
Research Article
J Fam Med. 2015;2(1): 1019.
Therapeutic Impacts of Tocotrienols and Lovastatin against Diabetic Dyslipidemia in a Rat Model
Wahid Ali1*, Pratibha Singh1, Sachil Kumar1, Abhilasha Mishra2, Mohd Wamique1 and Raj Mehrotra1
1Department of Pathology, King George’s Medical University, India
2Department of Biotechnology, Utkal University, India
*Corresponding author: Dr. Wahid Ali, Assistant Professor, P.G. Department of Pathology, King George’s Medical University, India
Received: July 17, 2014; Accepted: January 19, 2015; Published: January 22, 2015
Abstract
Introduction: Diabetic subjects are at an increased risk for developing Coronary Heart Disease (CHD), in part because of enhanced oxidation of low- density lipoproteins (LDL), which promotes atherogenesis. It is possible that increased atherogenecity of LDL during diabetes is associated with a preponderance of small dense (sd)-LDL subpopulation, that is more prone to oxidative modification than large buoyant (lb)-LDL.
Materials and Methods: In this study, we have investigated the hypolipidemic and antioxidant properties of dietary tocotrienols (Tocomin) and Lovastatin in diabetic-hyperlipidemic rats. In order to induce experimental diabetes, 28 overnight fasted rats were injected with streptozotocin (STZ), (freshly dissolved in 10 mM citrate buffer, pH 4.5, 6.0 mg/100 g body wt) intraperitonially.
Results: After 14 weeks of treatment with 6.0 mg Tocomin or 0.50 mg Lovastatin, diabetic control rats had a significant increase in plasma glucose and blood HbA1c, plasma TG, TC, VLDL-C, LDL-C, atherogenic non-HDL-C, while significant reduction in HDL-C, HDL2-C, HDL3-C. Tocomin and Lovastatin mediated a substantial decline in plasma and lipoprotein lipids without any significant change in plasma glucose, HbA1c and HDL-C, HDL2-C, HDL3-C, levels. Diabetes markedly increased the cholesterol and apoB content of sd- LDL including their percent share of LDL, which were significantly reduced in Tocomin or Lovastatin treated rats.
Conclusion: In the present investigation we have shown that the treatment of chronic diabetic rats with Tocomin or Lovastatin mediated a decline in blood glucose and HbA1 levels close to normal values. These results imply that there is a significant association between improved glycemic control and Tocomin or Lovastatin. Although a detailed investigation is needed to elucidate the possible mechanism(s) involved, it is intriguing to postulate that both Tocomin and Lovastatin being potent antioxidants may have effectively protected the β-cells from total damage by STZ and/or glucotoxicity.
Keywords: Tocotrienols; Hyperlipidemia; Lovastatin
Introduction
Hyperglycemia is the most important factor in the onset and progress of diabetic complications mainly by producing oxidative stress [1]. Although blood glucose is known to be highly predictive of micro vascular disease, the contribution of all the measured risk factors can explain no more than 25% of the excess macro vascular Coronary Heart Disease (CHD) associated with diabetes [2]. The excessive non-enzymatic glycosylation of proteins associated with markedly increased free radical production, stimulate formation of glycosylated haemoglobin and advanced glycosylation end products, which cause extensive cellular and tissue damage, including vascular injury [3]. The dyslipidemic profile of diabetics includes increased levels of plasma TG, TC, VLDL-C, LDL-C and sd-LDL-C, increased glycation of LDL and decreased plasma antiatherogenic HDL concentrations [4]. Previous reports indicate that altered plasma lipoprotein profile in the excess atherosclerosis associated with diabetes mellitus (DM) may be most critical, because at any total cholesterol level, in comparison to non diabetic subjects, diabetic patients have 3-to 5-fold higher CHD mortality rates [5]. In addition, 80% of all type 2 diabetics will die of an atherosclerotic event [4, 6]. It is possible that increased atherogenecity of LDL during DM is associated with a preponderance of sd-LDL subpopulation, that is more prone to oxidative modification than large buoyant (lb)-LDL [7]. Lipoprotein profiles that are relatively rich in sd-LDL particles are associated with up to 3-fold greater risk of myocardial infarction than those mainly consisting of lb-LDL particles [8]. Recently, Koba et al. [9] have reported that prognosis of CHD was closely linked not to the LDL particle size but to the concentration of highly atherogenic sd-LDL. Tocotrienols are found in certain cereals and vegetables such as palm oil, rice bran oil, coconut oil, barley germ, wheat germ and annatto [10, 11]. Palm oil and rice bran oil contain particularly higher amounts of tocotrienols (940 mg/kg and 465 mg/kg, respectively) [12]. Other sources of tocotrienols include grape fruit seed oil, oats, hazelnuts, maize, olive oil, Buckthorn berry, rye, flax seed oil, poppy seed oil and sunflower oil. Tocotrienols are thought to have more potent antioxidant properties than α-tocopherol [13, 14]. The unsaturated side chain of tocotrienol allows for more efficient penetration into tissues that have saturated fatty layers such as the brain and liver [15]. The multiple protective efficacies of Tocomin and Lovastatin on plasma TG, TC, VLDL-C, LDL-C, HDL-C, sd- LDL-C, lb-LDL-C; including non-HDL-C was investigated. In addition, quantification of cholesterol and apoB content in LDL and its subpopulation, sd-LDL and lb-LDL of normal, diabetic control and diabetic-hyperlipidemic rats treated either with Tocomin or Lovastatin has been done. Therapeutic role of Tocomin and Lovastatin in the amelioration of the above parameters was investigated.
Materials and Methods
Chemicals
Twenty five percent palmvitae oil suspension of tocotrienols containing d-α-tocopherol and purified individual d-α-tocotrienol (80%), d-γ- tocotrienol (90%), d-δ-tocotrienol (60%), and d-α- tocopherol (60%) as well as Refined bleached Deodorized (RBD) palm olein was supplied as a gift from CAROTECH BHD, Chemor, Malaysia. TocominR suspension (250 mg/g) contained 6.4% d-α- 1% d-β-tocotrienol, 10.2% d-γ-tocotrienol, 3.2% d-δ- tocotrienol and 5.7% d-α-tocopherol. Lovastatin was a gift from Saimira Innoform Pvt. Ltd., Chennai, India.
Animals/treatment
Male albino rats, weighing about 200-220g were conditioned to animal house environment prior to the experiment. The protocol of the study was approved by the animal ethical committee of the J N Medical College. The rats were given pelleted rat chow and water ad libitum. In order to induce experimental diabetes, 28 overnight fasted rats were injected with streptozotocin (STZ, freshly dissolved in 10 mM citrate buffer, pH 4.5, 6.0 mg/100 g body wt) intraperitonially. Rats in normal control group were injected with buffer only. After 12 days, 26 rats showed an average plasma glucose level of 257 mg/ dl. These rats were classified as diabetic and included in the present investigation. Tocomin and Lovastatin suspension in palmvitae oil was administered through gastric intubation in two divided doses (morning and evening) of 0.5 ml each/rat/day, containing 3.0 mg Tocomin or 0.25 mg Lovastatin.
Experimental design
In normal control group (N-C), eight rats were given 0.5 ml palmvitae oil for 14 weeks. Eight rats in diabetic control group (D-C) were administered 0.5 ml palmvitae oil. In diabetic Tocomin treated group (D-TT), eight rats were given 6.0 mg of Tocomin, whereas, eight rats in diabetic Lovastatin treated group (D-LT) were fed 0.50 mg of Lovastatin for 14 weeks. At the end of the treatment, overnight fasted rats in each group were anaesthetized and blood drawn by cardiac puncture. Blood was collected in heparinised tubes and plasma was prepared.
Measurement of glucose, glycosylated haemoglobin and lipids
Quantification of fasting plasma glucose, TG levels and glycosylated haemoglobin (HbA1c) in erythrocytes was done according to the standard procedures as described in commercial kits. Plasma VLDL-C was determined as described by Friedewald et al. [16]. Plasma LDL was isolated by precipitation method as described by Wieland and Seidel [17]. Sd-LDL and lb-LDL subfractions were isolated as described by Hirano et al [7]. Isolation of HDL and its subfractions, HDL2 and HDL3 were done by dual-precipitation method [18]. Total cholesterol content in plasma, LDL, sd-LDL, lb- LDL, HDL, HDL2 and HDL3 subfractions were determined by the method of Annino and Giese [19].
Determination of free radical scavenging activity (antioxidant capacity) of Tocomin, α-tocotrienol, γ-tocotrienol, δ-tocotrienol, α-tocopherol and Lovastatin
Antioxidant estimation: Free radical scavenging activity of Tocomin, α-T3, γ-T3, δ-T3, α-T and Lovastatin was determined by the method of Mellors and Tappel (1966) as modified by Khanduja and Bhardwaj (2003). The assay was carried out in a medium containing 40 mM tris buffer, pH 7.4 and 125μM ethanolic solution of 2, 2-diphenyl-1-picryl hydrazyl (DPPH). The reaction was started by the addition of ethanolic solution of Tocomin, α-T3, γ-T3, δ-T3, α-T and Lovastatin (5-100 μM) in a total volume of 2.0 ml. The samples were mixed thoroughly and the absorbance was recorded in dark at 517 nm (27±2°C) at 1 min time interval up to 10 min against absolute ethanol. A control blank containing all the above ingredients except the test compounds was used in order to monitor the absorption of DPPH. The percent inhibition of the DPPH by the above antioxidants was calculated according to the formula reported by Yen and Duh (1994).
Protein estimation: The protein content in plasma and lipoprotein fractions was determined by the method of Bradford [20], using bovine serum albumin as standard.
Statistical evaluation
The data was entered in Microsoft Excel sheet and checked for any inconsistency. The descriptive statistics (mean± sd) and average percent change from baseline to 14 weeks of treatment were analysed. An analysis of covariance with the baseline value as the covariate was done on post-treatment changes for each parameter studied. Bonferroni corrections were done to adjust for multiple comparisons. Statistical significance was accepted at a probability level of 0.05.
Results
The baseline and post supplementation of Tocomin and Lovastatin on plasma and lipoprotein lipids are shown in Table1. In comparison with the baseline values, total antioxidant, HDL-C, HDL2-C, HDL3-C levels significantly decreased in D-C, D-TT and D-LT treated groups and were significantly different from N-C. The post treatment total antioxidant, HDL-C, HDL2-C, HDL3-C levels were lower in D-C, D-TT and D-LT than N-C. However, after treatment with Tocomin and lovastatin for 14 weeks, the cholesterol content of TG, TC, VLDL, LDL, non-HDL significantly increased in D-TT and D-LT treated groups compared with the baseline. HBA1C was also significantly increased in treated group as compared to N-C.
Figure 1 demonstrates the average percentage decrease in antioxidant, HDL-C, HDL2-C, HDL3-C levels from baseline to 14weeks. The average percentage decrease in total antioxidant was significantly higher in D-C (41.4%, 95% CI=39.5-43.4) as compared to D-TT (33.8%, 95% CI=28.5-39.0), N-C (2.4%, 95% CI=1.7-3.1) and D-LT (1.1%, 95% CI=0.6-1.5). The average percentage in HDL was also significantly higher in D-C (16.9%, 95% CI=16.5-17.3) when compared with D-TT (12.8%, 95% CI=11.8-13.7), D-LT (8.3%, 95% CI=7.8-8.7) and N-C (0.4%, 95%CI=0.1-0.6). The average percentage decrease in HDL2-Cholesterol was <10% in DC (6.4%, 95% CI=2.3- 10.5), D-TT (3.5%, 95% CI=1.5- 5.6), D-LT (3.1%, 95% CI=1.5-4.7) and N-C (1.8%, 95% CI= -0.6-4.1). Similar observation was found for HDL3- Cholesterol [D-C: 9.3% 95% CI=8.6-10.0), D-TT: 6.9% (95% CI=2.9-10.9), DLT: 5.7% (95% CI=2.9-8.5) and N-C: 2.1% (95% CI=1.2-3.0)].
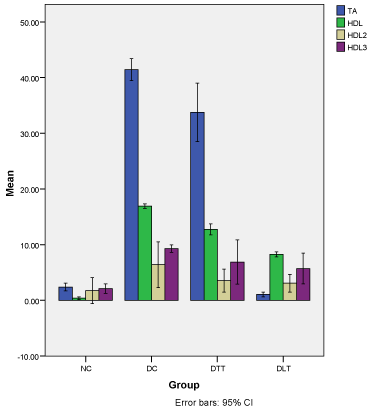
Figure 1: Percent decrease in plasma and lipoprotein lipids.
Demonstrates the average percentage decrease in antioxidant, HDL-C,
2, HDL3-C levels from baseline to 14 weeks. The average percentage
decrease in total antioxidant was significantly higher in D-C (41.4%, 95%
CI=39.5-43.4) as compared to D-TT (33.8%, 95% CI=28.5-39.0), N-C (2.4%,
95% CI=1.7-3.1) and D-LT (1.1%, 95% CI=0.6-1.5). The average percentage
in HDL was also significantly higher in D-C (16.9%, 95% CI=16.5-17.3) when
compared with D-TT (12.8%, 95% CI=11.8-13.7), D-LT (8.3%, 95% CI=7.8-
8.7) and N-C (0.4%, 95%CI=0.1-0.6). The average percentage decrease in
2holesterol was <10% in DC (6.4%, 95% CI=2.3-10.5), D-TT (3.5%,
95% CI=1.5- 5.6), D-LT (3.1%, 95% CI=1.5-4.7) and N-C (1.8%, 95% CI=
-0.6-4.1). The similar observation was found for HDL3- Cholesterol [D-C:
9.3% 95% CI=8.6-10.0), D-TT: 6.9% (95% CI=2.9-10.9), DLT: 5.7% (95%
CI=2.9-8.5) and N-C: 2.1% (95% CI=1.2-3.0)].
Figure 2 demonstrates the average percentage increase in TG, TC, VLDL-C, LDL-C and non-HDL-C levels from baseline to 14 weeks. The average percentage increase in TG was 42.8% (95% CI=42.3-43.2) in D-C group, 20.7% (95% CI=19.7-21.6) in D-TT, 10.8% (95% CI=9.7-11.9) in D-LT and 0.4% (95% CI= -0.4-0.7) in N-C group. Almost similar pattern was found for increase in the level of TC, VLDL-C, LDL-C and non-HDL-C from baseline to 14 weeks. The increase in HBA1C from baseline to 14 weeks treatment was also almost similar in D-C (54%, 95%CI=52.6-55.5), D-LT (53.3%, 95%CI=49.1-57.5) and D-LT (56.4%, 95% CI=51.3-61.5). However, in N-C group, there was small increase in HBA1C level (5.2%, 95%CI=0.6-9.8). The increase in HBA1C was significantly (p<0.0001) higher in D-LT group as compared to D-C, D-TT and N-C group (Table1).
Parameters
N-C (n=8)
D-C (n=8)
D-TT (n=8)
D-LT (n=8)
Total antioxidants (µmole/dl)
Baseline
51.54±0.82
52.36±0.92
52.56±0.99
52.60±1.05
14 Week
50.35±0.94
37.03±0.841,2,4,5
51.94±1.881,3
54.49±2.041,2,3
Triglycerides
Baseline
91.67±0.68
92.75±1.00
92.84±1.12
92.84±1.25
14 Week
92.01±0.43
162.00±0.511,2,4,5
117.01±0.601,2,3,5
104.07±0.591,2,3,4
Total cholesterol
Baseline
138.74±0.42
138.53±0.15
138.63±0.33
138.55±0.15
14 Week
139.09±0.38
246.05±0.491,2,4,5
176.67±0.321,2,3,5
159.30±0.121,2,3,4
VLDL-cholesterol
Baseline
18.08±0.40
18.28±0.43
18.14±0.55
17.98±0.31
14 Week
18.42±0.58
32.37±0.901,2,4,5
23.36±0.361,2,3,5
20.94±0.331,2,3,4
LDL-cholesterol
Baseline
97.78±0.54
98.40±0.36
97.91±0.50
98.00±0.52
14 Week
98.93±0.57
172.40±0.141,2,4,5
123.85±0.181,2,3,5
111.21±0.051,2,3,4
HDL-cholesterol
Baseline
33.12±0.05
33.58±0.17
32.97±0.36
33.06±0.04
14 Week
33.00±0.10
28.72±0.051,2,4,5
29.24±0.041,2,3,5
30.54±0.161,2,3,4
HDL2-cholesterol
Baseline
8.98±0.43
8.86±0.42
9.30±0.20
9.36±0.19
14 Week
8.66±0.39
8.33±0.091,2,4,5
8.98±0.111,2,3,5
9.08±0.091,3,4
HDL3-cholesterol
Baseline
22.21±0.38
22.47±0.15
21.92±0.74
22.16±0.36
14 Week
21.75±0.48
20.56±0.111,2,4
20.53±0.521,2,3
20.98±0.481,3,4
Non-HDL-cholesterol
Baseline
106.12±0.05
105.96±0.52
106.06±0.59
105.44±1.08
14 Week
106.26±0.08
217.78±0.191,2,4,5
147.46±0.111,2,3,5
128.76±0.071,2,3,4
Blood Sugar
Baseline
82.61±1.65
86.86±2.60
84.36±2.83
88.07±2.41
14 Week
84.34±1.44
329.94±1.971,2,4,5
320.44±2.861,2,3,5
322.74±3.141,3,4
HBA1C
Baseline
3.11±0.03
3.90±0.10
3.72±0.39
3.61±0.42
14 Week
3.29±0.20
8.50±0.281,2,4
7.97±0.371,2,3
8.31±0.291
Body Weight
Baseline
381.07±3.31
375.65±7.31
373.07±23.41
379.83±3.51
14 Week
385.51±2.70
205.68±9.261,2,4,5
196.16±3.571,2,3
196.27±5.151,3,4
HMG-Co A reductase†
Baseline
3.74±0.86
4.06±0.64
4.07±0.53
3.98±0.63
14 Week
4.45±0.49
7.21±0.571,2,4,5
5.07±0.541
4.67±0.371
1Significantly different from baseline, p<0.0001 (Bonferroni’s correction)
2Significantly different from NC, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
3Significantly different from DC, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
4Significantly different from D-TT, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
5Significantly different from D-LT, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
Table 1: Impact of Tocomin and Lovastatin on plasma and lipoprotein lipids.
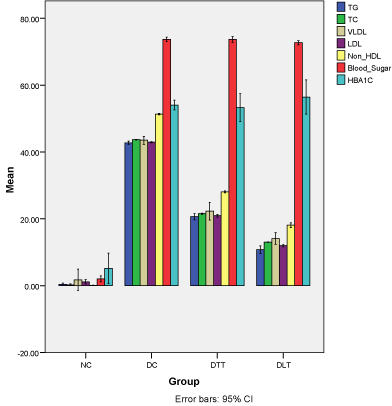
Figure 2: Percent increase in plasma and lipoprotein lipids.
Demonstrates the average percentage increase in TG, TC, VLDL-C, LDL-C
and non-HDL-C levels from baseline to 14 weeks. The average percentage
increase in TG was 42.8% (95% CI=42.3-43.2) in D-C group, 20.7% (95%
CI=19.7-21.6) in D-TT, 10.8% (95% CI=9.7-11.9) in D-LT and 0.4% (95%
CI= -0.4-0.7) in N-C group. Almost similar pattern was found for increase in
the level of TC, VLDL-C, LDL-C and non-HDL-C from baseline to 14 weeks.
The increase in HBA1C from baseline to 14 weeks treatment was also almost
similar in D-C (54%, 95% CI=52.6-55.5), D-LT (53.3%, 95% CI=49.1-57.5)
and D-LT (56.4%, 95% CI=51.3-61.5). However, in N-C group, there was
small increase in HBA1C level (5.2%, 95% CI=0.6-9.8). The increase in
HBA1C was significantly (p<0.0001) higher in D-LT group as compared to
D-C, D-TT and N-C group (Table-1).
Table 2 shows the effect of Tocomin and Lovastatin on cholesterol and apoB content of LDL, sd-LDL and Lb-LDL levels from baseline to 14 weeks of treatment. The LDL-apoB, sd-LDL-C, %LDL-C sd- LDL-apoB, %LDL-apoB %LDL-apoB, Lb-LDL-C were significantly increased from baseline in treated groups. However, Lb-LDL-apoB was significantly decreased from baseline to 14 weeks of treatment.
N-C (n=8)
D-C (n=8)
D-TT (n=8)
D-LT (n=8)
LDL-apoB
Baseline
132.82±1.04
133.66±1.36
132.80±1.05
133.88±1.28
14 Week
133.64±0.97
148.77±0.641,2,4,5
135.28±1.631,3,5
139.33±1.851,2,3,4
Sd-LDL-C
Baseline
29.89±0.68
30.97±1.24
29.74±1.01
31.01±1.20
14 Week
31.54±0.91
108.79±0.881,2,4,5
51.60±0.871,2,3,5
47.94±0.851,2,3,4
% LDL-C
Baseline
31.10±1.04
31.38±1.05
31.34±1.24
31.38±1.05
14 Week
31.89±0.70
63.03±1.251,4,5
41.55±0.631,2,3,5
43.07±1.641,2,3,4
Sd-LDL-apoB
Baseline
34.67±1.65
35.91±1.04
34.47±1.41
36.10±1.22
14 Week
36.42±1.11
92.42±0.961,2,4,5
51.83±0.691,2,3,5
54.71±1.121,2,3,4
% LDL-apoB
Baseline
26.22±1.03
26.93±1.11
26.54±1.25
26.33±1.19
14 Week
27.22±0.97
62.08±1.231,2,4,5
38.30±1.511,2,3,5
44.05±0.851,2,3,4
Lb-LDL-C
Baseline
61.66±1.10
63.58±1.06
63.30±1.27
62.97±0.92
14 Week
65.24±0.88
65.29±0.681,4,5
70.07±0.651,2,3,5
60.80±0.671,2,3,4
Lb-LDL-apoB
Baseline
90.68±0.58
90.70±0.66
90.79±0.66
90.78±0.36
14 Week
89.84±0.78
54.64±0.701,2,4,5
80.49±0.981,2,3,5
70.66±0.761,2,3,4
1Significantly different from baseline, p<0.0001 (Bonferroni’s correction)
2Significantly different from NC, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
3Significantly different from DC, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
4Significantly different from D-TT, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
5Significantly different from D-LT, p<0.0001 (Bonferroni’s correction, ANCOVA with baseline value as the covariate)
Shows the effect of Tocomin and Lovastatin on cholesterol and apoB content of LDL, sd-LDL and Lb-LDL levels from baseline to 14 weeks of treatment. The LDL-apoB, sd-LDL-C, %LDL-C sd-LDL-apoB, %LDL-apoB %LDL-apoB, Lb-LDL-C were significantly increased from baseline in treated groups. However, Lb-LDL-apoB was significantly decreased from baseline to 14 weeks of treatment.
Table 2: Effect of Tocomin and Lovastatin on cholesterol and apoB content of LDL-C, sd-LDL and lb-LDL*.
Figure 3 illustrates the percent increase/decrease from baseline to 14 weeks of treatment in cholesterol and apoB concentration of LDL sd-LDL and lb-LDL. The percentage increase in LDL-apoB was higher in D-C group (10.2%, 95% CI=9.2-11.1), than DLT (3.9%, 95% CI=2.3-5.4), D-TT (1.8%, 95% CI=0.8-2.8) and N-C group (0.6%, 95% CI= -0.1-1.3). Almost similar pattern was seen in other parameters.
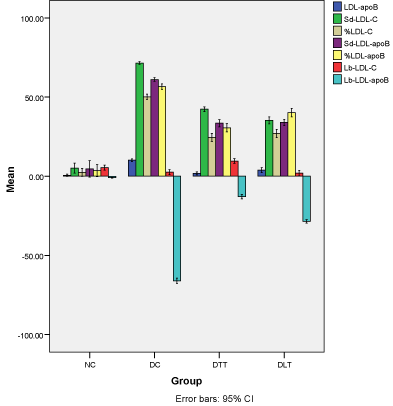
Figure 3: Average percent change in cholesterol and apoB content of LDL,
sd-LDL and lb-LDL.
Illustrates the percent increase/decrease from baseline to 14 weeks of
treatment in cholesterol and apoB concentration of LDL sd-LDL and lb-LDL.
The percentage increase in LDL-apoB was higher in D-C group (10.2%, 95%
CI=9.2-11.1), than DLT (3.9%, 95% CI=2.3-5.4), D-TT (1.8%, 95% CI=0.8-
2.8) and N-C group (0.6%, 95% CI= -0.1-1.3). Almost similar pattern was
seen in other parameters.
Discussion
The present study demonstrates that daily treatment of diabetic rats with either 6.0 mg dietary tocotrienols or 0.50 mg Lovastatin for 14 weeks did not influence the elevated fasting blood glucose and HbA1c levels in diabetic rats. Nazaimoon and Khalid [21] have initially reported that feeding a diet supplemented with Tocotrienols rich fraction (TRF) (1g/kg body weight) to STZ-induced diabetic rats for 12 weeks was associated with some reduction of blood glucose (32%) and HbA1c (24%) levels. Although, in principle, our results are in agreement with their findings but the hypoglycemic effect of Tocomin and Lovastatin was minimal, both the glucose and HbA1c levels after treatment were fully within the diabetic range [21]. In another report [22], feeding of vitamin E (d-l-α-tocopheryl acetate), probucol or Lovastatin supplemented diet to hyperlipidemic-diabetic hamsters failed to reduce elevated fasting blood glucose which is consistent with our findings. Our results appear to be inconsistent with an earlier study, where Vitamin E (d-α-tocopherol) could also exert its protective effect indirectly by reducing free radical mediated damage to islet of β-cells and thus improving insulin action leading to normalization of glycemic state [23]. As expected, treatment of D-C rats with Tocomin or Lovastatin significantly prevented the increase in plasma TG, TC, VLDL-C, LDL-C, including atherogenic non-HDL-C levels without any significant change in HDL-C and its subfractions, HbA2c and HbA3c. However, Lovastatin was more effective in selectively reducing the atherogenic non-HDL-C. Our results are consistent with earlier findings in type 2 diabetics [24] as well as hyperlipidemic diabetic hamster [22] where decrease in plasma HDL-C is exhibited. Analysis of LDL density subfractions in D-C revealed a substantial increase of 245% in sd-LDL-C with no change in lb-LDL-C. An increase >60% of LDL-C and LDL-apoB were recognized in sd-LDL subpopulation of diabetic rats. Both Tocomin and Lovastatin were equally effective in significantly reducing the cholesterol and apoB content of sd-LDL as well as their percent share of LDL. Most interestingly, Tocomin or Lovastatin treatment altered the LDL size profile, with a shift from more atherogenic sd- LDL to less atherogenic lb-LDL. Our results are consistent with a recent report [25], where administration of 1mg pitavastatin to type 2 diabetic patients for 3 months significantly reduced both LDL and sd-LDL. Mechanism wise, similar to pitavastatin [26, 27], induction of LDL receptors by tocotrienols or Lovastatin may stimulate the uptake of LDL and sd-LDL particles with a minimum effect on lb-LDL particle. Alternatively, similar to potent induction of LDL receptors by 2 mg pitavastatin, high dose of tocotrienols or Lovastatin may preferentially facilitate the removal of sd-LDL particles [27]. The efficient and preferential removal of sd-LDL than lb-LDL by Tocomin and Lovastatin may also be related to precursor product relationship. Triglyceride rich large VLDL1 and smaller VLDL2 are known to be a precursor of sd-LDL and lb-LDL22. As shown for fibrate [28], tocotrienols and Lovastatin may also reduce sd-LDL particles by suppressing the production of VLDL1 and stimulating the transfer from VLDL1 to VLDL2 via the activation of lipoprotein lipase. Consistent with these reports, our results show a concomitant decrease of both TG and sd-LDL in D-TT and D-LT rats. Thus, the specific decrease in sd-LDL-C without affecting lb-LDL-C implied significant increases in LDL buoyancy. However, present results indicate that the mechanism(s) of their cholesterol lowering effect in D-C rats may be quite different. Support for the existence of such a mechanism is obtained from a recent report [29], where rice bran oil containing γ-tocotrienol or γ-oryzanol have been shown to exert their hypolipidemic effects in diabetic rats by increasing fecal neutral sterol and bile acid excretion, via up regulating cholesterol synthesis and catabolism [29]. In the present study involving diabetic-hyperlipidemic rats, tocotrienols and Lovastatin may exert their hypolipidemic effects in a similar fashion. The combined results demonstrate a strong hypolipidemic effect of dietary tocotrienols and Lovastatin coupled with their potent antioxidative properties can provide additional benefit in the inhibition of oxidative stress,particularly in the resistance of highly atherogenic sd-LDL and hence in the prevention and treatment of diabetes linked hyperlipidemia with and without CHD and atherosclerosis. However, considering the host of side effects exhibited by Lovastatin [4, 30], use of dietary Tocomin as a multi therapeutic agent should be preferred. In addition, daily use of Tocomin as a dietary supplement will be cost effective as well as a good source of vitamin E.
Acknowledgements
We are thankful to the Vice Chancellor and Registrar of Aligarh Muslim University for funding the work as central university scholarship and to Professor Z.H. Beg for his guidance. The authors declare there is no conflict of interest.
References
- Giugliano D, Ceriello A, Paolisso G . Oxidative stress and diabetic vascular complications. Diabetes Care. 1996; 19: 257-267.
- Pyörälä K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987; 3: 463-524.
- Lyons TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes? Diabet Med. 1991; 8: 411-419.
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999; 100: 1134-1146.
- Stamler, J. 1987. Epidemiology, established major risk factors, and the primary prevention of coronary heart disease. In: Parmley, W. W., Chatterjee, K. eds. Cardiology. Philadelphia, Pa: J. B. Lippincott; p. 1-41.
- Garber AJ. The touch that kills. Clin Diabetes 1995; 3: 57-58.
- Hirano T, Ito Y, Koba S, et al. Clinical significance of small dense lowdensity lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler Thromb Vasc Biol 2004; 24: 558-563.
- Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996; 276: 875-881.
- Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, et al. Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis. 2006; 189: 206-214.
- Qureshi AA, Mo H, Packer L, Peterson DM. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem. 2000; 48: 3130-3140.
- Tan B, Brzuskiewicz L. Separation of tocopherol and tocotrienol isomers using normal- and reverse-phase liquid chromatography. Anal Biochem. 1989; 180: 368-373.
- Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010; 80: 1613-1631.
- Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991; 10: 263-275.
- Serbinova EA, Packer L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994; 234: 354-366.
- Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, et al. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993; 32: 10692-10699.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18: 499-502.
- Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. J Lipid Res. 1983; 24: 904-909.
- Patsch W, Brown SA, Morrisett JD, Gotto AM Jr, Patsch JR. A dual-precipitation method evaluated for measurement of cholesterol in high-density lipoprotein subfractions HDL2and HDL3 in human plasma. Clin Chem. 1989; 35: 265-270.
- Annino JS, Giese, RW. 1976 In: Clinical Chemistry. Principles and Procedures, IV Ed. Little, Brown and Company, Boston. p. 268.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248-254.
- Nazaimoon WM, Sakinah O, Gapor A, et al. Effects of olein tocopherol and tocotrienol on lipid peroxidation, lipid profiles and glycemic control in noninsulin diabetes mellitus patients. Nutrition Research 1996; 16: 1901-1911.
- El-Swefy S, Schaefer EJ, Seman LJ, van Dongen D, Sevanian A, Smith DE, et al. The effect of vitamin E, probucol, and lovastatin on oxidative status and aortic fatty lesions in hyperlipidemic-diabetic hamsters. Atherosclerosis. 2000; 149: 277-286.
- Wan Nazaimoon WM, Khalid BA. Tocotrienols-rich diet decreases advanced glycosylation end-products in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays J Pathol. 2002; 24: 77-82.
- Baliarsingh S, Beg ZH, Ahmad J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis. 2005; 182: 367-374.
- Tokuno A, Hirano T, Hayashi T, Mori Y, Yamamoto T, Nagashima M, et al. The effects of statin and fibrate on lowering small dense LDL- cholesterol in hyperlipidemic patients with type 2 diabetes. J Atheroscler Thromb. 2007; 14: 128-132.
- Saito Y, Teramoto, T, Yamada N, et al. Clinical efficacy of NK-104 (pitavastatin), a new synthetic HMG-CoA reductase inhibitor, in the doswe finding, double blind, three group comparative study. Rishoulyaku 2001; 17:829-855.
- Sone H, Takahashi A, Shimano H, Ishibashi S, Yoshino G, Morisaki N, et al. HMG-CoA reductase inhibitor decreases small dense low-density lipoprotein and remnant-like particle cholesterol in patients with type-2 diabetes. Life Sci. 2002; 71: 2403-2412.
- Milosavljevic D, Griglio S, Le Naour G et al. Preferential reduction of very low density lipoprotein-1 particle number by fenofibrate in type II B hyperlipidemia: consequences of lipid accumulation in human monocytederived macrophages. Atherosclerosis 2001; 155: 251-260.
- Chen CW, Cheng HH. A rice bran oil diet increases LDL-receptor and HMG-CoA reductase mRNA expressions and insulin sensitivity in rats with streptozotocin/nicotinamide-induced type 2 diabetes. J Nutr. 2006; 136: 1472-1476.
- Haffner SM. Management of dyslipidemia in adults with diabetes. Diabetes Care. 1998; 21: 160-178.