Introduction
In April 2020, the United States established Operation Warp speed to develop a Corona Virus Disease 2019 (COVID-19) vaccine and produce 300 million doses by January 2021. History suggest that the initiative is aptly named as Barney S. Graham notes, “Vaccine development is usually measured in decades, so having access to approved vaccines available for large scale distribution before the end of 2020 or even 2021 would be unprecedented. Emergency use of authorization for two Messenger RNA (m-RNA) vaccine-the BNT162b2 vaccine (Pfizer-BioNTech) and the mRNA-173 vaccine (Moderna) Marked important milestones in efforts to respond to the corona virus disease pandemic. The effect of vaccination on the preservation of the workforce has also been noticed.6Prospective cohorts of health care personnel first responder, and other essential and frontline worker over 13 weeks in eight U.S. location confirmed that authorized mRNA COVID-19 vaccines (Pfizer-BioNTech’s BNT162b2 and moderna’s mRNA-1273) are highly effective in real world conditions [7].
Methods
Reviewing Occupational Health Department(OHD) of the Moffitt Cancer Centre(MCC) we identified 274 employees who tested positive for SARS-CoV-2 infection between November 10 2020 and may 28 2021. Employees had positive RT-PCR antigen test. COVID vaccination of the MCC employees was started on 10th of December 2020. The OHD began recording vaccination status from 6th of January 2021. Employees in this study are divided into 2 phases the first phase: people who tested positive before 6th of January 2021 and the second phase: people who tested positive after 6th January 2021. Data was collected regarding employee’s vaccination status covid positive contact history, primary symptoms and area of work.
Results
274 employees who tested positive were included in the study. Among 274 employees, 133 employees were tested positive before 6th January 2021 in phase 1 and 142 were tested after 6th January 2021 in phase 2 (Figure 1). Employees were also asked about possible exposure and place with a SARS-CoV-2 positive person. Of 273 employees, total 143 (52.38%) employees had a known History of (H/O) exposure to SARS-CoV-2 positive cases. From those 143 employees, 136 employees were exposed to known SARS-CoV-2 positive person outside the hospital work environment either from family, friends or a social gathering. 7 employees had a known exposure with a SARS-CoV-2 positive person in the hospital work environment. 130 employees had no known exposure to a SARSCoV- 2 positive person (Figure 2). From the employees tested positive for SARS-CoV-2 in phase 2, 110(77.47%) employees had not received any vaccine and 32 (22.54%) employees had received one or two doses of vaccine (Figure 3). Among the employees vaccinated against SARS-CoV-2 , 21 employees had received a single dose of the vaccine and out of the 21 patients, 18 patients tested positive within 15 days of vaccination and 3 employees tested positive after more than 15 days of vaccination. 11 employees had received two doses of vaccine. Out of the 11 employees, 6 employees tested positive within 15 days of vaccination and 5 tested positive after 15 days of vaccination which is also called breakthrough infection (Figure 4 and 5).
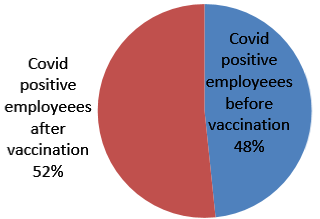
Figure 1:
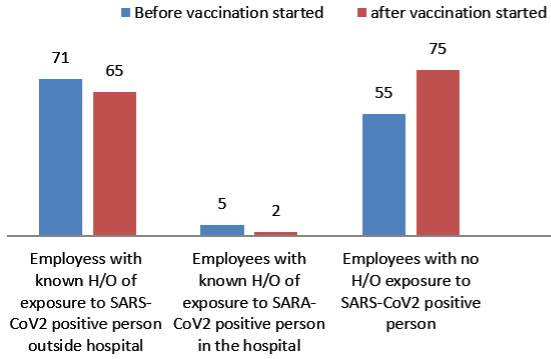
Figure 2:
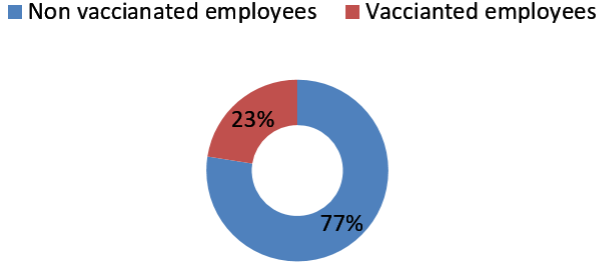
Figure 3:
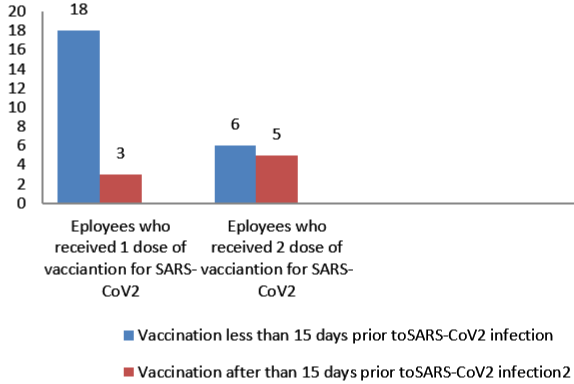
Figure 4:
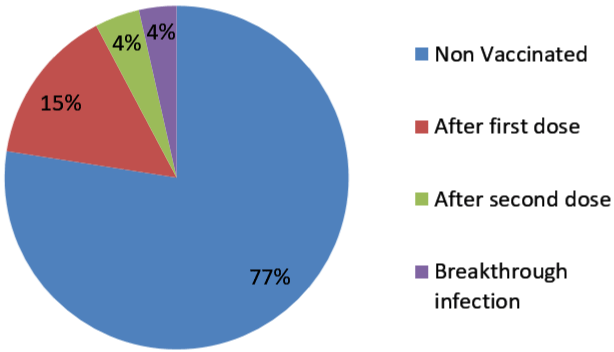
Figure 5:
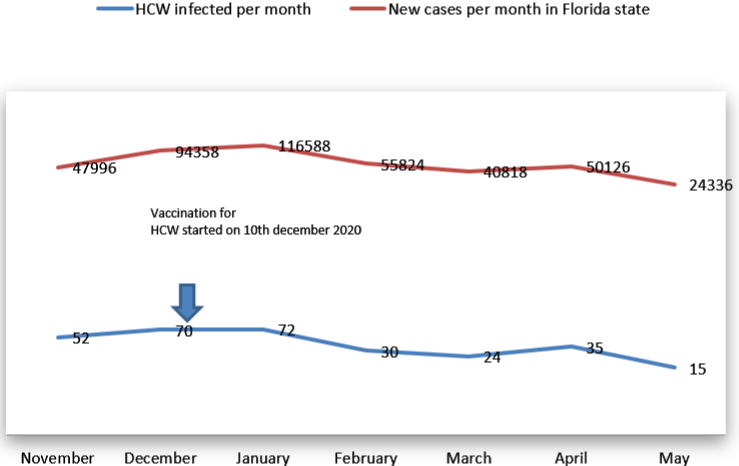
Figure 5 of 1:
Discussion
Despite unprecedented movement restrictions, social distancing measures, and stay-at-home orders enacted in many countries the COVID-19 pandemic has caused devastating morbidity and mortality. However, the vast majority of the global population remains susceptible to COVID-19, highlighting the need for an effective vaccine. To mitigate the mounting burden of COVID-19, vaccine development has occurred at an unprecedented pace. As of December 31, 2020, safety and efficacy results for a number of vaccines have been reported and Phase III clinical trials for several other candidates are underway [1].
As the global community continues to confront the Corona Virus Disease 2019(covid-19) pandemic, the potential for occupational exposure among healthcare workers remain a critical concern. Multiple studies comparing severe acute respiratory syndrome corona virus among healthcare workers and non-health care workers have found higher rates of infections among health care workers however other studies have observed no association. For example, at a large Belgian hospital with strong, early infection control measures, no occupational risk factors were associated with SARS-CoV-2 antibody positivity Health care workers, positivity was only associated with having household contact with suspected or confirmed covid 19.This finding suggests that little occupational transmission occurred but whether household contacts were infected before or after health care workers was not defined [3].To protect Health care practioners caring for patients with suspected or confirmed covid-19, healthcare facilities should continue to follow infection control guidelines and wear personnel protective equipment. Early recognition and prompt isolation including source control, for patients with possible infection can help minimize unprotected and high-risk exposure. These measures are crucial to protect Health care practioners and preserve the healthcare workforce [2].Most infections occurred at the early stage of epidemic, before protective measures were taken. So rigorous prevention measures are effective to prevent or limit transmission in a health care setting [4]. In Contrast, households are favorable environments for transmission. They are what are known as 3Cs environments, as they are closed spaces, where family members may crowd and be in close contact with conversation. There may be reduced use of personal protective equipment relative to other settings [5]. Prospective cohorts of health care personnel first responders, and other essential and frontline workers over 13 weeks in eight U.S. location confirmed that authorized mRNA COVID-19 vaccines (Pfizer-BioNTech’s BNT162b2 and moderna’s mRNA- 1273) are highly effective in real world conditions [7]. Reducing the risk of transmissible infection, which can occur among the persons with asymptomatic infection or among persons several days before symptoms onset is specially important among health care personnel, first responder and other essential and front line workers given their potential to transmit the virus through frequent close contact with patients and the public [7]. The efficacy of BNT162b2 against covid-19 is 52.4% after the first dose and 94.8% after more than 7 days of the second [8].
One of the aspects which define vaccine efficacy is breakthrough infections. The article by Hacisuleyman et al. reinforces concerns about SARS-CoV-2 variants and vaccine breakthrough. T-cell immunity is important in the control of SARS-CoV-2 and may be critical for immune protection against variants. MRNA vaccines generate weaker T-cell response than other vaccines platforms. Breakthrough infections itself does not distinguish between vaccine failure and breakthrough after successful vaccination. One of the proposed strategy for doing so: serial testing of asymptomatic persons, which has the potential to identify neutralizing antibodies in acute serum, along with assays testing the quality and duration of T-cell and B-cell responses [10]. According to reports, decline in the SARS-CoV-2 positivity was correlated with vaccination. In contrast, positivity rates also correlates with the regional trends over a longer follow-up period. These findings advocate for rapid vaccination of all eligible persons to quickly and safely achieve herd immunity, mitigate regional surges, and decrease the risk of sustained transmission and the evaluation of new more transmissible and deadlier variants [11].
Our findings confirm the efficacy of COVID-19 vaccination of HCWs mostly after 7 days of receiving the second dose. In addition, community acquisition of infection is much more common for HCWs compared to hospital acquired. This confirms that sure of isolation and PPE significantly reduces transmission of COVID-19 in the hospital work environment.
References
- Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clinical Infectious Diseases. 2021.
- Heinzerling A, Stuckey PMJ, Scheuer T, Xu K, Perkins KM, Resseger H, et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient-Solano County, California. 2020; 69: 472-476.
- Marshall K, Vahey GM., McDonald E, Tate JE, Herlihy R, Midgley CM, et al. Exposures Before Issuance of Stay-at-Home Orders Among Persons with Laboratory-Confirmed COVID-19-Colorado, Morbidity and Mortality Weekly Report. March 2020; 69: 847-849.
- Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA network open. 2020; 3: e209666-e209666.
- Madewell ZJ, Yang Y, Longini IM, Halloran ME, Dean NE. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA network open. 2020; 3: e2031756-e2031756.
- Daniel W, Nivet M, Warner J, Podolsky DK. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. New England Journal of Medicine. 2021; 384: 1962-1963.
- Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers-eight US locations, December 2020–March 2021. Morbidity and Mortality Weekly Report. 2021; 70: 495-500.
- Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. 2021; 384: 1576-1578.
- Graham BS. Rapid COVID-19 vaccine development. Science. 2020; 368: 945-946.
- Nixon DF, Ndhlovu LC. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. infection. 2021; 48: 9-5.
- Tosoni A, Mirijello A, Addolorato G. More on SARS-CoV-2 Infection after Vaccination in Health Care Workers. The New England journal of medicine. 2021; 385: e8.
