Abstract
Introduction: This systematic review article revolves around the assessment of the underlying factors and trends associated with the hyperglycemic control among the patients suffering from Diabetes mellitus Type 2.
Method: The review of research articles was performed between Feb. and may 2016 through meta-analysis and 40 out of 842 research papers were included in this study as they were most relevant and authentic. The validity of the research studies for their inclusion in the meta-analysis is performed through Newcastle Ottawa scale.
Result: The review of the research articles provided ample data regarding the benefits of disease-specific educational counseling of the patient suffering from Type 2 Diabetes.
Keywords: Diabetes self-care; Hyperglycemia; Patient education; Type 2 DM
Introduction
Diabetes mellitus type 2 can be considered as a group of metabolic diseases resulting due to hyperglycemia. This hyperglycemia may be accounted to defects in insulin sensitization and secretion. Type 2 Diabetes mellitus (DM) is suggested to be the leading cause of death among the population in the United States. It is reported that type 2 DM is a direct cause of mortality and has increased the death toll up to 73,000 per year. Moreover, DM type 2 has been suggested to be the underlying cause of 220,000 deaths annually. Type 2 Diabetes is also associated with various co-morbidities among which the most common are kidney failure and blindness observed among the adult population [1].
The American Diabetic association has reported that more than 20 million people have been diagnosed for Diabetes at present, whereas, 6 million of the population remain undiagnosed. The derangement of glucose homeostasis being observed among the population is responsible for the ultimate development of Diabetes. Hence, the multi-factorial analysis of genetic, ethnic and racial heritage and environmental factors contributing to the growing rate of Diabetes mellitus type 2 is needed. It is important to understand the precise interplay associated with all the contributing factors through generation of evidence based on long-term trials. Researchbased evidence regarding Diabetes mellitus will help in the effective prevention and management of Diabetes mellitus [1-9].
In 2012, the American Diabetes Association and the European Association for Diabetes provided a detailed position statement regarding the problem and for the management of hyperglycemia in patients suffering from type 2 DM specifically. This position statement is of dire need to pronounce the growing public health concern associated with the increasing need of managing Diabetes mellitus adequately. Moreover, scarcity of comparative treatment and management alternatives of type 2 DM evokes the need of conducting future longitudinal researches for producing long-term treatment outcomes [1,2,9].
Glycemic Targets for the Management of Type 2 Diabetes
Glucose control is the major target to be achieved during the management of type 2 Diabetes. Reducing hyperglycemia decreases the onset of microvascular complications but its effect on the reduction of cardiovascular complication is uncertain. It is suggested that long-term management of hyperglycemia may reduce the risk of cardiovascular complications. A personalized management approach for balancing the benefits and risks associated with glycemic control is necessary. It is mandatory to find out the age-related effects and the health status of patients who are being exposed to the adverse effects of lipid-lowering medications. The clinicians and other healthcare providers involve in the management of Diabetes have a greater responsibility to address the risk and consequences associated with an adverse event [2,3].
It is estimated that the usual HbA1c goal that is needed to be achieved is 53.0mmol/mol of plasma. Control of glucagon secretion and regulation of Paracrine mechanisms is another mechanism associated with the hyperglycemic control. These secretions help in the generation of positive feedbacks, which is helpful in providing favorable energetic conditions. Human cells are also known to release acetylcholine parallel to glucagon and this neurotransmitter is also helpful in the generation of the feedback loop. Such paracrine mechanisms control the secretion of glucagon from the a-cells that are responsible for releasing inhibitory factors. It should be notified that paracrine control does not depend upon capillary transport in the interstitial spaces and the microcirculation in the Islets of Langerhan is particularly important. Since glucose in the blood is responsible for the stimulation of insulin release, which in turn inhibits glucagon secretion from the a-cell [2,4].
It is further illustrated that inhibition of a-cells is secondary to the stimulation of β-cells and insulin can be considered as the first mediator of a-cells inhibition. Paracrine factors may be released from b-cells via two routes i.e. dependent Ca+2 releases from the secretary vessels or Ca+2 independent releases from b-cells via plasma membrane transporters. Insulin secretion is directly related to the level of glucagon response to hypoglycemia. It is estimated that glucagon release is inhibited to its maximum as the concentration of glucose is raised to 0–7 mM range as it indicates the secretion of Insulin [4,5].
In the Ca+2 process of release, paracrine influence is found on the secretion of glucagon in response to hypoglycemia. GABA is evident to release by activation through both the above mentioned routes. Studies conducted on the islets of Langerhan indicated that amount of glucose in the blood is known to control the GABA release from the cells. This reduction in GABA stimulates the increase of GHB, which in-turn inhibits the secretion of glucagon from the a-cells. Moreover, Somatostatin is also considered to be a potent inhibitor of both insulin and glucagon and was proposed to be regulator of insulin and glucagon release. Thus somatostatin mediates the inhibition feedback mechanism during hyperglycemia.
During the process of glycolysis, pyruvate is metabolized causing an increase in the cytoplasmic ATP/ ADP ratio, which closes the ATPdependent K+ channels leading to depolarization. This depolarization opens the Ca+2 channels causing a rise in the Ca+2 channels and is the main trigger of insulin release. The glucokinase activity plays a role similar to b-cells and may act as sensors for metabolic glucose [5].
The above mentioned mechanism demonstrates the heterogeneity of the metabolic conditions associated with the occurrence of Type 2 Diabetes mellitus. It should be noted that the pancreatic b-cells synthesizes and stores insulin at a regular basis, irrespective of the glucose levels in the blood. Insulin remains stored in the vacuoles and is released when it is triggered by an elevation of the blood glucose levels. An increase in the glucagon from the a-cells is mediated by a decrease in the insulin levels due to drop in the blood glucose. Glucagon stimulation promotes the conversion of glycogen to glucose for maintaining the normal blood glucose levels. The production of glucose to during the period of fasting requires rapid gluconeogenesis and glycogenolysis [4,5].
Three key defects in the process of glucose metabolism may result in the emergence of hyperglycemia causing the condition of Type 2 Diabetes mellitus. These defective processes include increased hepatic glucose production, diminished insulin secretion and impaired action of insulin. Hence, insulin resistance due to any of these defects resulted into higher levels of glucose in the blood. Insulin resistance can be defined as the delayed responses to Insulin receptors and this problem is generally post-receptor. This means that there is a problem in the response mechanism to Insulin release rather than its timely production, which leads to Type 2 Diabetes mellitus [5].
Insulin Signaling Mechanism
It is important to understand the insulin signaling mechanism in response to glucose plasma levels. Human Insulin receptors are heterodimer in nature and composed of two extracellular a-subunits along with two b-subunits, which are located into transmembranes. The ligand-binding domain is located into a-cells and is responsible for the regulation of the intracellular tyrosine kinase activity of b-subunits. The insulin receptor gene in mammals consisted of 22 axons that are responsible for the generation of isoforms by alternative splicing of axon-11; the isoforms (IRa) contains axon-11, whereas the b-isoform (IRb) omits axon-11. This IRb is responsible for binding to insulin on the receptor site and dominate the tissues that are insulin sensitive including adult liver muscle and adipose tissues. On the other hand, IRa binds to insulin growth factors in other tissues such as fetal tissues, hematopoietic cells and central nervous system. The growth factors and Insulin resides in the plasma membranes in the form of inactive covalent dimers, which increases the flexibility of the activation loop to allow ATP and causes active conformation by phosphorylation [6].
These activated insulin receptors further caused phosphorylation of the tyrosine residues in the cellular substrates. The phosphorylation of tyrosine sites provides a signaling cascade in which SH2 domains are binded to the effector proteins such as phosphoinositide 3-kinase, tyrosine kinase and phosphatase SHP2. The classic insulin cascade causes ensues the production of PI by phosphatidylinositol 3-kinase. phospholipid phosphatases and Protein phosphatases modulates the strength of insulin signals and dysregulation of these heterologous signaling mechanisms can causes glucose intolerance, hyperinsulinemia, insulin resistance and dysregulation of lipid metabolism. Translocation of the glucose transporters in the plasma membranes aids the transportation of glucose into the skeletal muscles and facilitates the diffusion of glucose in normoglycemic conditions [7].
Pathophysiology of T2DM
Skeletal muscle accounts for 75% of the glucose uptake that is regulated by insulin stimulation and defects in the skeletal muscle tissue plays a major role in the glucose homeostasis. It is reported that tyrosine phosphorylation by insulin receptor is found to be reduced in non-obese patients who have been suffering from type 2 diabetes mellitus. However, the peripheral insulin resistance is partly compensated by IRS1-independent pathway which helps in the transduction of insulin signals. It is shown through researchbased evidence that type 2 diabetic patients have impaired insulinstimulated tyrosine phosphorylation of IRS1 in skeletal muscles [7,8].
Mechanisms of Insulin Resistance
Dysregulation of Insulin receptors is a common feature that is identified during the anomaly of insulin resistance. The mechanisms for dysregulation might include tumor necrosis factors TNFamediating the down regulation of mRNA transcription. These signaling abnormalities resulted into an impaired glucose transport characterized by a decrease in the fat oxidation capacity of the body, which resulted into Type 2 Diabetes subjects. T2DM is characterized by an impaired flexibility towards body metabolism. It involves the inability of fatty acids to switch towards glucose oxidation in response to insulin. Therefore, a reduced fat oxidative capacity of the body causes insulin resistance in the skeletal muscles [7,9] (Figure 1).
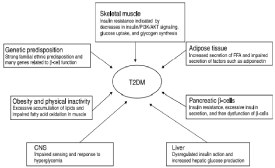
Figure 1: Pathogenesis of Type 2 Diabetes Mellitus.
Methods
Study design
A secondary qualitative method was used to conduct a review of the literature and various databases have been used for the collection of the most relevant literature. The review of research articles was performed between Feb. and may 2016. PubMed, Web of knowledge, Springer databases and Elsevier ScienceDirect were the databases used for literature searching. The papers related to type 2 diabetes mellitus incidence, causes and underlying mechanisms have been selected at first. Further, the research is narrowed down by selecting those articles which have demonstrated the feedback mechanism associated with hyperglycemia and insulin receptor impairment. The keywords used for searching include Type 2 Diabetes mellitus, T2DM, hyperglycemia, glucose metabolism disorders and insulin impairment etc [10].
Extraction of articles
The extraction process of the most relevant studies has been performed by examining the repeatability of articles. For this purpose, the titles and abstracts of the articles were browsed minutely and purposefully. It is confirmed that the inclusion criteria in the studies may include only those participants who are suffering from diabetes mellitus type 2. The exclusion criteria involve all those patients who have been suffering from type 1 Diabetes and those who have been suffering from Type 2 Diabetes [10].
Validity and reliability
The Newcastle Ottawa scale has been used to assess the quality of selected articles and their suitability for performing meta-analysis. This scale examined the quality of studies through three parameters, which include the selection of study groups, the comparability of study groups and the assessment of outcomes. Points were provided to studies through this point scale by from 1-7, where 4-6 is the moderate score for quality. High quality studies get criteria for high quality score i.e. greater than 7 [10].
Statistical analysis
The Statistical analysis of the extracted data was performed by examining heterogeneity among selected studies. The random effect model was used to calculate pooled OR and subgroup analysis was also conducted to assess the heterogeneity among selected studies. STATA version 12.0 was used for data analysis and the studies that were found beyond the 95% confidence interval were removed. A two tailed analysis was performed and P value is found to be 0.05, which was considered significant [10].
Results
The results of the Literature search are provided in Figure 2. Initially, 842 studies were identified and 110 papers were removed from the initial searches due to duplication. After examining the titles and abstracts of the studies for their heterogeneity, 40 research papers that are most relevant to the topic were identified. Other 692 papers were excluded from the study because they were not directly related to Type 2 DM. Finally, seven studies were selected for metaanalysis because others did not express results within 95% confidence interval. The prospective cohort studies with follow-up from 4 to 24 years were included in this literature review. The subjects of these studies included teenagers, adults and aged people suffering from Diabetes mellitus Type 2. Out of seven studies, two studies were of moderate quality, whereas, five studies were found to be of high quality on the Newcastle Ottawa scale. It was observed that no significant heterogeneity was performed among the study groups and the pooled ORs ranged between 1.30 at a confidence interval of 95%. The study results revealed that no single study has been conducted to substantially influence the total OR. It is suggested that hyperglycemia is a risk factor for developing Type 2 Diabetes mellitus. Trends that could establish glycemic control in the population include adherence to medication i.e. Insulin therapy, physical exercise, self-management through regular blood glucose testing [11].
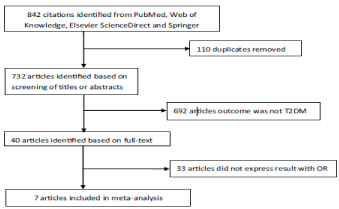
Figure 2: Flowchart for Literature Search.
Discussion
Diabetic patients are observed to have complications as a result of their lack of awareness regarding the disease. The scarcity of diseaserelated knowledge and poor insight of the diabetic patients is the cause of poor glycemic control among Diabetic patients. Therefore, patient education is highly needed for managing the disease and for reducing the co-morbidities associated with Diabetes mellitus. It is evident through the findings of various studies that many nonpharmacologic strategies such as patient education, diet changes, selfmonitoring training, telemedicine and psychological interventions are significant the risk of chronic Diabetes mellitus and for improving glycemic control among the patients. However, effectiveness of non-pharmacological assessment is still unclear in controlling hyperglycemia. Although, it is recommended that patient education is the most effective way of managing the disease and reducing the complication of Diabetes. The findings of a study reported that each 1% reduction of HbA1c causes a 37% decrease in the risk of microvascular complications and a 21% decrease in the risk of Diabetes [7,8].
Diabetes is a very difficult disease to manage as the difficulty lie in the fact that self-motivation and self-monitoring by the patients is essential for treatment. Hence, it is recommended in various study findings that Diabetes self-management education (DSME) is the key to improve the self-management capabilities of the patients suffering from Type 2 DM. Maintenance of normoglycemic values remains the cornerstone to the management of DM and nutritional education is essential for the comprehensive control of Diabetes education [8].
The findings of this study revealed that a significant improvement in the glycemic control of patients has been observed by conducting Diabetes management program as evident by the review of research literature selected. It is revealed that after educational sessions related to the management of Diabetes mellitus; the compliance of patients to healthy dietary regimens has considerably increased. Adherence to the dietary plan, avoidance of high fat foods and regular servings of carbohydrates in the meal is increased as a trend among the Diabetic patients after counseling sessions [9].
Various studies have concluded the fact that self-monitoring of blood-glucose (SMBG) represents an adjunct to HbA1c as it help in finding the fasting, pre-prandial and post-prandial hyperglycemia and glycemic conditions. As per observation, Diabetes self-education increases the prevalence of SMBG activities among patients resulting into greater glycemic control. Recent studies provided evidence regarding the use of SMBG and suggested it to be an excellent intervention for the prevention of Diabetic neuropathy. However, it is recommended that self-management of Diabetes through SMBG shall be incorporated after efficient educational intervention [10].
It is well-established through ample research-based evidence that regular physical exercise is associated with improved glycemic control among patients of Type 2 Diabetes. Exercise is found to increase insulin sensitivity and consequently lowers the blood glucose levels because of strenuous use of glucose and the up-regulation of glucose transport into the skeletal muscles. It is also stated that patients having Diabetes mellitus have stopped regular physical activity for a prolong period of time. Moreover, the Diabetes management educational programs have evolved the emergence of regular physical activity for a period of atleast 30 mins among Diabetic patients [11].
As discussed above, multifactorial health programs are a source of improvement among Diabetes patients. These educational counseling sessions are evident to improve the glucose and lipid levels particularly among patients with adverse HbA1c levels. The challenge of poor adherence to diabetic glycemic medications is also an issue of severe debate among the patients. Adherence to the prescribed antidiabetic medications is found to be poor resulting into higher HbA1c levels and cholesterol levels among Diabetic patients. Patient’s lack of adherence to their medication regimens is due to the concept that the role of patients in disease management is largely passive. The patient compliance issues have been a long standing concern for both patients and the healthcare providers. One of the best-suited strategies for Diabetes control is improving the patient’s adherence to the glucose lowering and lipid lowering medications as it will help in the optimization of metabolic control [8].
Findings of the previous literature also reported that there is a strong association between anxiety or depression and hyperglycemia. The risk of developing co-morbidities and complications also tends to increase the patient suffering and escalating healthcare costs.
Various studies have supported the fact that patient education, adherence to medications and self-management are the tools for the management of Diabetes mellitus. A study was conducted to assess the patterns of therapeutic interventions that have found to be extremely effective for the management of hyperglycemia. This study was conducted in the Hijaz region of Saudi Arabia and the treatment of patients in comparison to the standard guidelines for Diabetes was evaluated. Both the pharmacologic and nonpharmacologic interventions has been applied and later evaluated for their effectiveness [11].
In order to fulfill this aim, a surveillance of the physician’s prescription habits was performed and this study was conducted primarily in the health centers, hospitals and community pharmacies. Analysis of the physician’s prescription method was performed which revealed that there is a deviation between the standard and generally applied therapeutic protocol for the management of Diabetes. There was a considerable bias in using oral hypoglycemic patients instead of implementing lifestyle changes. The use of nutritional supplements is also overlooked usually by the physicians. The study revealed the fact that prescription of metformin and glimepiride for controlling hyperglycemia was the most commonly prescribed medications. The physicians need to develop patient treatment regimens in collaboration with other healthcare providers especially the pharmacists and the caregivers [11].
Risk factor management is another approach for avoiding complications in patients who have been newly diagnosed with Diabetes. A case-control study has been performed in healthcare centers in Jeddah, Saudi Arabia to determine the risk factors of Diabetes mellitus Type 2. The diabetic patients included in this study were recruited in the cases group, whereas, the non-diabetic patients have been recruited as controls. A questionnaire has been designed to collect data from 159 diabetic patients who are less educated, low salaried, married or divorced. the pre-diabetic, high control groups have been identified and counseled before the incidence of Diabetes to provoke prevention [12].
Prescription for Diabetes care is evaluated through a descriptive study that has been carried out on a primary healthcare clinic over a period of five months. A total of 160 female diabetic patients have been selected for this study from a medical complex in Saudi Arabia and data was collected from the medical records of the patients. It is evident that half of the Diabetic patients, who have been suffering from diabetes, have co-morbidities such as concomitant hypertension, hyperlipidemia and obesity. 82% of the sample population was older than 40 years and 57% of the participants were prescribed more than one drug for controlling hyperglycemia. The most common drugs that were found to be prescribed by most of the physicians include metformin and sulphonylureas. 70% of the Diabetic patients included in this study were treated with combination therapies such as ACE or ARBs and diuretics. Aspirin and Statins were used among 23.8% and 41% of the Diabetic patients. it is suggested that polypharmacy and self-monitoring of the type 2 diabetes are essential for the management of Diabetic care [13].
Another study supported the fact that continuous medical care is mandatory for improving the self-management skills of the patients, education and adherence to prescribed regimens. Hence, a study was conducted to assess the benefits of educational programs related to diabetes care in the management of the disease condition and its co-morbidities. Education materials were provided to the participants of this study and disease specific educational programs have also been conducted to improve the knowledge and compliance of patients towards diabetes therapies. The study participants were then interviewed through a structured interview portfolio and were later interviewed again after a period of 6 months for follow-up. The interview schedule included socio-demographic, diabetes selfmanagement, adherence to medications and individualized disease characteristics of patients and the impact of clinical education and counseling on the disease pattern of the population. The findings of the structured interviews revealed that after six months of diabetes education program, significant improvements in patient’s dietary plan, physical exercise and blood glucose monitoring and adherence to medications is observed. Further, implementation of educational programs on dietary plans, physical exercise, SMBG, adherence to medications are highly recommended and have been found significant in controlling HbA1c levels vand depression among Diabetic patients [14].
Conclusion
In conclusion, the results of this study indicated that diabetes education is directly associated with improvement in dietary regimens, physical exercise and adherence to the dosage regimens. Multifactorial assessment of the factors is important for the management of Diabetes and its co-morbidities.
Summary
This review paper reveals the current and past trends that are implemented to control hyperglycemia among diabetic patients. This paper consisted of a detailed meta-analysis of various research articles, which includes a study over the concepts of glycemic control among the Diabetic patients and the perception of Diabetes mellitus Type 2 treatment among the physicians. A detailed review of the literature is provided in this review article to address the underlying mechanisms associated with the regulation of insulin in the body via receptor pathways. Besides, the details of the intrinsic mechanism of the hormonal factors responsible for the regulation of increased blood glucose, the paper also include the defects in insulin secretion due to impaired hormonal mechanism. In the light of this background information, it is suggested that pharmacologic and nonpharmacologic interventions may reduce the risk of hyperglycemia and insulin intolerance in the body. Physical activity, adherence to diabetic medications and dietary changes are the key to hyperglycemic control among the patients of Diabetes mellitus Type 2.
References
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes care. 2012; 35: 1364-1379.
- Gylfe E, & Gilon P. Glucose regulation of glucagon secretion.Diabetes research and clinical practice. 2014; 103: 1-10.
- Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010; 59: 285-292.
- Malanda UL, Welschen L, Riphagen II, Dekker JM, Nijpels G, & Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. The Cochrane Library. 2012.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New England Journal of Medicine. 2009; 360: 129-139.
- Tabit CE, Chung WB, Hamburg NM, & Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Reviews in Endocrine and Metabolic Disorders. 2010; 11: 61-74.
- Parving HH, Persson F, & Rossing P. Microalbuminuria: A parameter that has changed diabetes care. Diabetes research and clinical practice. 2014.
- Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, & Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011; 54: 2506-2514.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010; 33: S62-S69.
- Wei X, Meng E, & Yu S. A meta-analysis of passive smoking and risk of developing Type 2 Diabetes Mellitus. Diabetes research and clinical practice. 2015; 107: 9-14.
- Zaki NM, & Maghrabi I. Trends in therapeutic interventions in patients with diabetes mellitus in Saudi Arabia. Int J Pharm Pharm Sci. 2013; 5: 171-177.
- Murad MA, Abdulmageed SS, Iftikhar R, & Sagga BK. Assessment of the Common Risk Factors Associated with Type 2 Diabetes Mellitus in Jeddah. International journal of endocrinology. 2014.
- ALHreashy FA, & Mobierek AF. Prescription Practice for Diabetes Management among a Female Population in Primary Health Care. International journal of family medicine. 2014.
- Hayek AA, Robert AA, Al Dawish MA, Zamzami MM, Sam AE, & Alzaid AA. Impact of an education program on patient anxiety, depression, glycemic control, and adherence to self-care and medication in Type 2 diabetes. Journal of family & community medicine. 2013; 20: 77.
