of the programme in 2023.
Method: DMD patients underwent a 5-day training program at the National Children’s Clinical Hospital “Okhmatdyt” in Kyiv, Ukraine, utilizing donated second-hand bilevel intermittent positive presure ventilators. Indications for NIV included: sleep-related symptoms, high Apnea-Hypopnea Index (AHI>10 events/hour), decreased Forced Vital Capacity (FVC=50%), low minimum saturation during sleep (SpO2<88%), or age above 17 years. Parallel Masterclass on NIV was conducted for Ukrainian healthcare professionals and patients’ parents.
Findings: Thirteen DMD inpatients, aged (mean ± standard deviation) 14.8±2.6 years and FVC of 1.8±0.4L (65.5±22.8% predicted value) were enrolled. Criteria for NIV initiation were: age>17 years (n: 4), FVC% =50% (n :6), AHI>10 (n :2) and SpO2<88% (n :5). Follow-up phone calls were conducted to monitor progress. Three months post-hospitalization, all 13 patients consistently adhered to nocturnal ventilator treatment with a mean use of 7.5 hours per night.
Conclusions: Despite the lack of equipment and system of reimbursement, the implementation of the comprehensive Stage 2 training programme supplying NIV for DMD patients in an LMIC such as in Ukraine, was a successful experience. The next steps in the home NIV programme will take place in the coming years.
Keywords: Developing country; Duchenne muscular dystrophy; Non-invasive ventilation; Home mechanical ventilation; Ukraine
Introduction
Duchenne Muscular Dystrophy (DMD), an X-linked genetic disorder, imposes significant challenges for affected individuals, initially characterized by early manifestations of proximal muscle weakness in childhood. Without intervention, the natural progression leads to wheelchair dependency by the age of 12, with cardiorespiratory complications emerging as a critical factor contributing to mortality during the late teens to early 20s [1]. Advancements in the management of DMD have brought attention to the potential benefits of Non-Invasive Mechanical Ventilation (NIV) and assisted coughing, provided by specially trained physicians and therapists. This approach has shown promise in improving outcomes and enhancing survival for individuals with DMD, surpassing the efficacy of invasive treatment methods [2]. Such interventions have become integral components of care for patients in developed countries grappling with the complexities of this genetic disorder. A comprehensive analysis of DMD patients at the Newcastle Muscle Centre over several decades further underscores the positive impact of NIV. The mean age of death witnessed a remarkable increase from 14.4 years in the 1960s to 25.3 years for those receiving ventilation since 1990. Coordinated care and the incorporation of nocturnal NIV emerged as pivotal factors, contributing to a notable 53% improvement in survival rates for patients ventilated since 1990 [3]. A pioneering study that delves into outcomes for DMD patients receiving 24-hour non-invasive mechanical ventilation revealed a mean survival extending to 31 years [4].
The strategic integration of NIV and cough-augmentation devices holds the promise of reducing the reliance on tracheostomy ventilation and mitigating hospitalizations associated with respiratory infections [5].
However, the availability and accessibility of NIV varies widely across different countries, depending on the resources, expertise, and policies of the health care systems [6]. In Ukraine, the situation of DMD patients is particularly challenging, as there is no state program or reimbursement for NIV, and the patients have limited access to such devices.
In this context, collaborative efforts involving Non-Governmental Organizations (NGO), state and regional authorities, business companies, scientists, and international partners are crucial. To adhere to and implement the guidelines for respiratory management in DMD patients, the NGO Duchenne Ukraine partnered with the author M.T., a Belgian expert in Home Mechanical Ventilation (HMV), and initiated the "1500 Years of Survival" project aimed at developing Home Mechanical Ventilation (HMV) in Ukraine, starting in November 2021 in Kropyvnytskyi city [7]. Indeed, the first step of our initiative involving 8 patients affected by DMD, was successfully conducted [7].
The first step of the project was a success in many respects, as it demonstrated that home mechanical ventilation was feasible for Ukraine’s DMD population. The aim of the second step of this project (Masterclass 2) was to prolong the introduction of NIV in DMD patients across Ukraine, and to evaluate its effectiveness on an even larger scale than in Step 1.
Material and Methods
Patients and Devices
Thirteen individuals diagnosed with DMD from different cities in Ukraine were invited to participate in a five-day hospitalization at the National Children's Clinical Hospital "Okhmatdyt" (Kyiv, Ukraine) in August 2023. Parents were included in this invitation to accompany their sons during the clinical intervention. Agiradom (Meylan, France), a French home health provider, generously provided 45 previously used ResMed S9 Vpap ST ventilators (ResMed, Saint-Priest, France). Complimentary accessories, including breathing circuits and interfaces, were contributed by local offices of UkrTeleMed (Kyiv, Ukraine) and Philips (Murrysville, USA) companies.
Adherence to the ethical principles outlined in the Helsinki Declaration of 1975 was ensured, with written informed consent obtained from both subjects and their parents. Written permission was additionally obtained from all individuals for the use of their photographs in publication. Local ethics approval was considered unnecessary for the successful completion of this project.
Current guidelines advocate for the initiation of NIV in DMD patients during nocturnal hypercapnic hypoventilation to mitigate the risk of uncontrolled respiratory decompensation and mortality [8]. Regrettably, the objective assessment of nocturnal hypoventilation at the National Children's Clinical Hospital "Okhmatdyt" was impeded by insufficient monitoring of blood carbon dioxide pressure (pCO2) during sleep.
Consequently, indications for elective nocturnal NIV were determined based on the available medical information. Criteria for initiation of home noninvasive ventilation included sleep-related symptoms such as morning headache or poor sleep quality, a high Apnea-Hypopnea Index (AHI) above 10 per hour, a decrease in Forced Vital Capacity (FVC) equal or lower than 50%, minimum oxygen saturation (SpO2) during sleep less than 88% or age greater than 17 years [8,9]. The exclusion criterion entailed the absence of DNA testing confirming the diagnosis of DMD.
An additional mini-review search of Medline/PubMed experiences for HMV programmes in Low-and Middle-Income Countries (LMIC), written in English and published between January 2014 and December 2023, was performed.
Step-by-Step Respiratory Device Integration
In the initial phase of the project, physicians introduced respiratory devices to the participants, providing both observational and hands-on experiences. This interactive approach allowed the children not only to understand the functionality of the devices but also to actively engage with them. Following the demonstration, a collaborative decision-making process took place, with patients, alongside medical professionals selecting optimal masks, conducting trial runs of the devices, and configuring preliminary settings in preparation for their first night in the new conditions (Figure 1). Prior to device initiation, patients underwent a comprehensive series of diagnostic procedures. This included chest radiography to evaluate pulmonary and cardiac status, spirometry to quantify respiratory function, and a complete blood count to assess systemic health indicators. Supplementary investigations encompassed blood gas analysis, pulse oximetry, densitometry, and abdominal ultrasound Doppler.
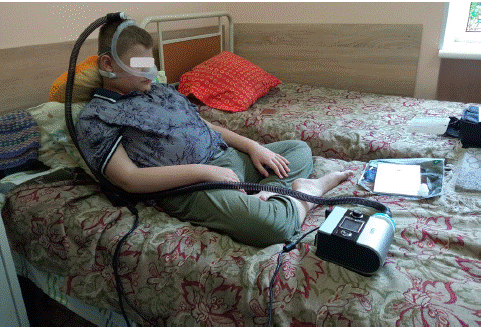
Figure 1: The collaborative decision-making process took place with patients, their families and the healthcare professionals.
On the subsequent day, physicians fostered active communication with the young patients. This included addressing inquiries, providing encouragement, and offering support - crucial elements that significantly contributed to the successful initiation of the project. The observed doctor-patient rapport, built on trust, played a pivotal role in positively influencing the children's reception of device utilization and easing their adaptation to the new conditions.
The third day started with a comprehensive training session led by the author I.T., leader of the project, aimed at fellow doctors and patients' parents. The session delved into the intricate operational principles of respiratory support devices, their composition, and the specific nuances of maintenance. Following this informative session, the physicians returned to the patients, conducting assessments, and adjusting device settings as necessary.
By the fourth day, a majority of patients had successfully acclimated to wearing masks and using ventilators. The medical team continued vigilant monitoring, conducted various assessments, and initiated preparations for patient discharge during the daytime.
The concluding day of the project featured a dynamic question-and-answer session, commencing with the children and later extending to their parents. This platform allowed the children to share their impressions, pose inquiries, and receive comprehensive responses from the medical team providing final clarifications. At the conclusion of the conference, every parent was offered a tailored appointment, meticulously customized to suit the specific needs of their child. This personalized approach ensured that each patient received the utmost attention and care, with parameters carefully selected to address their individual circumstances. In conclusion, the author I.T. presented parents with a cough augmentation device, underscoring its significance in the comprehensive care of patients with DMD.
Project Assessment
In the initial phase of the study, the focus was on assessing the use of NIV equipment, with a primary emphasis on quantifying total usage hours during a four-night hospital stay and an extended three-month home treatment period. The data collection methodology involved thorough follow-up phone calls to participants' homes. Additionally, a secondary outcome was addressed, concentrating on evaluating the resolution of symptoms experienced in the morning following nocturnal NIV use.
Results
In response to the invitation, 13 individuals diagnosed with DMD participated in the study at the National Children's Clinical Hospital "Okhmatdyt" in Kyiv, Ukraine. The mean age of DMD boys commencing NIV was 14.8±2.6 years (Table 1). No patients were excluded from the study. The mean (± standard deviation) weight, height, and body mass index were recorded as 58.4±12.8 kg, 151.1±11.9 cm, and 26,1±7.0, respectively. Prior to study inclusion, four patients underwent lung function tests, revealing a mean FVC of 1.8±0.4L, corresponding to 65.5±22.8% of the predicted FVC value. Mean (± standard deviation) AHI was 5.4±4.1 events/hour and minimum SpO2 was 86.5±4.4%
Patient
Age
Weight
Height
BMI
FVC
FVC
AHI
SpO2 min
#
Years
kg
cm
L
%
event/hour
%
1
16
80
165
29.4
1.54
2.7
91
2
11
45
145
21.4
2.13
89
0.5
87†
3
12
79
148
36.1
2.47
89
10.4†
83†
4
13
56
140
28.6
1.84
79
0.6
85†
5
15
48
168
17
1.94
48†
1.2
91
6
17
60
163
22.6
1.37
37†
8.8
88†
7
18
54
165
19.8
1.8
49†
1.8
90
8
18
50
138
26.3
1.55
60
9.5
82†
9
18
59
132
33.9
1.97
88
10.2†
89
10
13
45
160
17.6
1.67
50†
8.5
88†
11
13
79
142
39.2
1.71
70
8.1
76†
12
12
52
148
23.8
2.46
97
2.7
88†
13
16
52
150
23.1
1.03
30†
NA
NA
Mean
14.8
58.4
151.1
26.1
1.8
65.5
5.4
86.5
SD
2.6
12.8
11.9
7
0.4
22.8
4.1
4.4
BMI: Body Mass Index; FVC: Forced Vital Capacity; FVC %: Forced Vital Capacity in percentage of predicted value. AHI: Apnea-Hypopnea Index; SpO2: pulse oxymetry; NA: Not Available, SD: Standard Deviation; †: indicator for NIV implementation
Table 1: Anthropometric, spirometric and sleep parameters to indicate the need for NIV.
Indicators for the initation of NIV were: age older than 17 years in 4 boys, FVC% equal or lower than 50% in 6 boys, along with recurring headaches before the initiation of NIV. An additional 2 individuals had AHI>10 events/hour, and 5 reached SpO2<88% during sleep. No other subjective complaints were reported by the remaining patients. Seven patients commenced NIV with a facial mask on the first night, while the remaining six opted for nasal masks. During the hospital stay, four patients transitioned from facial to nasal masks.
The mode of ventilation was ‘Spontaneous/Timed’ (S/T) in all patients. Mean (± standard deviation) settings of S9 Vpap ventilators were: Inspiratory Pressure (IPAP): 10.1 ± 2.4 cmH2O; Expiratory Pressure (EPAP): 4.2 ± 0.8 cmH2O; rate: 14.9 ± 0.8 cycles/minute, and inspiratory time varied from 0.8 to 2 seconds in each individual.
After the first night, two patients reported skin redness, one experienced skin irritation, and one felt discomfort due to mask displacement. Additionally, one patient complained of a high leak, and another had difficulties adapting to the mask. However, these symptoms either improved or disappeared after four nights of NIV use.
The initial night of the study demonstrated favorable results, with 7 out of 13 patients achieving a minimum of 7 hours of sleep with NIV. Subsequently, an additional 5 patients successfully overcame initial challenges and adapted to NIV over the following 2 nights, consistently achieving an average of 7.6 hours of nocturnal NIV for the whole group at night 4.
Three months post-hospitalization, all 13 DMD patients consistently adhered to a regular nocturnal ventilator routine (mean 7.5±2 versus 7.5±1.6 hours a day 4 versus month 3, respectively), with patient #5 choosing increasing part-time usage (Table 2).
Patient #
Night 1
Night 2
Night 3
Night 4
Month 1
Month 2
Month 3
1
5
8
9
8
8
8
8
2
3.5
8
9
8
8
8
8
3
8
8
6.5
8
8
8
8
4
8
9
9
9
9
9
8
5
0.6
1
0.3
1
1
2
3
6
9
9
10
9
9
9
8
7
-
8
8
8
8
8
8
8
-
2
8
8
7
7
7
9
5.5
8
8
8
8.5
8.5
8.5
10
7.5
8
8
8
8
7.5
7.5
11
9
7
5
8
8
9
8.5
12
8
9
8
8
7
8
8
13
7
8
8
8
8
8
8.5
Mean
7.7
7.2
8.2
7.6
7.5
7.7
7.5
SD
1.4
2.6
1.3
2
2
1.9
1.6
Table 2: Number of hours of ventilator use during the first trimester after NIV initiation.
The results of the mini-review of recent HMV programmes in LMICs are presented in Table 3. It represents a total of 2199 patients in 13 developing countries whose mean gross domestic product [min-max] per habitant was 10,120 [2,257-26,821] USD. Eight studies targeted a pediatric population, 2 offered invasive ventilation only and 6 started HMV exclusively on the basis of clinical presentation, history, or after a stay in intensive care. The mean number of HMV users by center was 60.9 individuals. The percentage of patients affected by Neuromuscular Disorder (NMD) was 44.4% on average.
Country
Publication Date
GDP in
Patients
Centers
Mean cases
NMD
Start
Reimbursment of HMV
2020 USD
n
n
%
HMV Date
Policy
South Africa (1,2,7)
2016
7,055
55
1
55
60
1994
Public/private, Maldistributed
Turkey (1,7)
2017
9,661
61
1
61
31.1
2001
NA data
Hungary (2)
2018
18,728
384
17
23
11
1990
Public
Tunisia (2,4,5,6)
2018
3,807
27
2
14
NA
2004
NA data
Thailand (2,7)
2019
7,066
12
1
12
42
1995
Public ± 555 USD
Iran (1,2,7)
2019
4,091
67
1
67
31.3
NA
NA data
Malaysia (1,2,7)
2020
11,109
65
1
65
4.6
2001
NA data
Argentina (1,2,3,4,5)
2020
10,636
244
1
244
43
2007
Public in 84%
Serbia (1,2,3,4,5,6)
2020
9,230
105
NA
NA
75
2001
Public with restrictions of disorders and age
Czech Republic (2)
2021
26,821
125
NA
NA
NA
2003
Public (invasive solely)
India (1,2,3,4,5)
2020
2,257
57
NA
NA
68.4
2013
Private
Chile (2,4,5,6)
2021
16,265
1105
NA
NA
21.5
2008
Public 78%/ private
Ukraine (1,2,7)
2022
4,836
8
1
8
100
2021
No reimbursment
Total
2199
24
Mean
10,120
60.9
44.4
Table 3: Surveys on HMV conducted after 2015 in former and current developing countries.
Discussion
The "1500 Years of Survival" project's second phase marks a significant advancement in Duchenne Muscular Dystrophy (DMD) patient care in Ukraine. It demonstrates effective implementation and sustained adherence to NIV among a diverse patient group.
Thirteen DMD boys were offered NIV during a 5-day hospitalization in Kyiv. Criteria for NIV initiation improved as compared to Stage 1 of « 1500 years of survival » in 2021 [7]. Indeed, additional criteria included objective measurements such as: AHI>10 events/hour, FVC=50% and SpO2<88%. After 2015, only 5/13 studies conducted in LMIC used PCO2 measurements as criteria for NIV initiation (Table 3), suggesting that our NIV programme is in line with the majority (7/13) of studies conducted in LMIC. The absence of transcutaneous carbon dioxide monitoring, however, highlights the need for improvement in clinical practice and research.
The unavailability of NIV equipment in Ukraine is consistent with the low number of NIV users at home, internationally. Yet, the project was started in 2021, while the large majority of LMCI started NIV at home before 2010 (10/13), with 3 started before 2000. In addition, Ukraine had, in 2020, a GDP at 4,836 USD as compared to a mean 10,120 USD in the 13 LMCI reported in Table 3, which corresponds to almost 50% of the mean wealth of other LMIC. Finally, 8/13 LMCI in Table 3 reported a system of reimbursement of HMV with variable financial participation by the health services (public versus private, inequality of access to NIV, variables conditions of disorders). One has to remember that Ukraine is still lacking financial support from the authorities. A study in 2022 suggested that, despite the existing linear relationship between the GDP and the prevalence of HMV, the cost per HMV user remains quite low as compared to the costs attributed to inpatients using ventilators in intensive care units [6]. Table 3 also reflects the efforts of LMCI to build their HMV programmes.
In line with a recent review study in non-LMIC [6], Table 3 suggests that 44% of patients starting HMV in LMIC were affected by a Neuromuscular Disorder (NMD). This highlights the high prevalence of the NMD population as potential candidates for HMV. In our study, 100% of patients were affected by NMD (i.e. DMD).
• Interestingly, the 13 studies gave the following main messageHMV is feasible in LMIC despite socio-economic challenges [10]
• HMV allows discharge of stable and dependent patients at home [11]
• There is a need for dedicated HMV centers [12]
• There is a reduction of 83% stay in ICU in the COPD population [13]
• The best strategy: the health-care home visit that significantly improved survival [14]
• HMV is more cost-effective than ICU [15]
• Children on HMV have poorer HRQoL than healthy control [16]
• 1/3 HMV users live >500Kms far from the hospital [17]
• HMV is feasible in LMCI, even when reimbursment is lacking [18]
• HMV is cost-effective [19]
• The key factors for success of HMV are a multidisciplinary approach [20]
• The prevalence of HMV in LMIC is similar to West-Europe [21]
• HMV is feasible in LMCI, even when reimbursment is lacking [7]
Those messages are of high importance. They support the expected widespread use of HMV in LMIC and non-LMIC. In short, the main messages are that HMV is feasible in LMIC, it is cost-effective, HMV costs less than stays in Intensive Care Units (ICU) and reduces the use of ICU.
Clearly, future efforts should focus on integrating more comprehensive diagnostic tools and adaptable protocols to address varying healthcare infrastructure levels.
Feedback underscores the importance of communication and education in medical interventions. Transitioning towards quieter, focused training sessions ensures effective information delivery without distractions.
Looking ahead, exploring long-term outcomes of NIV in DMD, ongoing healthcare provider education, and alternative respiratory support technologies are essential. Research should address effective NIV implementation practices, particularly in low-resource settings, and adapt them to meet patient needs.
Limitations of the Study
The present study is subject to several limitations, primarily arising from the absence of noninvasive Transcutaneous Carbon Dioxide (TcCO2) monitoring in the healthcare facilities in Ukraine. This hindered the precise determination of criteria for initiating Noninvasive Ventilation (NIV). In the absence of this monitoring, medical professionals relied on spirometry results, the loss of ambulation status, and contemporary information regarding the overall health of the patients, to guide the decision-making process. Given the absence of Duchenne muscular dystrophy patients older than 18 years due to respiratory or cardiac insufficiency-related mortality, the medical team opted to include younger patients in the study when criteria were met, with the aim of extending their life expectancy and enhancing their quality of life. Continuous monitoring by medical professionals is in place to reassess the appropriateness and effective setup of NIV. Learning from the challenges encountered in the first phase of the project, where parents and patients found it challenging to focus on ventilator usage amidst a crowded hospital setting with the presence of doctors and media, the learning process in this second phase was meticulously organized in a quieter environment. Noisy training sessions involving doctors and media were limited to fixed periods, while the doctors involved in the study allocated dedicated time to meet with patients and their families, ensuring a thorough clarification of all necessary information.
Conclusion
The implementation of a comprehensive two-stage training programme using NIV for DMD patients in an LMIC such as in Ukraine was a successful experience. The next steps in the home NIV programme will take place in the coming years.
Author Statements
Acknowledgements
The authors thank Deborah Robins, Cairns, Australia, for editing the English manuscript. The author M.T. of this publication is member of the European Reference Network for Neuromuscular Diseases - Project ID N° 870177.
Disclosure
The authors declare no conflict of interest.
Authors Contribution
I.T., M.M. and R.S. designed the study and performed experiments; I.T and M.Y. collected the data; M.T. analyzed the data and M.Y and M.T wrote the manuscript. All authors read and approved the final manuscript.
Ethical Issues
All authors have contributed to the work and agree with the presented findings.
The work has not been published before nor is being considered for publication in another journal.
Trials on human subjects are done in accord with the Helsinki Declaration of 1975.
The current study did not receive financial/material support.
Conflict of Interest
The authors declare to have no potential conflicts.
The authors declare no conflict of interest.
References
- Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. J Paediatr Child Health. 2015; 51: 759-4.
- Ishikawa Y, Miura T, Ishikawa Y, Aoyagi T, Ogata H, Hamada S, et al. Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul Disord. 2011; 21: 47-51.
- Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002; 12: 926-9.
- Toussaint M, Steens M, Wasteels G, Soudon P. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J. 2006; 28: 549-55.
- Simonds AK. Home Mechanical Ventilation: An Overview. Ann Am Thorac Soc. 2016; 13: 2035-44.
- Toussaint M, Wijkstra PJ, McKim D, Benditt J, Winck JC, Nasilowski J, et al. Building a home ventilation programme: population, equipment, delivery and cost. Thorax. 2022; 77: 1140–8.
- Tsarenko A, Trofimov I, Shatillo A, Kostiukova D, Kobylinskyi S, Melnyk S, et al. Mechanical ventilation in Duchenne muscular dystrophy: A pilot project in Ukraine. Pediatr Int. 2022; 64: e15225.
- Finder JD, Birnkrant D, Carl J, Farber HJ, Gozal D, Iannaccone ST, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004; 170: 456-65.
- Hull J, Aniapravan R, Chan E, Chatwin M, Forton J, Gallagher J, et al. British Thoracic Society guideline for respiratory management of children with neuromuscular weakness. Thorax. 2012; 67: i1-40.
- van der Poel LAJ, Booth J, Argent A, van Dijk M, Zampoli M. Home Ventilation in South African Children: Do Socioeconomic Factors Matter? Pediatr. Allergy Immunol Pulmonol. 2017; 30: 163-70.
- Ertugrul A, Baykaci B, Ertugrul I, Kesici S, Yalcin EG. Clinical Evaluation of Invasive Home Mechanical Ventilation Dependent Pediatric Patients. Iran J Pediatr. 2017; 27: e9531.
- Valko L, Baglyas S, Gal J, Lorx A. National survey: current prevalence and characteristics of home mechanical ventilation in Hungary. BMC Pulm Med. 2018; 18: 190.
- Toujani S, Dabboussi S, Snene H, Mjid M, Kamoun S, Hedhi A, et al. Ventilation noninvasive à domicile au cours de la broncho-pneumopathie chronique obstructive [Home non-invasive ventilation for chronic obstructive pulmonary disease]. Rev Pneumol Clin. 2018; 74: 235-41.
- Saiphoklang N, Kanitsap A, Ruchiwit P, Pirompanich P, Sricharoenchai T, Cooper CB, et al. Patient characteristics and outcomes of a home mechanical ventilation program in a developing country. Lung India. 2019; 36: 207-11.
- Hassani SA, Navaei S, Shirzadi R, Rafiemanesh H, Masiha F, Keivanfar M, et al. Cost-effectiveness of home mechanical ventilation in children living in a developing country. Anaesthesiol Intensive Ther. 2019; 51: 35-40.
- Tan LT, Nathan AM, Jayanath S, Peng Eg K, Thavagnanam S, Lum LCS, et al. Health-related quality of life and developmental outcome of children on home mechanical ventilation in a developing country: A cross-sectional study. Pediatr Pulmonol. 2020; 55: 3477-86.
- Leske V, Guerdile MJ, Gonzalez A, Testoni F, Aguerre V. Feasibility of a pediatric long-term Home Ventilation Program in Argentina: 11 years’ experience. Pediatr Pulmonol. 2020; 55: 780-7.
- Basa M, Minic P, Rodic M, Sovtic A. Evolution of Pediatric Home Mechanical Ventilation Program in Serbia-What Has Changed in the Last Decade. Front Pediatr. 2020; 8: 261.
- Gajdoš O, Rožánek M, Donin G, Kamensky V. Cost-Utility Analysis of Home Mechanical Ventilation in Patients with Amyotrophic Lateral Sclerosis. Healthcare (Basel). 2021; 9: 142.
- Kinimi I, Shinde SS, Rao NM. Home Mechanical Ventilation in Children: A 7-year Experience. Indian J Sleep Med. 2020; 15: 46–50.
- Maquilón C, Antolini M, Valdés N, Andrade M, Canales K, Rabec C, et al. Results of the home mechanical ventilation national program among adults in Chile between 2008 and 2017. BMC Pulm Med. 2021; 21: 394.
