Abstract
Background: The National Center for Preventive Programs and Disease Control (CENAPRECE) reports that in Mexico more than 20,000 new cases and 2,000 deaths from tuberculosis were diagnosed in the last years. The balance in Mexico shows that the success of treatment is 78%, closer to 85% goal set by the Global Plan to Stop Tuberculosis.
Objective: To describe the follow-up of new cases of pulmonary tuberculosis from the family medicine unit #27 (FMU 27) in Tijuana, Mexico.
Design and Setting: Descriptive cross-sectional study.
Methods: Descriptive cross-sectional study. The FMU 27 epidemiology census of patients diagnosed as a new case of Pulmonary Tuberculosis was reviewed from January 2014 to December 2018. The records of patients who do not have epidemiological study or incomplete information were excluded. Sociodemographic variables, comorbidities, follow-up (based on 5 criteria: clinical control, medical consultations, blood analysis, radiological and bacteriological analysis) and treatment outcome were analyzed. Descriptive statistics were used; for quantitative variables, analysis of central tendency and dispersion were made; for qualitative, frequencies and percentages.
Results: 350 new cases of pulmonary tuberculosis were found. The result of treatment was as follows: cure 35%, complete treatment 42%, treatment failure 1%, death 2%, loss of follow-up 17% and not evaluated 3%.
Conclusion: The results of the success/cure rate are similar to the international literature.
Keywords: Pulmonary Tuberculosis; Treatment; New Case
Introduction
Tuberculosis (TB) is a chronic infectious disease caused by a group of Actinomycetal bacteria of the Mycobacteriaceae family. The M. tuberculosis complex includes M. tuberculosis, M. africanum, M. bovis, M. microti and M. canetii [1]. Pulmonary TB has nonspecific clinical manifestations, the most frequent symptoms are: cough, expectoration, chest pain and general symptoms (fever, sweating, asthenia, anorexia and weight loss) [2]. The diagnosis of pulmonary tuberculosis begins with a clinical examination and risk factors. Paraclinical analysis includes: microscopic study of acid-alcohol resistant bacilli (BAAR), sputum culture and mycobacterial identification. The shortened primary treatment of tuberculosis includes: Isoniazid (H), Rifampicin (R), Pyrazinamide (Z) and Etambutol (E), it is granted to any new case that has never received treatment [3-4].
The shortened primary treatment should be administered for approximately 25 weeks (105 doses), divided into intensive phase, 60 doses (daily from Monday to Saturday with HRZE); and maintenance phase, 45 doses (intermittent, 3 times a week, with HR). According to the result of treatment according to the World Health Organization (WHO) and PanAmerican Health Organization (PAHO) all cases of bacteriologically confirmed and clinically diagnosed TB should be assigned to a result, except those with drug-resistant and multi-drug resistant TB, which are placed in a scheme with second line medications [5].
The classification according to the result is: cure, patient with pulmonary TB with bacteriology confirmed at the beginning of the treatment and who has negative baciloscopy or culture in the last month of treatment; complete treatment, patient with TB who completed the treatment without evidence of failure, but without result of baciloscopy or sputum culture in the last month of treatment and at least on a previous occasion they were negative; treatment failure, patient with TB whose baciloscopy or culture is positive in month five or later during treatment; death, patient with TB who dies for any reason before starting or during treatment; loss of follow-up, patient with TB who did not initiate treatment or discontinued treatment for two consecutive months or more; not evaluated, a patient with TB who has not been assigned the treatment outcome, this last category includes cases transferred to another treatment center and also cases whose treatment outcome is unknown [6].
According to the current standards for Tuberculosis care in Mexico, the case follow-up is carried out properly by the following points: clinical control, is carried out every month or in less time when the patient requires it to review the general condition, evaluation of symptoms, verification of intake and tolerance to drugs, detection of signs of toxicity and registration of adjustments for approximately 6 consultations, with blood tests. Bacteriological control: a monthly baciloscopy is required. The evaluation will be favorable when the baciloscopy is negative from the second month of treatment or before and persists negative until the end of the treatment; and unfavorable when it persists positive from the fourth month of treatment (suspected failure or drug resistance) or presents positive baciloscopy in consecutive months after a period of negativization. Radiological control: if possible, every 2-3 months. Evaluation of the primary treatment and case classification: when completing the primary treatment (6 months) the case should be classified as cure, term of treatment, failure or death; cases that do not end treatment are classified as abandonment, transfer or death [7].
In Mexico there are no previous studies evaluating the follow-up of cases in patients diagnosed with tuberculosis. The WHO World Report (2018) on tuberculosis says that the outcome of treatment for TB cases in the Americas (2016) was: 75.4% success, no evaluation 8.3%, loss of follow-up 8.6%, deaths 7.3% and failures 0.5% [8]. The objective of the research was to describe the follow-up of new cases of pulmonary tuberculosis in FMU 27 in Tijuana, Baja California, Mexico.
Methods
Study design and population
A cross-sectional descriptive study was carried out to describe the follow-up of new cases of pulmonary tuberculosis from January 2014 to December 2018. The research was developed in FMU 27 of the Instituto Mexicano del Seguro Social (IMSS), primary care unit and main center of health care in the city. Medical records of patients diagnosed with a new case of tuberculosis who started primary treatment were included. The files of patients who did not have an epidemiological study in a clinical records or incomplete information were excluded. The information was obtained from the epidemiology census of this health center.
Variables
The variables were obtained through the standardized treatment sheet for each of the patients diagnosed as a new case of pulmonary tuberculosis. Other sources of variable collection were the epidemiological study carried out by the epidemiology department, medical notes of the family physician through the Family Medical Information System (SIMF) and laboratory studies through the portal "WINlab" system of laboratory results on line. The radiological results were collected from the information described in the medical care notes. Follow-up criteria were used according to the operational definitions of the “Standards for Tuberculosis Care in Mexico” [7].
The criteria to evaluate a case follow-up were five: criterion 1, monthly clinical control, where the intake and tolerance to the drugs is verified, detection of signs of toxicity, evaluation of the primary treatment and classification of case; criterion 2, number of medical evaluations (at least 6); criterion 3, blood test, blood count, glucose, creatinine, urea nitrogen and liver function; criterion 4, radiological control at the beginning and two months after the start of treatment; criterion 5, bacteriological control in the first month, second, fourth and at the end of treatment; the compliance of the follow-up of the new cases was integrated with all the previous points and was expressed as an average follow-up on a scale of 0-100%. The final treatment classification was cure, complete treatment, treatment failure, death, loss of follow-up and not evaluated.
Statistical analysis
The data obtained was integrated into the data collection sheets and analyzed using the SPSS program version 21 in Spanish. We perform descriptive statistics; for qualitative variables frequencies and percentages; for quantitative variables, mean and standard deviation.
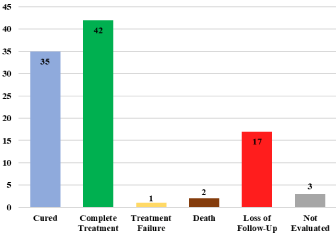
Figure 1: Distribution according to the treatment result.
Ethics
The study was approved by the local health research and ethics committee #204; with registration number R-2019-204-012. The research was conducted under the general health law on health research, bioethical principles and the Helsinki declaration.
Results
350 new cases of pulmonary tuberculosis were found. In the sociodemographic variables 61% (n=215) were men and 39% (n=135) women, the age ranged from 31-45 years. The state of birth with the highest number registered was Baja California 32% (n=112), Chiapas 12% (n=43) and Sinaloa 11% (n=40). According to comorbidities, 24% (n=83) had Diabetes Mellitus, 6% (n=20) human immunodeficiency virus infection, 7% (n=23) alcoholism and 1% (n=4) history of use of intravenous drug. An average of 67.55% of the total case follow-up percentage was obtained. According to the treatment result (Figure 1) the following results were obtained: cure 35% (n=123), complete treatment 42% (n=146), treatment failure 1% (n=5), death 2% (n=7), loss of follow-up 17% (n=59) and not evaluated 3% (n=10).
Discussion and Conclusion
The most important result of our study was a success/cure rate very similar to national and international literature. Our success/cure percentage was 77% considering cases of cure and complete treatment. According to WHO (2016), the outcome of treatment successful/Cured for TB cases in the Americas was 75% and in the Central America and Mexico region 82% [8]. According to the National Center for Preventive Programs and Disease Control (CENAPRECE), the states with the highest incidence of new cases of pulmonary tuberculosis in Mexico (2016) were Veracruz (n=1,895), Baja California (n=1,587), Nuevo León (n=1,261), Chiapas (n=1,115), Guerrero (n=1,087) and Tamaulipas (n=1,014) [9]. Our study identified that the most frequent birth states are Baja California, Chiapas and Sinaloa, which is similar to the national incidence.
It was observed that 146 (52%) patients finished the treatment and 59 (17%) there was a loss of follow-up, this impacts the follow-up of the case since it is unknown if the patients developed drug resistance. In the literature reviewed, no study has been identified where patients with tuberculosis are followed up; García, in Cuba, only identified the main weaknesses in the follow-up of patients with tuberculosis, they defined as a control indicator when there were 6 visits, relating it to sociodemographic variables such as sex and age group [10]. Torres and Herrera in Chile assessed the treatment outcome of 134 audits according to the profile of the patient leaving the tuberculosis treatment, identifying as possible factors male sex, the age between 15 and 45 years and being a new case [11]. Muñoz-Sánchez, in Colombia focused his research on describing the actions that were carried out for the early diagnosis and monitoring of tuberculosis based on microscopy, identifying that of 273 health workers, only 52% were monitored for contacts with tuberculosis, 34% verified the follow-up and 41% requested baciloscopy from patients with symptoms [12].
Patient follow-up has an epidemiological and economic impact on the IMSS and society. Baja California is the state with the highest incidence rate of this disease in the country, as well as high migration zone. It is recommended to carry out strategies to adequate follow-up by primary care doctors. There is an area of opportunity to implement strategies and programs to encourage, verify and evaluate the knowledge of primary care physicians to monitor patients with tuberculosis and find risk factors associated with abandonment of treatment.
References
- Sánchez I, Ussetti P, Melero C. Tuberculosis: aspectos epidemiológicos. Etiopatogenia. Manifestaciones clínicas. Diagnóstico. Medicine. 1998; 7: 3666-3671.
- Diagnóstico y Tratamiento de Casos Nuevos de Tuberculosis Pulmonar. México: Secretaria de Salud; 2009.
- Ramírez-Lapausa M, Menéndez-Saldaña A, Noguerado-Asensio A. Tuberculosis extrapulmonar, una revisión. Rev Esp Sanid Penit. 2015; 17: 3-11.
- Mendoza-León C. Diabetes mellitus mal controlada como factor de riesgo para tuberculosis resistente en el Hospital Nacional Daniel Alcides Carrión durante el periodo 2010-2012. Interciencia. 2014; 5: 35-40.
- Nathanson E, Nunn P, Uplekar M. MDR Tuberculosis Critical Steps for Prevention and Control. NEJM. 2010; 363: 1050-1058.
- World Health Organization (WHO). Definiciones y marco de trabajo para la notificación de Tuberculosis; 2013.
- Secretaria de Salud. Estándares para la Atención de la Tuberculosis en México. Mexico: Secretaría de Salud; 2013.
- Pan American Health Organization. Tuberculosis in the Americas 2018. Washington, D.C.: PAHO; 2018.
- Centro Nacional de Programas Preventivos y Control de Enfermedades (CENAPRECE). Cifras Oficiales tuberculosis agosto de 2016; 2016.
- García-de la Rosa RG, Davis-Norales A, Rodríguez-Rodríguez O. Control de la calidad del seguimiento de contactos de tuberculosis en Camagüey. MEDISAN. 2014; 18: 355-363.
- Torres Z, Herrera T. Perfil del paciente con tuberculosis que abandona el tratamiento en Chile. Rev Chil Enf Respir. 2015; 31: 52-57.
- Muñoz-Sánchez AI, Puerto-Guerrero AH. Acciones programáticas relacionadas con el diagnostico precoz y seguimiento de pacientes con tuberculosis en dos localidades de Bogotá. Colombia. Investigaciones Andina. 2014; 29: 1045-1058.
