Abstract
In this manuscript, we present a series of four patients treated for cancer pain, all of whom had prior or concurrent substance use disorders. We describe our clinical experience creatively balancing pain relief with harm reduction for at-risk patients and introduce three strategies to augment the ‘universal precautions’ for opioid prescribing. This manuscript illustrates the need for establishing an external structure, utilizing the skills of the entire care team and practicing meticulous inter- and intra-team communication when caring for these patients. By utilizing the entire team and its associated resources, including consultation with colleagues in addiction medicine, we demonstrate strategies for successfully treating cancer pain in patients with substance use disorders.
Keywords: Cancer Pain; Addiction Disorders; Diagnosis
Introduction
Pain is highly prevalent in patients with cancer. A recent systematic review by van den Beuken-van Everdingen found the prevalence of pain in all stages of cancer to be 50.7% [1]. Moreover, pain can be debilitating, negatively impact quality of life and impair patients’ ability to tolerate disease-directed therapies. Opioids remain the mainstay of treatment for cancer pain [2-4], yet using opioids to treat pain is complicated, particularly in patients with a history of a substance use disorder (SUD) for whom the risk of potential misuse of opioids is higher. In the United States, the number of people who died from overdoses of prescription medications in 2014 was over 14,000 [5].
In 2005, Gourlay et al. published ten universal precautions to more safely manage chronic non-cancer pain [6]. These recommendations are based primarily on expert opinion and exclude patients with cancer pain. However, emerging data suggest that misuse of opioids in the cancer population is higher than initially thought [7]. Barclay et al. found that over a one-month period 21% of patients screened in their Palliative Care Clinic using the Opioid Risk Tool were found to be at high risk for opioid misuse and 22% at medium risk [7]. They also found that greater than 12% of patients screened had a history of prescription drug misuse [7].
Fundamentally, it is important to recognize that patients with the dual diagnoses of cancer and an SUD have two potentially life threatening conditions which need to be managed simultaneously. Physicians have an ethical obligation both to relieve pain and suffering and to do no harm. A number of publications have outlined basic principles for treating patients with cancer pain and addiction [2-4,8-11]; however, few have outlined specific strategies to put these principles into clinical practice.
In this manuscript, we present a series of four patients treated for cancer pain, who also had prior or concurrent SUDs. We describe our clinical experience creatively balancing pain relief with harm reduction for at-risk patients and introduce the following strategies as ways to augment the ‘universal precautions’ for opioid prescribing [6]:
1. Provide an external structure
2. Utilize the skills of the entire team
3. Practice meticulous inter- and intra-team communication
The palliative care team described in this paper is made up of both inpatient and outpatient providers. The patients described in this series were cared for in both settings. The team sees patients in both inpatient and outpatient settings and is made up of board certified palliative medicine physicians, advance practice clinicians, nurses, social workers, pharmacists and chaplains. The palliative care team is housed at a large academic center that provides consultation to a large number of cancer patients annually with concurrent access to physicians certified in pain medicine and addiction, as well as addiction psychiatrists and addictions counselors. All patient information has been de-identified, including fictionalizing initials and personal details to retain patient privacy.
Case 1, MN
Strategy 1) Provide an external structure
MN had insight into her addiction and greatly feared losing control of her opioid analgesic use. To safely manage MN’s pain, our first step was to provide an external structure to her care that served to intensify her existing medical and social supports. We began seeing her weekly in clinic to monitor her pain experience, functional status, response to interventions and coping. Given her financial limitations, our clinic worked with her insurance company to arrange/fund scheduled rides aligned with a regular visit schedule. Seeing MN weekly helped to establish a trusting therapeutic relationship that augmented her emotional support and enhance overall coping strategies (Box 1).

Box 1: Case 1, MN.
For her analgesic regimen, we utilized a non-traditional transdermal fentanyl prescribing pattern of M-W-F application and removal of patches, as well as scheduled, rather than intermittent, as needed doses of immediate-release hydromorphone. This schedule allowed the leveraging of her local pharmacy to deliver small amounts of medication on an M-W-F schedule, giving her access to only small quantities of opioids at any given time. This strategy improved the opioid safety profile and decreased her risk of misuse, diversion and repeat overdose. It also freed her from the daily decision-making inherent in “as needed” dosing. For some patients with a prior history of addiction, who fear a loss of control with regards to their opioid analgesics, this additional structure provides a comforting sense of reassurance/safety.
Strategy 2) Utilize the skills of the entire care team
The benefit of team care for MN cannot be over-emphasized. While mental health resources are scarce commodities, involving psychiatry in the care of patients with SUD can be critical. Prior to psychiatric consultation, none of MN’s providers recognized that her anxiety was a manifestation of her under-managed bipolar disorder. Psychiatry clinicians adjusted her medications which resulted in significant functional improvements to her overall mental health and coping. Practically, this also reduced MN’s reliance on chemical coping with opioids or benzodiazepines. We also leveraged the interdisciplinary team members within our clinic to maximize efficiency and minimize disruption to the daily function of the clinic. Patients with dual diagnoses of cancer pain and SUDs require intensive services that can rapidly overwhelm an already full clinic schedule. The simple act of utilizing the proxy function on our state’s prescription monitoring program allowed the nursing staff to print out the prescribing data for the physician to review, saving the physician significant time.
Strategy 3) Practice meticulous inter- and intra-team communication
Effective team-based approaches must be interdisciplinary. The initial lack of communication regarding MN’s plan of care amongst all of MN’s care providers, led to a lack of awareness of the co-prescribing of benzodiazepines and opioids that contributed to her inadvertent overdose. Effective communication in such complex clinical scenarios requires a “quarterback” – a clinician (or clinical group) who ensures thorough, timely communication with all providers so that everyone knows next steps and everyone’s respective role in those steps.
Case 2, JP
Strategy 1) Provide an external structure
JP’s social and clinical circumstances were complex. His mild cognitive dysfunction, impulsivity in decision-making and lack of insight into his chemical dependency limited his capacity to plan ahead, making safe management of his cancer pain particularly challenging. Unlike MN, who relished the safety net of external structure, JP found external structure personally challenging. Given his long treatment course, which initially had curative-intent, we deferred direct management of his SUD, other than to employ harmreduction strategies. To that end, we not only set up a tight external structure with twice-weekly visits and scheduled medications; we also took the structure a step further and intervened when his inadequate planning compromised his care. For example, after several missed visits due to his inability to secure transportation, we engaged our social worker to set up transportation through county resources and connected him with a local county case-worker (Box 2).

Box 2: Case 2, JP.
As we remained firm in our insistence on external structure, we remained flexible and creatively personalized that structure to meet JP’s needs. Accordingly, after 8 weeks of demonstrated stability, we transitioned to two pre-arranged visits per week, one in our palliative care subspecialty clinic and one locally with his PCP. This change maintained the external structure required to manage his pain safely, while lessening the burden of travel to our subspecialty clinic an hour away from his home. In making this transition, we worked with his local primary care office to arrange the appointments in advance as to not rely on JP to make the appointments himself.
Strategy 2) Utilize the skills of the entire care team
Incumbent to the above strategy was close communication with his entire clinical team. Social work played a key role in identifying and working through financial/logistical barriers to visits and shared prescribing with his PCP improved overall compliance. His psychiatrist managed his mental health needs and understood that we anticipated that JP would require chemical dependency treatment once his cancer treatment was complete. Our nursing staff provided JP with frequent, proactive phone calls to remind him of appointments and transportation and to check on his pain. Through these phone calls and by being regularly present during his clinic visits, the nurses fostered a trusting relationship with JP that became increasingly invaluable. He began to see the nurses as the first point of contact prior to going to the ER.
Strategy 3) Practice meticulous inter- and intra-team communication
Communication amongst all team members was critical to the success of treatment. Prior to JP’s transition to shared visits between palliative care and his PCP, a physician-to-physician phone call established a shared set of roles and expectations. Regular inter-team communication was maintained with his psychiatrist and oncologist to keep all team members current regarding the plan of care. As with MN, the palliative care team assumed the “quarterback” role for communication liaisons. The palliative care nursing staff augmented the physician-to-physician communication with regular, secure e-mail updates that included all treating providers.
Case 3, KO
]Strategy 1) Provide an external structure
Case 3 demonstrates the power of addiction and the profound impact addiction can have on those suffering from concurrent lifethreatening illness. KO’s addiction threatened his life both directly and indirectly by interfering with his ability to comply optimally with chemotherapy. For KO, the external structure needed to be robust enough to co-manage his cancer pain and his active addiction (Box 3).

Box 3: Case 3, KO.
To create structure, we arranged weekly visits in palliative care clinic and asked KO’s father to dispense his opioids. The opioids were kept in a locked box at home, and his father had the only key. His father provided KO with a one-day supply of opioids at any given time. Early in KO’s care, we considered methadone for pain management but rejected it for two reasons: 1) there was a serious drug-drug interaction between his chemotherapy and methadone, and 2) safe prescribing of methadone requires rigorous medication compliance, which we had not yet established with KO. KO’s illicit injection of opioids during the course of his cancer and cancer pain treatment resulted in hospitalization and a life-threatening infection.
While his SUD presented ongoing risk of overdose, the high level of external structure may have prevented a fatal opioid overdose due to his access to small amounts of opioid medication at any given time.
Strategy 2) Utilize the skills of the entire care team
Starting from the first visit, we utilized a team-based approach to KO’s care, initially meeting with KO and his father as a collaborative team that included palliative care, pain medicine, nursing and a clinical nurse specialist with addiction training. This collaborative practice model was possible due to our group practice design and allowed for ongoing co-management throughout KO’s case. This allowed for shared visits, frequent de-briefing and may reduce team-splitting and clinician burnout. While formal addiction treatment during chemotherapy is rarely possible, establishing these relationships early in the course of treatment and planning a transition into intensive addiction treatment helps prevent surprises in the therapeutic management plan.
KO could have been discharged from the palliative care clinic for non-compliance or other aberrant behaviors. However, for patients with a concurrent serious illness, discharging them has potentially life-threatening ramifications for the patient and fails the rest of the team (e.g. KO’s hematologist) and the health system.
Strategy 3) Practice meticulous inter- and intra-team communication
KO’s endocarditis demonstrates the dangers of communication failures, as well as how challenging, but critical, rigorous communication can be even in a multidisciplinary group with a shared electronic medical record. KO’s opioid prescribing physicians were unaware that he had been prescribed injectable testosterone and provided with a prescription for needles. It was also apparent that the prescribing endocrinologist was unaware of KO’s severe addiction and history of IV drug use. While a shared electronic medical record is a helpful tool, it does not replace the need for direct communication in patients with high medical complexity. Robust team-based care demands rigorous communication at all times and an insistent “quarterback” to champion it.
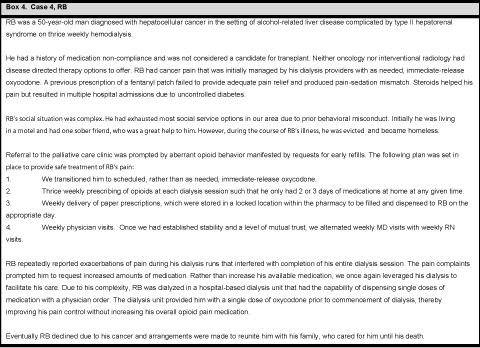
Case 4, RB
Strategy 1) Provide an external structure
In caring for RB, we creatively utilized his hemodialysis schedule, facilitating prescription of small amounts of opioids at each dialysis appointment without undue provider or patient burden. Prescribing a small number of opioids at a time minimized his risk of self-adjusting his medications and thereby either overdosing or running out early. If he did deplete his medication supply early, it was only a short wait until the next refill (Box 4).
Box 4: Case 4, RB.
Changing RB from an intermittent, as needed dosing schedule to a fixed dosing schedule further minimized his ability to selfadjust his medications. The fixed schedule also made calculating the number of tablets to prescribe with each refill straight forward. Just how effective this strategy was, not only from a stand point of safety but also in terms of effective pain control, became apparent over his course of treatment. After several months of stability, we mutually agreed to shift to weekly opioid prescribing rather than thrice weekly. Almost immediately, RB reported lack of adequate pain control and began requesting more opioid pain medication. We suspected he was running out early and therefore having worsening pain at the end of the week. At our insistence, we resumed thrice weekly prescriptions, and his pain control improved back to baseline without any increase in overall dosage.
RB began to report worsening pain during his dialysis run, which necessitated early discontinuation of hemodialysis and generated considerable conflict between RB and the dialysis staff. Initially, it was intended that RB would take his last dose of medication just prior to arriving at dialysis. In response to his persistent complaints of pain during hemodialysis, we creatively leveraged his hospital-based dialysis environment to address the pain: We added a single dose of oxycodone, dispensed by the dialysis nurses, prior to his hemodialysis run. We simultaneously decreased his total home prescription accordingly, such that his total opioid dosing remained unchanged. This additional step of provider structure improved both RB’s quality of life and treatment compliance.
Strategy 2) Utilize the skills of the entire care team
Members of the palliative care team visited RB at dialysis weekly. Initially, this was a physician visit. Once we established some stability and trust, we alternated visits between physicians and nurses, eventually graduating to monthly physician visits and thrice monthly nurse visits. This was time and labor-intensive. If a problem was detected during a nurse visit, the physician would assess RB. While this added an unplanned visit into the physician’s calendar, it occurred only rarely. The consistent provider presence focused on pain management garnered two benefits: 1) it prevented the dialysis staff having to engage in ‘battles’ about pain medication, improving their therapeutic relationship with RB and allowing them to focus on his overall medical cares and 2) it provided RB with ongoing continuity from the team managing his pain. Our consistent presence demonstrated our commitment to him and fostered bi-directional trust. By utilizing the whole team in his care, we lessened the care burden on each individual staff member.
Strategy 3) Practice meticulous inter- and intra-team communication
To be effective, RB’s care plan required ample communication between the palliative care and the dialysis staff. Both provider groups needed to agree on the plan of care and their respective roles in order to present a consistent message to RB and to prevent him from splitting the teams. The palliative care providers touched base with the dialysis advance practice clinician after every visit to update the plan of care. Additionally, there was ongoing communication with pharmacy, whose support of prescription management was critical.
Conclusion
It is essential to treat cancer pain effectively and safely in all patients, and nowhere is the challenge greater than with patients with a history of SUDs. Cancer pain can affect functioning and adherence to disease-directed therapies in patients with SUD creating a need to develop individualized plans to treat symptoms and ensure patient safety. To identify and successfully manage complex patients with dual diagnoses, care teams must have a heightened awareness of the risks and warning signs of aberrant opioid use and recognize addiction as a life-threatening illness in its own right that requires assessment, management, planning and care coordination. Maintaining respect is the foundation required to build rapport and may help decrease defensive responses from patients and their loved ones.
These cases demonstrate the need for establishing an external structure, utilizing the skills of the entire care team and practicing meticulous inter- and intra-team communication. By utilizing the entire team and its associated resources, including consultation with colleagues in the addictions field, patients with SUDs can have their cancer pain successfully treated.
References
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J Pain Symptom Manage. 2016; 51: 1070-1090.
- Bruera E, Paice JA. Cancer pain management: safe and effective use of opioids. Am Soc Clin Oncol Educ Book. 2015; .
- Portenoy RK. Treatment of cancer pain. Lancet. 2011; 377: 2236-2247.
- Dalal S, Tanco KC, Bruera E. State of art of managing pain in patients with cancer.Cancer J. 2013; 19: 379-389.
- CDC. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2016.
- Gourlay DL, Heit HA, Almahrezi A. Universal Precautions in Pain Medicine: A Rational Approach to the Treatment of Chronic Pain. Pain Medicine. 2005; 6: 107-112.
- Barclay JS, Owens JE, Blackhall LJ. Screening for substance abuse risk in cancer patients using the Opioid Risk Tool and urine drug screen. Support Care Cancer. 2014; 22: 1883-1888.
- Arthur JA, Haider A, Edwards T, Waletich-Flemming J, Reddy S, Bruera E, et al. Aberrant Opioid Use and Urine Drug Testing in Outpatient Palliative Care. J Palliat Med. 2016; 19: 778-782.
- Kircher S, Zacny J, Apfelbaum SM, Passik S, Kirsch K, Burbage M, et al. Understanding and treating opioid addiction in a patient with cancer pain. J Pain. 2011; 12: 1025-1031.
- Koyyalagunta D, Burton AW, Toro MP, Driver L, Novy DM. Opioid abuse in cancer pain: report of two cases and presentation of an algorithm of multidisciplinary care. Pain Physician. 2011; 14: E361-371.
- Modesto-Lowe V, Girard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag. 2012; 8: 167-175.
