Abstract
A 41-year-old obese male presented with rapid weight gain (7kg in one week) associated with bilateral oedema to the knees. He had nephrotic range proteinuria of 9 grams per day with impaired kidney function, serum creatinine 176umol/L. He was diagnosed with Type II Diabetes Mellitus (HbA1C 12.1%) and renal biopsy confirmed class III diabetic nephropathy with nodular glomeurlosclerosis.
Introduction
The incidence and prevalence of diabetes mellitus have grown significantly throughout the world, primarily due to the increasing prevalence of Type II Diabetes Mellitus [1]. This increase in the number of people developing diabetes has had a major impact on the development of diabetic kidney disease (DKD). Although kidney disease attributable to diabetes is referred to as DKD, diabetes and various kidney diseases are common chronic conditions. Thus, people with diabetes may have other aetiologies of chronic kidney disease (CKD) in addition to diabetes. Notably, DKD remains one of the most frequent complications of both types of diabetes, and diabetes is the leading cause of end-stage kidney disease (ESKD), accounting for approximately 50% of cases in the developed world (Figure 1).
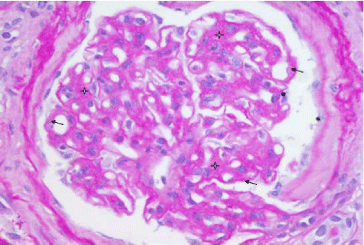
Figure 1: Class III diabetic nephropathy. Diffuse expansion of mesangium
(star) and diffuse thickening of the glomerular basement membrane (arrow).
PAS stain, X400.
The overall costs of care for people with DKD are extraordinarily high, due in large part of the strong relationship of DKD with cardiovascular disease (CVD) and development of ESKD [2]. For example, overall Medicare expenditures for diabetes and CKD in the mostly older (>65 years of age) Medicare population were approximately $25 billion in 2011. At the transition to ESKD, the per person per year costs were $20,000 for those covered by Medicare and $40,000 in the younger (<65 years of age) group. Increased albuminuria and decreased glomerular filtration rate (GFR) are each independently and additively associated with an increase in all-cause and CVD mortality, and, in fact, most of the excess CVD of diabetes is accounted for by the population with DKD.
The following article discusses the epidemiology, clinical manifestations and treatments associated with diabetic kidney disease. It illustrates the spectrum of diabetic kidney disease which General Practitioners need to appreciate.
Messages
- Diabetic Kidney Disease (DKD) is the most common cause of End Stage Kidney Disease in Australia.
- Diabetic nephropathy is heralded by the onset of proteinuria.
- Diabetic kidney disease represents a heterogeneous group of diseases.
- Proteinuria and impaired renal function are strong predictors of developing renal failure, cardiovascular morbidity and mortality.
- Certain ethnic groups – especially Australian Aborigines and Torres Strait Islanders – are genetically predisposed to DKD.
- Patients with diabetic kidney disease are susceptible to acute kidney injury.
- Recurrent episodes of acute kidney injury in diabetic kidney disease result in progressive chronic kidney disease.
- Heart disease is common in diabetic kidney disease. Diabetic kidney disease worsens diabetic heart failure; heart failure worsens diabetic kidney disease.
- Glycaemic control and blood pressure control help reduce the development and progression of both diabetic kidney disease and heart disease.
- ACEI and angiotensin II receptor blockers are useful in diabetic nephropathy and diabetic heart disease.
- Preparation for End Stage Kidney Disease requires careful planning.
- DKD has notable characteristics that distinguish it from other forms of CKD.
- Adynamic bone disease more apparent in DKD.
- Reliance on the Glomerular Filtrations Rate equations, which estimates creatinine clearance, is rife with misinterpretation in DKD.
- There are clinical indications to perform a kidney biopsy in a DKD patient.
- Patients with DKD are more predisposed to urinary tract infections.
Diabetes is the most common cause of end stage kidney disease (ESKD) in Australia
Diabetes remains the leading cause of end stage kidney disease in Australia. Approximately 5000 Australians receive renal replacement therapy (RRT) as a consequence of diabetes [3]. For every diabetic patient receiving RRT, there are approximately fifty diabetic patients with earlier stages of diabetic kidney disease.
Diabetic nephropathy is heralded by the onset of proteinuria
Classical diabetic nephropathy is defined by the onset of proteinuria (>300mg/day). In the early stages of diabetic nephropathy (incipient nephropathy), microalbuminuria is present (= 30mg/day - <300mg/day). Without specific interventions, microalbuminuria will progress over time to diabetic nephropathy, which heralds the onset of a progressive decline in renal function. Actions to slow down the progression of diabetic renal disease including, blood pressure and blood glucose control, are important treatments to reduce the incidence of ESKD.
Diabetic kidney disease represents a heterogeneous group of diseases
Classical diabetic nephropathy is not the only cause of diabetic kidney disease. Irreversible kidney disease can result from ischaemic damage to the kidneys as a consequence of repeated episodes or persistent reduction in renal blood flow as found in conditions such as heart failure, renal artery stenosis and hypertension [4]. Ischaemic nephropathy can coexist with classical diabetic nephropathy. Additionally, patients with diabetes may have other causes of renal disease including other glomerular diseases and obstructive uropathy [5].
Proteinuria and impaired renal function are strong predictors of developing renal failure, cardiovascular morbidity and mortality
Proteinuria and GFR are each significant predictors of ESKD in T2DM, and the combination of proteinuria and GFR is significantly better than either measure alone [6]. Proteinuria and impaired renal function are also well-recognized predictors of increased cardiovascular risk and mortality in diabetes [7-9]. The magnitude of proteinuria predicts an increased likelihood of cardiovascular events. In the RENAAL study, baseline proteinuria was found to be the strongest predictor of heart failure with an almost linear relationship between the two such that an increase of 1.0g/day proteinuria was associated with 26% risk for heart failure [10].
Certain ethnic groups – especially Australian Aboriginals and Torres Strait Islanders – are genetically predisposed to DKD
Incidence rates of ESKD are higher for Indigenous people compared with non-Indigenous people in all states and territories in Australia, with the highest rates recorded for Indigenous people living in the Northern Territory (1,549 per 1,000,000), Western Australia (1,194 per 1,000,000) and South Australia (876 per 1,000,000) [11]. The high rates of ESKD are a major public health problem for Indigenous people, particularly those living in remote parts of Australia. The incidence of ESKD varied greatly with remoteness. The incidence of ESKD for Indigenous people was especially high in remote and very remote areas of Australia with rates of almost 18 times and 20 times compared with non-Indigenous counterparts [11].
Patients with diabetic kidney disease are susceptible to acute kidney injury
Patients with diabetic kidney disease are at higher risk of developing acute kidney injury (AKI) compared to patients with diabetes alone. The more severe the kidney disease, the greater is the risk of AKI [12]. Potential insults to the kidney include cardiovascular disease, heart failure, medications such as antihypertensive agents, diuretics, episodes of sepsis, and increased exposure to surgical procedures or iodinated contrast agents. Patients with diabetic kidney disease are often exposed to multiple events that lead to AKI [12].
Recurrent episodes of AKI in diabetic kidney disease result in progressive chronic kidney disease (CKD)
Approximately 30% of diabetic patients with an episode of AKI will experience another AKI episode [13]. Each episode of AKI doubles the risk of progressive diabetic kidney disease and increases the risk of developing advanced stages of CKD. The effect of AKI is independent of other major risk factors of kidney disease progression [13].
Heart disease is common in diabetic kidney disease. Diabetic kidney disease worsens diabetic heart failure; heart failure worsens diabetic kidney disease
The kidney and the heart regulate extracellular fluid volume, blood pressure (BP), cardiac output, natures is and GFR. Kidney and cardiac dysfunction frequently coexist, reflecting common factors in their aetiology including diabetes, hypertension, atherosclerosis and left ventricular hypertrophy [14]. The cardio-renal syndrome describes the bidirectional relationship between the heart and kidneys whereby dysfunction in either may cause deterioration in the function of the other [14-16].
Glycaemic control and blood pressure control help reduce the development and progression of both diabetic kidney disease and heart disease
Good glycaemic and blood pressure control reduces the development and progression of diabetic nephropathy [17,18]. There is a continuous relationship between glycaemic exposure and the development of heart failure (HF), such that for each 1% reduction in HbA1c, there was an associated 16% decrease in hospitalization for HF [17]. “Tight” BP control (achieved BP 144/82 mmHg) was associated with a 56% reduction in the risk for heart failure compared with less tight control (achieved BP 154/87mmHg) [18,19]. In patients with diabetic nephropathy, systolic blood pressure and heart failure hospitalization were directly related such that achieving a 20mmHg lower systolic blood pressure was associated with a 25% reduction in heart failure events [20].
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) are useful in diabetic nephropathy and diabetic heart disease
A large body of evidence supports the use of ACEIs and ARB to treat diabetic nephropathy and diabetic heart failure [21-23]. Pivotal trials have reported a 20-30% reduction in the incidence of progressive renal disease, end stage kidney disease, and the rate of first hospitalization for new-onset HF in patients who had overt nephropathy and had received ARB compared to standard BP control. This protection is despite similar BP control between ARB and standard BP treatment.
Preparation for ESKD requires careful planning
Many patients with diabetic kidney disease will require renal replacement therapy. To reduce the risk of developing ESKD requires multi-disciplinary care across primary and secondary care. Care is aimed at optimizing blood pressure, glycaemic control and reducing other risk factors associated with progressive renal and cardiac disease. Specific attention to minimize the risk of acute kidney injury is essential to preserve renal function in diabetes. Despite effective clinical care many patients develop ESKD. Ideally ESKD should be anticipated such that the most appropriate form of treatment can be planned for and implemented [24].
DKD has notable characteristics that distinguish it from other forms of CKD
Patients are often more anaemic in CKD than in non-diabetic CKD. Inflammatory inhibitors of erythropoiesis and proteinuria, with losses of iron bound to iron-carrying proteins, have been variably implicated as causative [25]. However, the less well appreciated interstitial compartmental lesion of DKD may be the more important factor. Progressive fibrosis of this region of the kidney precludes the adaptive increase of endogenous erythropoietin production in response to increasing levels of hypoxia sensed by the organ. When inflammation, proteinuria, or both are also present, DKD patients become highly susceptible to the anaemia in CKD. This is a substantial risk multiplier for patients with diabetes, with high prevalence rates of left ventricular hypertrophy or heart failure. The addition of iron supplementation together with erythropoietin stimulating agents offermore optimal control of anaemia in patients with DKD.
A dynamic bone disease is more apparent in DKD patients
This form of CKD mineral and bone disorder is characteristic by a marked decrement in bone turnover and in the absence of osteoid accumulation. A dynamic bone disease (ABD) occurs more frequently in DKD and may be apparent before CKD Stage V [26]. This lowvolume bone disorder is associated with lower parathyroid hormone levels, increased fracture risk, and cardiovascular calcification. The pathophysiology of ABD in DKD and other forms of CKD remains enigmatic. However, the adverse consequences that attend ABD are not. Principally, when calcium and phosphorus are not set in bone, they migrate to ectopic valvular and vascular sites, aggravating coronary artery disease and peripheral artery disease.
Reliance on the Glomerular Filtration Rate (GFR) equations, which estimates creatinine clearance, is rife with misinterpretation in DKD
The GFR equations was not validated in a patient population with a large proportion of diabetics. The equation must be normalised to a body surface area of 1.72m², which is almost never done and is especially important in obese individuals because of the weight term in the numerator. Glycaemic control must be evident at the time of creatinine clearance estimation because hyperglycaemia can either promote hyper filtration or induce extracellular fluid volume depletion, thereby nullifying the validity of the creatinine clearance.
There are clinical indications to perform a kidney biopsy in the DKD patient
The routine presumption that DKD is the cause of kidney impairment in diabetic patients may be inaccurate; however, the threshold for kidney biopsy varies amongst nephrologists. A routine kidney biopsy is not required for patients fulfilling the clinical criteria for DKD (proteinuria, normal urinary sediment, normal kidney size and diabetes duration >10 years), and vascular nephropathy (normal urine status, normal or near normal protein excretion, shrinkage of kidney, renal artery stenosis on ultrasonography) [27]. A kidney biopsy should be considered in diabetic patients with chronic kidney disease in addition to the following features:
- Absence of diabetic retinopathy
- Short duration of diabetes (<5 years)
- Absence of typical chronology (e.g. acute onset of proteinuria, progressive decline in kidney function)
- Presence of haematuria
- Presence of other systemic disease
- Nephrotic syndrome
Patients with DKD are more predisposed to urinary tract infections
Predisposition to urinary tract infections in patients with diabetes mellitus results from several factors. Susceptibility increases with longer duration and greater severity of diabetes. High urine glucose content and defective host immune factors predispose to infection. Hyperglycaemia causes neutrophils dysfunction by increasing intracellular calcium levels and interfering with actin and thus diapedesis and phagocytosis [28]. The most common organisms include Escherichia coli, Klebsiella pneumoniae, and Candida [28].
Conclusion
DKD has emerged as a major aftermath of the worldwide diabetes pandemic. Therefore, diabetes prevention must remain at the cornerstone of reducing DKD. Care of patients with DKD is extraordinarily challenging due to multiple co-morbidities, disparities and complexities of healthcare delivery systems. Identification of DKD depends upon screening for increase albuminuria and low eGFR. Prevention of CVD, a major cause of death in DKD, centres upon management of LDL cholesterol and BP. Medical home models and integration of multiple risk factor management, keeping the patient-at-centre, are of great potential for high-risk groups. Importantly, team care including health care professionals from various disciplines and effective communication are requirements for successful attainment of integrated, whole-person care.
References
- de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011; 305: 2532-2539.
- US Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013: 1.7.2; 1.8.2.
- White S, et al. Diabetic kidney disease in Australia: Current burden and future projections. Nephrology. 2014; 19: 450-458.
- Chonchol M, et al. Diagnosis and Management of Ischemic Nephropathy. Clin J Am Soc Nephrol. 2006; 1: 172-181.
- Teng J, et al. Spectrum of renal disease in diabetes. Nephrology. 2014; 19: 528-536.
- Berhane AM, et al. Albuminuria and Estimated Glomerular Filtration Rate as Predictors of Diabetic End-Stage Renal Disease and Death. Clin J Am Soc Nephrol. 2011; 6: 2444-2451.
- Ninomiya T, et al. Albuminuria and kidney function predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009; 20: 1813–1821.
- Afkarian M, et al. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013; 24: 302–308.
- Debella YT, et al. Chronic kidney disease as a coronary disease equivalent–a comparison with diabetes over a decade. Clin. J. Am. Soc. Nephrol. 2011; 6: 1385-1392.
- de Zeeuw D, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004; 110: 921-927.
- Australian Institute of Health and Welfare. Aboriginal and Torres Strait Islander health performance framework 2014: detailed analyses. 2015; Canberra: Australian Institute of Health and Welfare.
- Matthew AM, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with AKI. Am J Kidney Dis. 2015; 66: 602–612.
- Thacker CV, et al. Acute Kidney Injury Episodes and Chronic Kidney Disease Risk in Diabetes Mellitus. Clin J Am Soc Nephrol. 2011; 6: 2567-2572.
- Sarnak MJ, et al. A Patient with Heart Failure and Worsening Kidney Function. Clin J Am Soc Nephrol. 2014; 9: 1790-1798.
- Ronco C, et al. Cardio-renal syndromes: Report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010; 31: 703-711.
- Gilbert RE, et al. Heart Failure and Nephropathy: Catastrophic and Interrelated Complications of Diabetes. Clin J Am Soc Nephrol. 2006; 1: 193–208.
- Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000; 321: 405–412.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998; 317: 703–713.
- Adler AI, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ. 2000; 321: 412–419.
- Berl T, et al. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol. 2005; 16: 2170–2179.
- Brenner BM, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Eng J Med. 2001; 345: 861-869.
- Parving HH, et al. The effect of ibresartan on the development of diabetic nephropathy in patients with Type 2 diabetes. N Engl J Med. 2001; 345: 870-878.
- Lewis E, et al. Renoprotective effect of the angiotensin receptor antagonist ibresartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345: 851-860.
- Andrew S. Optimal Preparation for ESRD. Narva Clin J Am Soc Nephrol. 2009; 4: S110–113.
- Gansevoort RT, Vaziri RD, de Jong PE. Treatment of anaemia of nephrotic syndrome with recombinant erythropoietin. Am J Kidney Dis. 1996; 28: 274-277.
- Weiss G, Goodnough LT. Anaemia of chronic disease. N Eng J Med. 2006; 352: S1011-1023.
- Biesenbach G, Bodlaj G, Pieringer H, et al. Clinical versus histological diagnosis of diabetic nephropathy – is renal biopsy required in type 2 diabetic patients with renal disease? QJM. 2011; 104; 771-774.
- Chen SL, Jackson SL, Boyko EJ. Diabetes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol. 2009; 182: S51-56.
