Abstract
General Practitioners (GPs) in Western societies diagnose a great number of patients with anemia, i.e. a lowered concentration of hemoglobin in the blood. The high prevalence of anemia combined with the need for effectively establishing the underlying etiology in order to initiate proper management, calls for an optimal diagnostic strategy. Due to the significant role of the primary care sector in anemia assessment, we chose to focus this review on anemia and evidence-based clinical guidelines for its diagnosis and management as they pertain to the GP in the Western world, i.e. Europe, the USA, Canada and Australasia. A PubMed literature search was performed using PubMed’s MeSH terms of relevance to the most common anemia types in primary care in regard to diagnostic strategies and management.
A panel of 18 routine blood tests, which will enable the GP to diagnose anemia and the underlying etiology were presented and elaborated upon. Based on a smaller basic initial set of these tests, two flowcharts were also presented to help the GP reach the correct etiology effectively. Finally, different strategies on how the GP may order a relevant set of laboratory tests were discussed.
Keywords: Anemia; General Practice; Diagnosis; Iron Deficiency; Vitamin B12; Vegetarians
Abbreviations
Alanine Aminotransferase (ALAT); Anemia of Chronic Disease (ACD); Chronic Kidney Disease (CKD); Colorectal Cancer (CRC); C-Reactive Protein (CRP); General Practitioners (GPs); Glucose-6-Phosphate Dehydrogenase (G6PD); Inflammatory Bowel Disease (IBD); Iron Deficiency Anemia (IDA); Lactate Dehydrogenase (LDH); Mean Corpuscular Hemoglobin (MCH); Mean Corpuscular Volume (MCV); Methylmalonic Acid (MMA); Myelodysplastic Syndromes (MDS); Nonsteroidal Anti-Inflammatory Drugs (NSAID); Point-of-Care Testing (POCT); Randomized Controlled Trial (RCT); Red blood cells (RBCs); Red Blood Cell Distribution Width (RDW); Subclinical Vitamin B12 Deficiency (SCCD); Transient Erythroblastopenia of Childhood (TEC); World Health Organization (WHO)
Introduction
Anemia (i.e. a lowered concentration of hemoglobin in blood) is a common finding in general practice and is associated with a wide range of benign and malignant conditions [1-3]. The World Health Organization (WHO) defines anemia as a hemoglobin level of less than 13 g/dL (8.1 mmol/L) in men (15 years of age and above) and less than 12 g/dL (7.5 mmol/L) in non-pregnant women (15 years of age and above) and in children 12 to 14 years of age. Pregnant women and children below the age of 12 have different hemoglobin reference intervals [4]. As estimated by the WHO, roughly 20% of children less than 5 years of age and around 25% of pregnant women in Europe are anemic [5]. Anemia in pregnancy is not considered within the scope of this review and will not be discussed any further. Furthermore, it is reported that an overall 17% of the elderly (aged 65 years or above) in developed countries have anemia [6]. Despite the WHO definition, the cutoff values for a low concentration of hemoglobin vary between countries and laboratories because the cutoff values must reflect the population for whom they are used [7].
To understand anemia, a basic understanding of erythropoiesis, and the process by which Red Blood Cells (RBCs) are produced is essential. Erythropoietin, a crucial regulatory hormone facilitating erythropoiesis, is primarily produced in the kidneys and stimulates both the production and maturation of erythroid precursor cells in the bone marrow. Also critical to a normal erythropoiesis is the availability of key nutrients, such as iron, vitamin B12 and folate, as well as a healthy bone marrow, that is without bone marrow failure syndromes, and a normal hemoglobin type [8].
Anemia is traditionally classified based on etiology or cell morphology. The 3 main etiological causes of anemia are 1) increased RBC loss (bleeding), 2) decreased RBC production (nutritional deficiency and bone marrow failure syndromes) and 3) increased RBC destruction (hemolysis). These causes may again present themselves morphologically in RBCs through different *(MCV), resulting in microcytic (<80 fL), normocytic (80 to 100 fL) and macrocytic (>100 fL) types of anemia [9,10] (Figure 1).
General Practitioners (GPs) give advice concerning management including dietary recommendations and even refer patients to secondary care in severe or complicated cases. However, unless considered severe, anemia is often overlooked by the primary care physician. It is therefore critical to recognize that even mild anemia may be an indication of a serious underlying condition such as malignancy [1,8].
The high prevalence of anemia combined with the need for effectively establishing the underlying etiology in order to initiate proper management, calls for an optimal diagnostic strategy. Due to the significant role of the primary care sector in anemia assessment, we therefore chose to focus this review on anemia and its diagnosis and management as they pertain to the GP in the Western world, namely Europe, the USA, Canada and Australasia.

Figure 1: Classification of anemia based on pathophysiological or morphological characteristics. RBC: Red Blood Cell.
Methods
A PubMed literature search was performed using PubMed’s MeSH terms: vitamin B12/cobalamin deficiency, folic acid deficiency, megaloblastic anemia, iron deficiency anemia, autoimmune hemolytic anemia, cold agglutinin/antibody disease, congenital/hereditary hemolytic anemia, chronic disease, inflammatory/secondary anemia, general/family practice/practitioners, primary health care and primary care physicians. After producing 878 search results, all the article abstracts were then screened to assess whether the search results were of relevance to the focus of this review. Systematic reviews Randomized Controlled Trials (RCTs) and cohort studies were prioritized. Moreover, articles focusing on anemia in primary care were given priority over anemia in secondary care. In order to get a broad perspective on anemia, articles with a variety of patient groups were included, namely adults, the elderly, children and vegetarians, although Western society patients were prioritized. Accordingly, articles including patients from Europe, the USA, Canada and Australasia were favored. As the number of relevant articles, references and keywords increased along with even more relevant articles presented through the ‘Similar articles’ tab on PubMed, our literature search on PubMed was refined. This approach produced a final pool of 50relevant research articles including4 systematic reviews, 1 RCT, 14other original articles, 30 reviews and 1 clinical report, with a publishing span from 1983 to 2020. Contradicting results and perspectives on the subject were noted and presented.
Clinical Diagnosis
History Taking
Identifying key risk factors as well as uncovering relevant symptoms in the clinical history are an important first step. Some adults and most children with mild anemia will be asymptomatic, exhibiting no clinical signs or symptoms [11,12]. In acute presentations, patients may display symptoms of dizziness, hypotension and syncope. In chronic anemia, the onset is usually more insidious. The elderly cannot increase heart rate, cardiac output and other compensatory mechanisms as readily as younger people, with fatigue, dyspnea, weakness and worsening of comorbid conditions (angina, heart failure, Chronic Kidney Disease (CKD), chronic obstructive pulmonary disease etc.) becoming more common as chronic anemia worsens. As the latter conditions may cause many of the same symptoms as anemia, a high clinical suspicion is necessary, particularly in older adults [11, 13].
Children
A thorough history concerning children should address following prioritized risk factors: nutritional status, infections, chronic diseases, family history of anemia including ethnicity and finally symptoms and objective findings indicative of leukemia and other hematological malignancies in children [12] (Table 1).
Nutritional deficiency and excessive cow’s milk consumption are indicative of Iron Deficiency Anemia (IDA). Anemia of Chronic Disease (ACD) should be suspected in the case of infections, autoimmune disorders or chronic diseases such as Inflammatory Bowel Disease (IBD), celiac disease or renal diseases. In addition, recurrent diarrhea raises suspicion of malabsorption and occult blood loss occurring also in celiac disease and IBD. A consideration of hereditary disorders, such as hereditary spherocytosis and thalassemia, is warranted in long-standing anemia, especially in patients with immigrant backgrounds. Leukemia and other hematological malignancies are critical not to overlook and will be specified further below. Lastly, recent prescription drug use may suggest Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency or a plastic anemia, and recent viral illness may suggest Transient Erythroblastopenia of Childhood (TEC) [14].
Adults
Questions regarding adults and the elderly could be prioritized as follows: blood loss, nutrition and dietary habits, chronic (and acute) diseases, alcohol consumption, medication, gastrointestinal disorders, such as recurrent diarrheas, and malignancies including Myelo Dysplastic Syndromes (MDS).
Melena, hematochezia and unintentional weight loss may indicate gastrointestinal bleeding of various causes, and hematuria is to be suspected in the case of dark or red urine. Dietary history is important, not only with the purpose of identifying a potential IDA, but also in order to assess the increased risk of vitamin B12 deficiency in patients following strict vegan diets. Infections, autoimmune disorders, CKD and other chronic inflammatory diseases are also associated with anemia in adults, and chronic alcohol consumption is linked to an increased risk of folate deficiency, bleeding peptic ulcer disease and bleeding varices. A careful medication review may be useful, paying particular attention to Non steroidal Anti-Inflammatory Drugs (NSAID), warfarin and other drugs increasing the risk of bleeding [8,13]. Finally, it is important not to overlook signs of more rare, but serious illness, such as underlying malignancy and MDS, especially in older adults [15]. Therefore, recurrent infections and skin bleeds suggestive of a more broad bone marrow failure as well as so-called B symptoms (e.g. fever, night sweats and weight loss) indicating a potential malignancy should always be taken into consideration [11] (Table 2). The presence or absence of these risk factors should guide further evaluation.
Risk factors
Differential diagnoses
Nutritional deficiency
Excessive cow's milk consumptionIron deficiency
Infections
Chronic diseases
Autoimmune disordersACD (e.g. IBD, celiac disease, renal diseases, pneumonia)
Recurrent diarrhea
Malabsorption (e.g. celiac disease)
Occult blood loss (e.g. IBD)Family history of anemia
Ethnicity
Long-standing anemiaHereditary disorders (e.g. hereditary spherocytosis, thalassemia)
B symptoms (e.g. fever, night sweats and weight loss)
Recurrent infections
Skin bleedsMalignancy - leukemia and other hematological malignancies
Recent prescription drug use
G6PD deficiency
Aplastic anemiaRecent viral illness
TEC
ACD: Anemia of Chronic Disease; IBD: Inflammatory Bowel Disease; G6PD: Glucose-6-Phosphate Dehydrogenase; TEC: Transient Erythroblastopenia of Childhood.
Table 1: Key risk factors to address in the history taking when suspecting anemia in children.
Risk factors
Differential diagnoses
Melena
Hematochezia
Unintentional weight lossGastrointestinal bleeding of various causes
Dark or red urine
Hematuria
Nutritional deficiency
Iron deficiency
Vitamin B12 deficiency (e.g. due to strict veganism)Infections
Chronic diseases
Autoimmune disordersACD (e.g. IBD, celiac disease, renal diseases, pneumonia)
Chronic alcohol consumption
Folate deficiency
Peptic ulcer disease
Bleeding varicesMedication (e.g. NSAID, warfarin)
Recent surgeryInternal bleeding
Recurrent diarrhea
Malabsorption (e.g. celiac disease)
Occult blood loss (e.g. IBD)B symptoms (e.g. fever, night sweats and weight loss)
Recurrent infections
Skin bleedsBone marrow failure (e.g. MDS)
Malignancy - both hematological and solid tumors
ACD: Anemia of Chronic Disease; IBD: Inflammatory Bowel Disease; MDS: Myelodysplastic Syndrome; NSAID: Nonsteroidal Anti-Inflammatory Drug.
Table 2: Key risk factors to address in the history taking when suspecting anemia in adults.
Clinical Examination
The clinical examination of anemic patients mainly revolves around uncovering conjunctival pallor since other signs such as increased heart rate and shortness of breath may be indicative of the anemic state per se, but are not very specific. Conjunctival pallor correlates well with severe anemia, and the observation of this phenomenon in a patient, even without other information suggesting an anemic state, should lead the GP to determine the hemoglobin concentration [16]. Other noteworthy clinical findings include accompanying jaundice, which in the presence of anemia points to an etiology of hemolysis, as well as lymphadenopathy, hepatosplenomegaly, bone tenderness, petechiae and ecchymoses [8].
Laboratory Diagnosis
Based on a panel of 18 routine blood tests (Table 3), the medical history and physical examination, the GP will be able to unravel the underlying etiology and decide to either continue evaluation and treatment in primary care or to refer the patient to secondary care.
From a pure pragmatic perspective, there are different strategies for the GP to achieve a relevant set of the mentioned laboratory tests: The routine-strategy in which the tests are ordered by the GP step by step, the extensive-strategy in which all the laboratory tests are ordered by the GP all at once, and finally analgorithmic-strategy in which an anemia-workup test is ordered by GP and the laboratory performs an algorithm-based analysis of relevant succeeding tests depending on the previous test results (see Supplementary Information for more details on diagnostic strategies) [17]. Ultimately, the choice of diagnostic strategy relies on the country and its laboratories as well as the organization of the primary health care including insurance and reimbursement.
A basic set of initial laboratory tests and two flowcharts are presented in the following sections to help the GP identify the underlying etiologies of anemia. The flowcharts are presented independently of the strategy used by the GP to order the laboratory tests and are based on the understanding that many anemia types may be multifactorial, and that identifying the underlying causes conclusively may not always be possible.
The basic set of initial laboratory tests (highlighted in blue in Table 3) includes hemoglobin and, if anemia is documented, mean corpuscular volume, Red Blood Cell Distribution Width (RDW), leukocytes, platelets and reticulocyte counts. The MCV allows placement of the anemia into one of the standard classifications of microcytic, normocytic or macrocytic anemia [10,11,14]. The reticulocyte count distinguishes a hypoproliferative anemia (decreased RBC production) from an anemia due to blood loss or hemolysis (increased RBC destruction). An elevated reticulocyte count suggests an appropriate response by the bone marrow to the anemia, whereas a low reticulocyte count may signal bone marrow disorders or aplastic crisis [8,14].
Blood tests
Comments
Hemoglobin
Confirms the anemia.
MCV
Allows placement of the anemia as microcytic, normocytic or macrocytic.
RDW
Anisocytosis. High values support ongoing changes in RBC size (either as a result of development of microcytic/macrocytic anemias or as a response to treatment of microcytic/macrocytic anemias). In anemias due to deficiencies, the RDW becomes abnormal before the hemoglobin and MCV do.
Leukocytes
Leukocytosis is present in chronic diseases and infections. Abnormal counts are also common in bone marrow disorders (e.g. leukemia and MDS).
Thrombocytes/platelets
Thrombocytosis is present following blood loss. Moderate thrombocytosis is present in acute and chronic inflammatory responses and often in IDA. Abnormal counts are also common in bone marrow disorders (e.g. leukemia and MDS).
Reticulocyte count
Distinguishes a hypoproliferative anemia (decreased RBC production) from an anemia due to blood loss or hemolysis (increased RBC destruction).
ReticMCH
Mean corpuscular hemoglobin content. Low in IDA and thalassemia.
CRP
Inflammation (acute phase reactant). Also allows more correct interpretation of ferritin.
Creatinine
Low hemoglobin combined with an elevated creatinine supports anemia due to renal disease.
Ferritin
Evaluates the level of iron storage under normal conditions (may act as an acute phase reactant).
Iron and transferrin
The transferrin saturation is indicative of the availabiblity of iron, i.e. a low saturation suggests IDA. Iron as a solitary test is not a valid marker of IDA and should not be used in the assessment of anemia.
Vitamin B12 (MMA)
Vitamin B12 deficiency. Methylmalonic acid only if vitamin B12 is inconclusive (grey zone result).
Folic acid
Folate deficiency.
LDH, Bilirubin (ALAT)
Hemolysis. Normal ALAT excludes liver disease as a cause of high LDH.
Haptoglobin
Hemolysis (binds free hemoglobin and is thus low in hemolytic states).
Blue highlights basic laboratory tests to obtain in the initial blood test. ALAT: Alanine Aminotransferase; CRP: C - reactive protein; IDA: Iron Deficiency Anemia; LDH: Lactate Dehydrogenase; MCH: Mean Corpuscular Hemoglobin; MCV: Mean Corpuscular Volume; MMA: Methylmalonic Acid; RBC: Red Blood Cell; RDW: Red Blood Cell Distribution Width.
Table 3: A panel of routine blood tests relevant for GPs in the diagnosis of anemia and the underlying etiology.
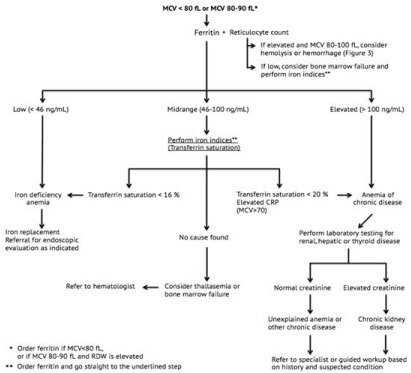
Figure 2: Flowchart for the diagnosis of microcytic and normocytic anemia. CRP: C-reactive protein; MCV: mean corpuscular volume; RDW: red blood cell
distribution width.
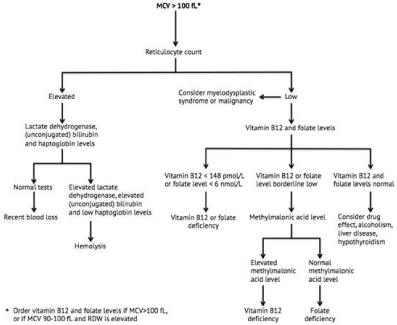
Figure 3: Flowchart for the diagnosis of macrocytic anemia. Adapted with permission from Bross MH, et al. [13] MCV: mean corpuscular volume; RDW: red blood
cell distribution width.
Microcytic and Normocytic Anemia
The most prevalent types of anemia in primary care are IDA (16-19 %) and ACD (20-31%), the latter being immune-driven and developing secondary to acute and chronic infections, autoimmune disorders, renal failure, heart failure and malignancies and becoming increasingly prevalent with age [17-19]. Despite microcytic anemia being classically associated with IDA, and normocytic anemia with anemia of chronic or unknown disease, there is still a remarkable overlap between the manifestations of these diseases [11,20,21] (Figure 2). For instance, up to 25% of anemias of chronic disease depict microcytic cells, and up to 40% of IDA cases are estimated to be normocytic [21, 22]. A reason for this may be the fact that ACD and IDA can both be present concurrently in the same patient [23]. In normocytic anemias with elevated reticulocyte counts, hemolysis and hemorrhage should always still be suspected. This topic will be further discussed under macrocytic anemias (Figure 3), where these conditions are also seen commonly.
Ferritin measurement is a sensitive parameter for the assessment of iron stores in patients. A value of less than 15 µg/L confirms the diagnosis of IDA, whereas a value above 100 µg/L rules out IDA and points to ACD. Since ferritin is an acute phase reactant, concentrations may be elevated in the presence of inflammation or infection, thus a simultaneous measurement of C-Reactive Protein (CRP) is recommended to rule out inflammation [13,24]. A ferritin level below 35 µg/L is still highly suggestive of IDA [18,20]. In older adults a cutoff of 45 µg/L has even proven to have a higher sensitivity [25]. Some countries and laboratories aim to save costs by only requesting ferritin in certain cases; here the RDW becomes a useful parameter (Table 3). Ferritin should be ordered if the MCV is below 80 fL, but a MCV of 80 to 90 fL combined with a high RDW may be the first signs of IDA and should also prompt the GP to order a ferritin measurement. The same analogy applies to borderline macrocytic cases (MCV of 90 to 100 fL) with high RDWs, which should prompt the GP to order vitamin B12 and folate levels.
Endoscopic evaluation should be considered in patients with IDA, particularly in the elderly, as it may well be a sign of gastrointestinal bleeding or malignancy. In a British RCT from 2002, Colorectal Cancer (CRC) was identified in 7% of referred patients with IDA [26]. Another large British primary care case-control study from 2008 confirmed IDA as an independent predictor of CRC, with a positive predictive value of 13% (95% CI: 9.7 to 18) for men and 8% (95% CI: 5.7 to 11) for women aged over 60 years [27].
When ferritin levels are in the midrange of approximately 45 to 100 µg/L, neither IDA nor ACD can be excluded [18,28,29]. In these patients, certain iron indices should be performed to distinguish between IDA and ACD. The transferrin saturation is here the critical laboratory test and is calculated based on transferrin and the iron level. A low transferrin saturation signifies an iron supply insufficient to support normal erythropoiesis. Even though transferrin saturation is low in both IDA and ACD, it tends to be even lower in IDA (<16%) compared to ACD (<20%) [1,30]. Transferrin is usually elevated in IDA and low in ACD [18].The iron level is low in both IDA and ACD, and it increases after recent oral intake, thus making it an unspecific measurement. Consequently, iron should not be used in the evaluation of anemia. A few other important parameters, however, may be very useful, especially when suspecting ACD. For example, the MCV is rarely less than 70 in ACD, and patients with ACD generally have mildly or moderately decreased hemoglobin levels of around 9.5 g/dL (5.9 mmol/L) to 8 g/dL (5 mmol/L). Furthermore, the reticulocyte count is remarkably low for the degree of anemia reflecting decreased RBC production. Lastly, increased inflammatory markers such as CRP support the diagnosis of ACD [1,18,21]. In older adults, ACD is often caused by CKD. This can be assessed by obtaining a basic metabolic panel, including creatinine and glomerular filtration rate and also testing for hepatic and thyroid function in more rare cases [11].
If the cause of anemia remains obscure, thalassemias and bone marrow failure should be suspected. A very low reticulocyte count is characteristic of bone marrow failure, such as MDS and leukemia, which are often accompanied by other abnormalities in peripheral blood counts and warrants prompt referral to a hematologist [31]. Where leukemia is encountered in people of all ages, MDS is much more common in the elderly. Myelodysplastic syndromes should always be a diagnostic consideration when anemia is accompanied by white cell or platelet abnormalities, but isolated anemia is also seen in MDS [1,15,30]. Thalassemia, a hemoglobinopathy, is suspected when there is a family history of achronic, mild and Microcytic Anemia (MCV of 75 fL or less) not responsive to iron supplements [1,32]. Due to the presence of Hemoglobin F at birth, newborns with thalassemia should be expected to be asymptomatic until Hemoglobin A becomes predominant at 6 months of age [33]. Performing a hemoglobin electrophoresis is required to confirm the thalassemia diagnosis [34].
Macrocytic Anemia
Although rare in children, macrocytic anemia is less uncommon among adolescents and adults. Approximately up to 2% of the elderly US population has clinical vitamin B12 deficiency, and up to 20% has Subclinical vitamin B12 Deficiency (SCCD) [35,36]. The reticulocyte count obtained in the initial laboratory tests is the first step in the evaluation of macrocytic anemia. A decreased reticulocyte count should lead the GP to obtain the vitamin B12 and folate levels as well as consider bone marrow disorders and malignancies as differential diagnoses. On the contrary, an elevated reticulocyte count is associated with an increased RBC production by the bone marrow and thus indicates hemolysis or recent blood loss, please refer below [13,37].
Vitamin B12 and folate deficiency anemia, commonly referred to as megaloblastic anemias due to the appearance of structurally abnormal immature RBCs, require thorough laboratory evaluation by the GP due to various cutoff values and hence sensitivities and specificities for current diagnostic biomarkers [38,39]. Conventionally, a decreased vitamin B12 or folate level is indicative of the diagnosis of vitamin B12 or folate deficiency, respectively. If these biomarkers appear in the lower part of the reference range or are equivocal in any other way, Methylmalonic Acid (MMA) levels are more sensitive for the diagnosis of vitamin B12 deficiency. MMA levels are elevated in vitamin B12 deficiency but not in folate deficiency. Measurement of intrinsic factor antibodies should be obtained if pernicious anemia is suspected [40]. Clinical data show that approximately 2.5% to 5.2% of patients diagnosed with vitamin B12 deficiency have vitamin B12 levels above a commonly used lower cutoff value of 148 pmol/L. In addition, an even higher proportion of patients with abnormal MMA levels display vitamin B12 levels above 148 pmol/L [36,38]. Nonetheless, the sensitivity of this cutoff value amounts to 95-97% for clinical vitamin B12 deficiency, and even though its specificity has not yet been formally determined (but estimated <80%), 148 pmol/L is still the cutoff value generally used by clinicians and scientists. The sensitivity of an elevated MMA is above 95% in clinical vitamin B12 deficiency, but this biomarker is also more expensive than a vitamin B12 measurement. Still, due to vitamin B12 being remarkably less sensitive in diagnosing SCCD compared to clinical vitamin B12 deficiency, MMA is recommended in subclinical cases if possible [38,41]. In the literature, the lower folate cutoff values are included in the range of 6-11 nmol/L, with values below 6-7 nmol/L being associated with folate-responsive megaloblastic anemia. A large retrospective Belgian study from 2013 showed that changes in hemoglobin and RBC indices including MCV can be detected by folate levels of 11 nmol/L and below [39]. Taking all this into account, vitamin B12 and folate are important first choice diagnostic biomarkers, but MMA can be highly crucial inequivocal and subclinical cases.
Various drug effects, alcoholism, liver disease and hypothyroidism should be suspected in the case of a low reticulocyte count and normal vitamin B12 and folate biomarkers. Bone marrow disorders and malignancies are also severe differential diagnoses and are mentioned previously under microcytic and normocytic anemia. Drug therapy and alcoholism account for around half of the cases of macrocytosis and a plethora of drugs may cause macrocytic non-megaloblastic anemia. This includes treatment with azathioprine, chemotherapy, antiretroviral therapy and anticonvulsants, to name a few [37]. Alcoholism is often related to alcoholic liver disease and poor nutrition resulting in folate deficiency. Even in the absence of these causes, chronic alcohol abuse is known to lead to the development of macrocytosis, also before the occurrence of anemia. Accordingly, some 2 to 4 months of abstinence from alcohol usually results in the resolution of the macrocytosis [42].
Hemolytic anemia, an innate or acquired condition with increased RBC destruction,is a common reason for macrocytic or normocytic anemias with reticulocytosis [37,43]. When the latter is identified, laboratory testing should therefore include measurement of lactate dehydrogenase, (unconjugated) bilirubin levels as well as haptoglobin. The constellation of reticulocytosis, increased lactate dehydrogenase levels, increased (unconjugated) bilirubin levels and decreased (often immeasurable) haptoglobin levels confirms the diagnosis of hemolysis. Such patients should be readily discussed and referred to a hematologist for further workup. Absence of these findings or normal hemolytic tests should prompt a search for other possible causes, such as recent blood loss [43]. In children, a rapid onset of anemia or significant hyperbilirubinemia in the neonatal period should also warrant consideration of hemolytic anemia [43].
Vegetarians and Vegans - An Important Risk Group
In recent years, the number of consumers following a vegetarian or vegan diet has increased remarkably in many Western countries, with an estimated prevalence of individuals following these diets varying between 1% and 10% in the EU, the USA and Canada [44,45]. Although some plant-based food contains vitamin B12, it may not provide a sufficient amount to meet the recommended dietary allowance of 2.4 µg per day. Vegetarians and vegans adhering to only plant-based food are in this way at risk of inadequate intake of vitamin B12 and consequently susceptible to vitamin B12 deficiency [46,47]. Previously, it was thought that only vegans are susceptible to vitamin B12 deficiency, but studies have since found that vegetarians develop vitamin B12 deficiency regardless of demographic characteristics, residency, age or type of vegetarian diet [47]. Nonetheless, studies still report a higher prevalence of deficiency among vegans compared to vegetarians according to a systematic review from 2014 [48]. Vegans, vegetarians and those consuming food low on vitamin B12 as well as children breastfed by vitamin B12 deficient mothers typically develop a mild deficiency [49]. Mild deficiencies manifest as fatigue and anemia, indices suggesting vitamin B12 deficiency, and an absence of neurological features. Moderate deficiencies may present apparent macrocytic anemias with symptoms like glossitis and subtle neurological features, such as distal sensory impairment. Due to relatively high body storage of vitamin B12 of about 1-5 mg, noticeable clinical signs of vitamin B12 deficiency from diminished intake or absorption may not develop for years after the cessation of intake [50].
Conclusion
GPs in Western societies diagnose a majority of cases of anemia, with IDA and ACD being the most common types encountered in primary care. Thorough history taking plays a crucial role in leading the GP closer to the underlying cause of anemia. Following the clinical evaluation and documentation of anemia at the first consultation, a panel of 18 routine blood tests will enable the GP to diagnose the underlying etiology. An initial basic set of these laboratory tests include hemoglobin and, if anemia is documented, mean corpuscular volume, red blood cell distribution width, leukocytes, platelets and reticulocyte counts. In microcytic and normocytic anemias, a low ferritin is typically suggestive of IDA, whereas an elevated ferritin is indicative of ACD. Aferritin in the mid range requires further iron indices such as transferrin saturation for more clarification. Vitamin B12 and folate levels should be performed in the case of a macrocytic anemia with a low reticulocyte count. A macrocytic or normocytic anemia with an elevated reticulocyte count on the other hand should prompt consideration for hemolysis or hemorrhage. Either way, a very low reticulocyte count should always alert the GP of potential bone marrow failure, such as MDS and leukemia. Future research in anemia-workup in primary care will shed more light on how important risk groups are best identified and monitored as well as how laboratory tests in anemia-workup are used optimally.
Author’s Contributions
EP co-designed the study, collected, analyzed and interpreted data and drafted the manuscript. CLA co-designed the study, analyzed and interpreted data. BSL co-designed the study, analyzed and interpreted data. All authors revised the manuscript critically for important intellectual content, approved the final version to be submitted, and take public responsibility for appropriate portions of the content.
Supplementary Information
The GP can document a suspected anemia with Point-of-Care Testing (POCT) during the very first patient consultation immediately following the history taking and physical examination. If POCT is not available, the anemia should be documented with a regular blood test. Reaching the underlying etiology may then be accomplished through following laboratory diagnostic strategies:
One strategy is the routine-strategy in which the 18 laboratory tests presented in Table 3 are ordered by the GP step by step. The patient therefore risks having to return for several consultations as the more common causes of anemia are being excluded [17].
A second strategy is the extensive-strategy, in which all the 18 laboratory tests are ordered by the GP all at once. This strategy may be more convenient in terms of consultations. However, it may also lead to the request of many unnecessary laboratory tests [17].
Finally, a third strategy is an algorithmic-strategy’, where an anemia-workup test is ordered by the GP at the first consultation, after which the laboratory performs an algorithm-based analysis of relevant succeeding tests depending of the previous test results. The algorithmic approach thus has the benefit of taking both the number of patient consultations and the number of laboratory tests into account. Anemia may already be documented after one consultation/blood test, and if the initial blood test does not confirm anemia, further laboratory analysis of anemia is immediately stopped. If the initial blood test documents anemia, however, a stepwise analysis of relevant laboratory tests is conducted by adding ferritin in a suspected microcytic anemia or vitamin B12 in a suspected macrocytic anemia, etc.
References
- Kujovich JL. Evaluation of Anemia. Obstet Gynecol Clin North Am. 2016; 43: 247-264.
- Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is Common in Heart Failure and is Associated with Poor Outcomes. Circulation. 2003; 107: 223-225.
- Lipschitz D. Medical and Functional Consequences of Anemia in the Elderly. J AM Geriatr Soc.2003; 51: S10-S13.
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. 2011.
- World Health Organization. The Global Prevalence of Anaemia in 2011.
- Gaskell H, Derry S, Moore RA, Henry J McQuay. Prevalence of Anaemia in Older Persons: systematic review. BMC Geriatr. 2008; 8: 1.
- Beutler E, Waalen J. The Definition of Anemia: What is the Lower Limit of Normal of the Blood Hemoglobin Concentration? Blood. 2006; 107: 1747-1750.
- Powell DJ, Achebe MO. Anemia for the Primary Care Physician. Prim Care - Clin off Pract. 2016; 43: 527-542.
- Greenburg AG. Pathophysiology of Anemia. Am J Med. 1996; 101: 7S-11S.
- Bessman JD, Gilmer PR, Gardner FH. Improved Classification of Anemias by MCV and RDW. Am J Clin Pathol. 1983; 80: 322-326.
- Lanier JB, Park JJ, Callahan RC. Anemia in Older Adults. Am Fam Physician. 2018; 98: 437-442.
- Wang M. Iron Deficiency and Other Types of Anemia in Infants and Children. Am Fam Physician. 2016; 93: 270-278.
- Bross MH, Soch K, Smith-Knuppel T. Anemia in Older Persons. Am Fam Physician. 2010; 82: 480-487.
- Irwin JJ, Kirchner JT. Anemia in children. Am Fam Physician. 2001; 64: 1379-1386.
- Hansen JW, Sandholdt H, Siersma V, Andreas Due Ørskov, Staffan Holmberg, Ole Weis Bjerrum, et al. Anemia is Present Years Before Myelodysplastic Syndrome Diagnosis: Results from the Pre-diagnostic Period. Am J Hematol. 2017; 92: E130-E132.
- Sheth TN, Choudhry NK, Bowes M, Allan S. Detsky MD. The Relation of Conjunctival Pallor to the Presence of Anemia. J Gen Intern Med. 1997; 12: 102-106.
- Schop A, Kip MMA, Stouten K, Soraya Dekker, Jurgen Riedl, Ron J van Houten, et al. The Effectiveness of a Routine Versus an Extensive Laboratory Analysis in the Diagnosis of Anaemia in General Practice. Ann Clin Biochem. 2018; 55: 535-542.
- Weiss G, Goodnough LT. Anemia of Chronic Disease. N Engl J Med. 2005; 352: 1011-1023.
- Guralnik JM, Eisenstaedt RS, Ferrucci L, Harvey G Klein, Richard C Woodman. Prevalence of Anemia in Persons 65 Years and Older in the United States: Evidence for a High Rate of Unexplained Anemia. Blood. 2004; 104: 2263-2268.
- Guyatt GH, Oxman AD, Ali M, A Willan, W McIlroy, C Patterson. Laboratory Diagnosis of Iron-Deficiency Anemia: An Overview. J Gen Intern Med. 1992; 7: 145-153.
- Gangat N, Wolanskyj AP. Anemia of Chronic Disease. Semin Hematol. 2013; 50: 232-238.
- Bermejo F, Garcia-Lopez S. A Guide to Diagnosis of Iron Deficiency and Iron Deficiency Anemia in Digestive Diseases. World J Gastroenterol. 2009; 15: 4638-4643.
- Batchelor EK, Kapitsinou P, Pergola PE, Csaba P Kovesdy, Diana I Jalal. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J Am Soc Nephrol. 2020; 31: 456-468.
- Baker RD, Greer FR. Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0-3 Years of Age). Pediatrics. 2010; 126: 1040-1050.
- Guyatt GH, Patterson C, Ali M, J Singer, M Levine, I Turpie, et al. Diagnosis of Iron-Deficiency Anemia in the Elderly. Am J Med. 1990; 88: 205-209.
- Logan ECM, Yates JM, Stewart RM, K Fielding, and D Kendrick. Investigation and Management of Iron Deficiency Anaemia in General Practice: A Cluster Randomised Controlled Trial of a Simple Management Prompt. Postgrad Med J. 2002; 78: 533-537.
- Hamilton W, Lancashire R, Sharp D, T J Peters, K K Cheng, T Marshall. The Importance of Anaemia in Diagnosing Colorectal Cancer: A Case-Control Study Using Electronic Primary Care Records. Br J Cancer. 2008; 98: 323-327.
- Kis AM, Carnes M. Detecting Iron Deficiency in Anemic Patients with Concomitant Medical Problems. J Gen Intern Med. 1998; 13: 455-461.
- Koulaouzidis A, Cottier R, Bhat S, Elmuhtady Said, Barry D Linaker, Athar A Saeed. A Ferritin Level >50 μg/L is Frequently Consistent with Iron Deficiency. Eur J Intern Med. 2009; 20: 168-170.
- Smith DL. Anemia in the Elderly. Am Fam Physician. 2000; 62: 1565-1572.
- Palmblad J, Siersma V, Lind B, Ole Weis Bjerrum, Hans Hasselbalch, Christen Lykkegaard Andersen. Age-Related Prevalence and Clinical Significance of Neutropenia - Isolated or Combined with Other Cytopenias: Real World Data from 373 820 Primary Care Individuals. Am J Hematol. 2020; 95: 521-528.
- Muncie HL, Campbell J. Alpha and Beta Thalassemia. Am Fam Physician. 2009; 80: 339-344.
- Jain S, Kamat D. Evaluation of Microcytic Anemia. Clin Pediatr (Phila). 2009; 48: 7-13.
- Martin A, Thompson AA. Thalassemias. Pediatr Clin North Am. 2013; 60: 1383-1391.
- Janus J, Moerschel SK. Evaluation of Anemia in Children. Am Fam physician. 2010; 81: 1462-1471.
- Yetley EA, Pfeiffer CM, Phinney KW, Regan L Bailey, Sheena Blackmore, Jay L Bock, et al. Biomarkers of Vitamin B-12 Status in NHANES: A Roundtable Summary. Am J Clin Nutr. 2011; 94: 313S-321S.
- Savage DG, Ogundipe A, Allen RH, S P Stabler, J Lindenbaum. Etiology and Diagnostic Evaluation of Macrocytosis. Am J Med Sci. 2000; 319: 343-352.
- Carmel R. Biomarkers of Cobalamin (Vitamin B-12) Status in the Epidemiologic Setting: A Critical Overview of Context, Applications, and Performance Characteristics of Cobalamin, Methylmalonic Acid, and Holotranscobalamin II. Am J Clin Nutr. 2011; 94: 348S-358S.
- De Bruyn E, Gulbis B, Cotton F. Serum and Red Blood Cell Folate Testing for Folate Deficiency: New Features? Eur J Haematol. 2014; 92: 354-359.
- Savage DG, Lindenbaum J, Stabler SP, R H Allen. Sensitivity of Serum Methylmalonic Acid and Total Homocysteine Determinations for Diagnosing Cobalamin and Folate Deficiencies. Am J Med. 1994; 96: 239-246.
- Stabler SP. Vitamin B12 Deficiency. N Engl J Med. 2013; 368: 2041-2042.
- Ballard HS. The Hematological Complications of Alcoholism. Alcohol Health Res World. 1997; 21: 42-52.
- Phillips J, Henderson AC. Hemolytic Anemia: Evaluation and Differential Diagnosis. Am Fam Physician. 2018; 98: 354-361.
- Janssen M, Busch C, Rodiger M. Motives of Consumers Following a Vegan Diet and Their Attitudes Towards Animal Agriculture. Appetite. 2016; 105: 643-651.
- McEvoy CT, Temple N, Woodside JV. Vegetarian Diets, Low-Meat Diets and Health: A Review. Public Health Nutr. 2012; 15: 2287-2294.
- Chandra-Hioe MV, Lee C, Arcot J. What is the Cobalamin Status among Vegetarians and Vegans in Australia? Int J Food Sci Nutr. 2019; 70: 875-886.
- Pawlak R, Parrott SJ, Raj S, Diana Cullum-Dugan, Debbie Lucus. How Prevalent is Vitamin B12 Deficiency among Vegetarians? Nutr Rev. 2013; 71: 110-117.
- Pawlak R, Lester SE, Babatunde T. The Prevalence of Cobalamin Deficiency among Vegetarians Assessed by Serum Vitamin B12: A Review of Literature. Eur J Clin Nutr. 2014; 68: 541-548.
- Green R. Vitamin B12 Deficiency from the Perspective of a Practicing Hematologist. Blood. 2017; 129: 2603-2611.
- Hunt A, Harrington D, Robinson S. Vitamin B12 Deficiency. Br Med J. 2014; 349: g5226.
