Abstract
Paget-Schroetter syndrome is a rare, though serious, cause of upper extremity deep venous thrombosis (DVT) often seen in a physically active younger patient population seen in the primary care setting. Significant morbidity occurs if not recognized and treated expeditiously. The literature commonly states that younger males are at highest risk. This review presents current approaches to diagnosis and management of this often misdiagnosed syndrome, with special emphasis on under represented risk factors in women.
Keywords: Paget-Schroetter Syndrome; Effort-Related Upper Extremity Deep Vein Thrombosis; Idiopathic Upper Extremity Deep Vein Thrombosis; Women; Exercise-Induced Upper Extremity Deep Vein Thrombosis
Introduction
Paget-Schroetter syndrome (PSS), also known as “effort thrombosis” is a rare condition characterized by the presence of spontaneous thrombosis in the axillary-subclavian venous system, typically after strenuous activity or repetitive motion in the affected limb [1]. The incidence has been estimated from data in Sweden to be approximately 2 per 100,000 people per year; this is roughly equivalent to about 3-6,000 cases per year in the United States [2]. Upper extremity DVTs in general constitute approximately 11% of all DVTs [3]. Etiology can be further classified into primary or secondary causes. Paget-Schroetter is considered a primary cause and though it constitutes less than 20% of cases of upper extremity thrombosis, it affects a particularly youthful and active patient population, unlike most other patients at risk for thrombosis [1].
Sequelae of Paget-Schroetter syndrome can include residual venous obstruction of the upper extremity causing persistent pain and disability (post-thrombotic syndrome), early exercise fatigue, or even more concerning, pulmonary embolism [2,4]. Given that the population most often associated with PSS is often healthy at baseline, preventing long term morbidity largely guides clinical management.
The purpose of this updated review is twofold: Firstly, it is critical to continue to increase awareness among primary care physicians of this condition, which can be easily misdiagnosed and thus substantially increase morbidity in an otherwise healthy population. Secondly, those who are aware of this syndrome typically associate it with an active male population—-as a consequence, female patients are possibly at greater risk for a delay in diagnosis of a syndrome that demands prompt recognition and management (Figure 1) [5].
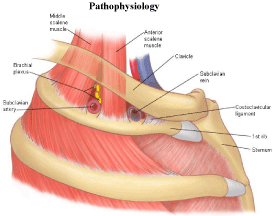
Figure 1: Simplified anatomy of the thoracic outlet [5].
The subclavian vein emerges between two bony structures, namely the clavicle anteriorly and the first rib postero-inferiorly [6]. Two muscles, the subclavius and the anterior scalene border the vein anteriorly and laterally respectively [6]. The costoclavicular ligament forms the medial border of this area collectively known as the thoracic outlet. Compression of the subclavian vein is known as venous thoracic outlet syndrome (VTOS).
Several anatomical factors can be implicated in mechanical compression of the subclavian vein. A congenital variation of the costoclavicular ligament has its insertion point lateral to the more commonly seen variant [6]. Scar tissue from previous clavicular fractures, presence of a cervical rib, and fibromuscular bands can also be contributing factors [7].
Even in the absence of any anatomical variant, the subclavian vein can be compressed transiently by abduction of the arm, often in the overhead position [2]. Over time, repetitive upper extremity motion, is thought to damage to the vessel wall in several ways. It has been hypothesized that strenuous exercise causes physiologic hypertrophy of the anterior scalene and subclavius muscles, causing compression of the vessel, much like the anatomical variants described above [8].
Chronic compression results in thickening and fibrosis. Concurrently, the intima of the vessel is disrupted, leading to an irregular thrombogenic surface encouraging clot formation [9].
It is unknown whether an underlying anatomical variant must be present or if PSS is strictly a physiologic phenomenon due to overuse of the limb [2]. Cases of PSS have been reported without either of these two factors in women [10], emphasizing the need for careful consideration in this population.
Clinical Presentation
Initially, a “bursting feeling”has been described in the affected limb during intense physical activity, thought to be secondary to venous hypertension [9]. Prior to consolidation of thrombus, multiple “herald”events can occur whereby subocclusive thrombosis and resolution occur causing transient pain [9]. Once the vessel has thrombosed, often triggered by an increase in blood viscosity, (typically dehydration during intense physical activity), the classic presentation is of a young patient (mean age early 30’s) who presents with a heavy, discolored, painful arm [2]. Pain is often severe and prompts patients to seek evaluation urgently usually within 24 hours of intense activity [11]. Anywhere from 60-80% of patients will report recent physical activity in the affected limb [2].
Though PSS patients most commonly present with local signs of venous compression, some exhibit classic symptoms typical of thoracic outlet compression including numbness, muscle weakness and parathesias [7]. Rarely, the patient does not present with isolated local symptoms, but rather only after embolisation, i.e., with pleuritic chest pain and progressive dyspnea [7].
Signs suggestive of PSS are visible venous collaterals noted on physical exam, most clearly seen with the patient in the “surrender” position [9]. This venous engorgement over the affected area is known as Urschel’s sign [12]. Palpable cords over the basilic and axillary veins can be tender [13]. Additional signs include blue or red discoloration of the affected limb and Adson’s sign, or loss of the radial pulse when the head is hyperextended and rotated ipsilaterally [9,14].
Diagnosis
The diagnosis of DVT in the upper extremity can often be made with color duplex ultrasound (CDU), which tends to be widely available and is noninvasive. Sensitivity of this modality is estimated to be 78-100% and specificity 82-100% [12]. In PSS patients, while CDU cannot definitively establish the diagnosis, it can visualize larger clots that extend into the axillary system and thus prompt rapid further investigation.
There are several important limitations of this modality when considering PSS in the differential diagnosis. One is the inability to reliably visualize the proximal portion of the subclavian vein, a common site for thrombosis in PSS [15]. An acute occlusion can be echolucent, and thus not apparent [11]. Moreover, the clavicle obstructs this critical area and does not allow for compressibility [15]. Ultrasound evaluation is often technician dependent and a well developed collateral can be mistaken for subclavian patency in this rare presentation [15].
Given the limitations of ultrasound, the gold standard for diagnosis of PSS is contrast venography, which allows for optimal viewing of the subclavian posterior to the obstructing clavicle [15]. Contrast venography can demonstrate areas of stenosis as well as allow for compression of collaterals in various positions [15].
Magnetic resonance venography can be considered for certain patients in whom contrast is contraindicated, namely pregnant patients, those with contrast allergy, or renal insufficiency.
Management
Historically the management of PSS was conservative and consisted largely of anticoagulation with low molecular weight heparin or vitamin K antagonists, and elevation [15,16]. Subsequent studies indicated that anticoagulation was not adequate in reducing the incidence of long term sequelae in PSS, likely because it does not restore patency of the vessel [12].
Clinical outcomes were poor and a large proportion of patients experienced considerable pain and disability [11]. Rates of pulmonary embolism as high as 6-15% have been reported with anticoagulation alone [12].
Thrombolysis was introduced in the 1980’s in order to mitigate this risk and restore patency of the vessel [17]. Systemic thrombolysis, however, carried an unacceptably high risk of serious complications including cerebral hemorrhage, pulmonary embolism and prolonged bleeding, and this approach was later substituted with catheter directed thrombolysis (CDT) often with tPA (tissue plasminogen activator) [12]. The optimal timing of this procedure has been discussed and early treatment within 1-2 weeks appears to be more favorable [12,18]. Beyond that, a substantial risk of fibrosis within the vessel wall can lead to chronic edema and clinically significant disability [18].
More recently, Shah et al reported the first known implementation of power-pulse spray thrombectomy in PSS patients with the goal of debulking the thrombus rapidly [13]. This newer technique combines tPA pharmacotherapy with a mechanical high velocity saline jet to break up the clot quickly [13]. Though data is limited, this approach could significantly decrease the lysis time and subsequent bleeding risk [13].
Reocclusion after CDT is, according to Thomson et al, to be expected if the cause of the mechanical compression is not addressed [9]. Risk of rethrombosis and/or persistent symptoms is not insignificant; some reports indicate as many as a third to one half of patients face these risks if treated with thrombolysis alone [2,17]. Surgical decompression of the thoracic outlet via first rib resection (FRR), which has a high likelihood of restoring full function, after CDT emerged as the standard of care of PSS in experienced centers, largely due to this risk [13,17].
However, both the timing and need of FRR have been widely debated. Molina et al reported 100% success rate with CDT followed by immediate FRR and finally 8 weeks of anticoagulation in 126 PSS patients treated from 1985 through 2008 [18]. A few dissenting opinions have claimed that since not every patient reoccludes, the FRR can be performed as needed in the case of recurrent thrombosis [17]. While randomized controlled trials are lacking, most experts support CDT with immediate FRR followed by anticoagulation for 6 months [11].
Discussion
Historically, Paget-Schroetter syndrome was associated with physically active male patients involved in repetitive overhead use of the arm or strenuous exercise [15]. The current medical literature largely perpetuates this notion that the classic presentation of PSS is in younger males [1,12,19,20]. Many reported cases of spontaneous thrombosis involve rock climbers, golfers, musicians, wrestlers, pitchers, in addition to overhead workers like plumbers, and painters [7,12,21].
Female patients, however, merit new consideration with respect to PSS both for clinical and anatomical reasons that have, thus far, not been highlighted in the current literature. Women are increasingly involved in the types of activities that have placed their male counterparts at risk. An uptick in the presentation of PSS in young women at the University of Southern California’s Center for Vascular Care prompted an internal review of patients presenting between 2003 and 2005 [15]. Exercise was the precipitating factor in all 14 patients and the distribution of male and female patients was nearly even [15]. This challenges the rather widespread assumption that males remain at highest risk in the future.
Anatomically, the thoracic outlet in some women is smaller [10], and thus more prone to thrombosis. It has also been reported that women have an increased risk for recurrence of upper extremity thrombosis, a finding not seen in men, and thereby underscores the importance of taking a thorough clinical history [22]. Moreover, a subset of patients with PSS do not present with the typical risk factors of strenuous/overhead activity or anatomical abnormalities: these patients are more likely to be female and have an underlying hypercoagulable disorder [10].
Artificial reproductive technology appears to be a significant risk factor due to increased risk of ovarian hyperstimulation syndrome [3]. Pregnancy, a known hypercoagulable state, has been associated with the development of upper extremity DVT early on, as opposed to the more common lower extremity DVT seen later and even postpartum [3]. In addition, pregnant patients appear to be at higher risk for clot extension despite adequate anticoagulation [3]. And finally, effort thrombosis has been reported in women taking oral contraceptives [23], thereby suggesting that other forms of exogenous estrogen like hormone replacement therapy (HRT) could reasonably play a role.
Conclusion
Paget-Schroetter syndrome is a rare cause of upper extremity DVT that, if not treated promptly, can be disabling and potentially life-threatening in an otherwise active and youthful population [24]. It is of critical importance that primary care physicians include Paget- Schroetter Syndrome in the differential diagnosis of upper extremity swelling or pain, as they are often the first point of care for many patients. The medical literature more commonly associates this clinical picture with young active males. Much has changed since the mid 1900’s when Sir James Paget and Leopold von Schroetter were jointly credited with this syndrome by British surgeon Hughes [10]. Women are an increasingly important population with a myriad of risk factors that deserve further consideration when the clinician encounters this unusual constellation of symptoms.
References
- Kucher N. Deep-Vein Thrombosis of the Upper Extremities. NEJM. 2011; 364: 861-869.
- Illig K, Doyle A. A comprehensive review of Paget-Schroetter Syndrome. J Vasc Surg. 2010; 51: 1538-1547.
- Chan WS, Ginsberg JS. A review of upper extremity deep vein thrombosis in pregnancy: unmasking the ‘ART’behind the clot. J Thromb Haemost. 2006; 4: 1673-1677.
- Lee, JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C. Long-term thromobotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006; 43: 236-243.
- First Rib Image Galleries.
- Urschel HC, Patel AN. Surgery Remains the Most Effective Treatment for Paget-Schroetter Syndrome: 50 Years’Experience. Ann Thorac Surg. 2008; 86: 254-260.
- Bliss S, Weinberger S, Meier M, Saint S. The Unusual Suspect. NEJM. 2002; 347: 1876-1881.
- Fleta GE, Blanco AT, Palones FG, Monzon EO. Combined non-surgical treatment for Paget-Schroetter syndrome: a case report. Journal of Medical Case Reports. 2016; 10: 171.
- Thompson JF, Winterborn RJ, Bays S, White H, Kinsella DC, Watkinson AF. Venous Thoracic Outlet Compression and the Paget-Schroetter Syndrome: A Review and Recommendations for Management. Cardiovasc Intervent Radiol. 2011; 34: 903-910.
- Likes K, Rochlin D, Nazarian SM, Streiff MB, Freischlag JA. Females With Subclavian Vein Thrombosis May Have an Increased Risk of Hypercoagulability. JAMA Surg. 2013; 148: 44-49.
- Mall NA, Van Thiel GS, Heard WM, Paletta GA, Bush-Joseph C, Bach Jr BR. Paget-Schroetter Syndrome: A Review of Effort Thrombosis of the Upper Extremity From a Sports Medicine Perspective. Sports Health. 2013; 5: 353-356.
- Naeem M, Soares G, Ahn S, Murphy TP. Paget-Schroetter syndrome: A review and Algorithm. Phlebology. 2015; 30: 675-686.
- Shah A, Bajakian DR, Olin JW, Lookstein RA. Power-Pulse Spray Thrombectomy for Treatment of Paget-Schroetter Syndrome. AJR. 2007; 188: 1215-1217.
- Urschel HC, Razzuk MA. Paget-Schroetter Syndrome: What Is the Best Management? Ann Thorac Surg. 2000; 69: 1663-1669.
- Shebel ND, Marin A. Effort thrombosis (Paget-Schroetter syndrome) in active young adults: Current concepts in diagnosis and treatment. J Vasc Nurs. 2006: 24: 116-126.
- Flinterman LE, Van Der Meer FJM, Rosendaal FR, Doggen CJM. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost. 2008; 6: 1262-1266.
- Lugo J, Tanious A, Armstrong P, Back M, Johnson B, Shames M, et al. Acute Paget—Schroetter Syndrome: Does the First Rib Routinely Need to Be Removed after Thrombolysis? Ann Vasc Surg. 2015; 29: 1073-1077.
- Molina JE, Hunter DW, Dietz CA. Protocols for Paget-Schroetter Syndrome and Late Treatment of Chronic Subclavian Vein Obstruction. Ann Thorac Surg. 2009; 87: 416-422.
- Shimada T, Tounai T, Syoji T, Fukumoto Y. Acute Pulmonary Embolism due to Paget-Schroetter Syndrome. Intern Med. 2015; 54: 1875-1879.
- Ljaopo R, Oguntolu V, DCosta D, Garnham A, Hobbs S. A case of Paget-Schroetter syndrome (PSS) in a young judo tutor: a case report. Journal of Medical Case Reports. 2016; 10: 63.
- Lutter C, Monasterio E, Schöffl V. Rock climbing-related subclavian vein thrombosis. BMJ Case Reports. 2015; 10: 1136.
- Flinterman LE, Van Hylckama Vlieg A, Rosendaal FR, Doggen CJM. Recurrent Thrombosis and Survival After a First Venous Thrombosis of the Upper Extremity. Circulation. 2008; 118: 1366-1372.
- Stricker SJ, Sowers DK, Sowers JR, Sirrige MS. “Effort thrombosis”of the subclavian vein associated with oral contraceptives. Ann Emerg Med. 1981; 11: 596-599.
- Seeger M, Bewig B. Paget-Schroetter Syndrome. NEJM. 2010; 363: 3: e4.
