Abstract
Background: The causes of age-related poor urine concentration capacity favouring low-grade chronic dehydration in aged populations are not well understood.
Objectives: To explore links between age and plasma and urine osmolarity levels and a possible picture of peripheral resistance to arginine vasopressin (R-AVP) in an aged population.
Design: observational cross-sectional study. Study population: communitydwelling subjects aged 70 years and older. Data collection: Blood and urine samples collected after 10 hours night fasting were analysed for osmolarity and copeptin levels (AVP surrogate). R-AVP was established based on a urinary osmolarity/copeptin ratrio<35.
Results: 237 subjects were recruited (mean age 75.7 years, 52.7% women). Plasma osmolarity was similar between the sexes and age groups (70-79 and ≥80 years); whereas urine osmolarity was lower in women and in the older age group. Plasma hyperosmolarity (>295 mOsm/L) was present in no women and in 4.5% of men, and was significantly 14 times greater in the older group. R-AVP prevalence was 12.7% in the younger group vs 20.7% in the older group (p=0.252). Subjects with R-AVP, compared to without R-AVP, presented higher plasma osmolarity (287.6 vs 285.4 mOsm/L; p=0.023) and higher prevalence of plasma hyperosmolarity (8.7% and 0.7%; p=0.053). R-AVP was also related with IL-6 and creatinine levels and with loop diuretic use.
Conclusions: Urine concentration capacity decreases and plasma hyperosmolarity increases after the age of 80 years. Fourteen percent of ≥70 year’s old population present R-AVP, which greatly increases the risk of plasmahyperosmolarity and is related with loop diuretic use, and IL-6 and creatinine levels.
Keywords: Hyperosmotic stress; Plasma osmolarity; Urine osmolarity; Peripheral resistance to AVP; Aged
Abstract
Background: The causes of age-related poor urine concentration capacity favouring low-grade chronic dehydration in aged populations are not well understood.
Objectives: To explore links between age and plasma and urine osmolarity levels and a possible picture of peripheral resistance to arginine vasopressin (R-AVP) in an aged population.
Design: observational cross-sectional study. Study population: communitydwelling subjects aged 70 years and older. Data collection: Blood and urine samples collected after 10 hours night fasting were analysed for osmolarity and copeptin levels (AVP surrogate). R-AVP was established based on a urinary osmolarity/copeptin ratrio<35.
Results: 237 subjects were recruited (mean age 75.7 years, 52.7% women). Plasma osmolarity was similar between the sexes and age groups (70-79 and ≥80 years); whereas urine osmolarity was lower in women and in the older age group. Plasma hyperosmolarity (>295 mOsm/L) was present in no women and in 4.5% of men, and was significantly 14 times greater in the older group. R-AVP prevalence was 12.7% in the younger group vs 20.7% in the older group (p=0.252). Subjects with R-AVP, compared to without R-AVP, presented higher plasma osmolarity (287.6 vs 285.4 mOsm/L; p=0.023) and higher prevalence of plasma hyperosmolarity (8.7% and 0.7%; p=0.053). R-AVP was also related with IL-6 and creatinine levels and with loop diuretic use.
Conclusions: Urine concentration capacity decreases and plasma hyperosmolarity increases after the age of 80 years. Fourteen percent of ≥70 year’s old population present R-AVP, which greatly increases the risk of plasmahyperosmolarity and is related with loop diuretic use, and IL-6 and creatinine levels.
Keywords: Hyperosmotic stress; Plasma osmolarity; Urine osmolarity; Peripheral resistance to AVP; Aged
Introduction
Approximately 55-60% of the body mass is water, with some variations depending on age, sex, and the fat mass/lean mass ratio. Water in the body is mainly distributed between the intracellular and extracellular water compartments, flowing from one to the other according to osmotic pressure and maintaining balanced osmolarityon both sides of the cell membrane [1]. Because plasmaosmolarity needs to remain within a very narrow range (280-295 mOsm/L), small variations are strictly controlled by osmoreceptors that stimulate thirst, Arginine-Vasopressin (AVP),and other hormones in the central nervous system [2,3]. When plasma osmolarity is above 295 mOsm/L, water tends to leave the intracellular compartment, causing cells to shrink and affecting the cytoskeleton and enzymatic activity because of alterations in protein folding, structure, and functioning [4,5]. Hyperosmotic stress causes cell damage and, when persistently high, may even cause cell death. Intracellular water depletion has been linked to inflammation, increased reactive oxygen species production, intracellular catabolic effects, insulin resistance, and cardiovascular, kidney, and muscular disorders [5,6]. Muscle, as the main water reservoir in the body, is one of the first organs affected by dehydration, which interferes with muscle contractile capacity and metabolic functions, decreasing cell glucose uptake and glycogen and protein synthesis [7,8]. Reduced intracellular water content in lean mass has also been associated with poorer handgrip and functional capacity, and with frailty in aged populations [9-10], so plasma hyperosmolarity has been proposed as an early marker of frailty [11].
It is well known that body water content decreases with age, and that aged people are at an increased risk of dehydration (in turn associated with greater disability and morbi-mortality), for which estimated prevalence is 20-30% [12-14]. Moreover, dehydration signs and symptoms are obvious in some cases, but there is a suspicion that there may be cases of low-grade chronic dehydration, with few clinical manifestations but with possible long-term effects [15]. Different circumstances may favour low-grade dehydration in the aged population, including a reduced thirst sensation, a reduced capacity to concentrate urine [16], alterations in the reninangiotensin- aldosterone and a trial natriuretic peptide systems [17], hyperglycaemia, and use of drugs, such as diuretics, that affect water balance [18].
In this study we focus especially on the reduced capacity of aged people to concentrate urine, as the corresponding mechanisms are poorly understood. It has been suggested that the reason for decreased urine concentration in the elderly is neither decreased AVP secretion nor reduced glomerular filtration [19]; rather, the presence of diluted urine along with elevated AVP levels would suggest a clinical picture of peripheral (renal) resistance to AVP (R-AVP); however, this is a little known and described clinical condition. The objectives of this study were, for a community-dwelling population aged 70 years and older, to explore links between age and plasma and urine osmolarity levels and to explore a possible picture of peripheral R-AVP.
Methodology
Study Design and Population
A population-based cross-sectional study was performed of community-dwelling subjects aged 70 years and older recruited between January and March 2020. A sample was randomly preselected from the database for three primary care centres in the Maresme region (Barcelona, Spain). Pre-selected subjects were invited by telephone to a visit with their primary care physician. During the visit eligibility criteria were checked, patients were informed about the study, and if they agreed to participate, signed a consent form. Exclusion criteria were active malignancy, neuromuscular disease, dementia or serious mental illness, life expectancy of less than six months, bilateral knee or hip prosthesis use, in palliative care, or institutionalized. The local ethics committee approved the study protocol (code CEIm CSdM 65/19).
Data Collection
Sociodemographic data (age, sex, and education level), main comorbidities, current chronic treatments, and tobacco and alcohol consumption data were obtained directly from the participant and/ or electronic medical records. Drugs were classified in the following groups that may affect water homeostasis: corticosteroids, loop diuretics, thiazide-type diuretics, potassium-sparing diuretics, Selective Serotonin Receptor Inhibitors (SSRIs), Angiotensin Converting Enzyme Inhibitors (ACEIs), Angiotensin Receptor Blockers (ARBs), beta-blockers, oral hypoglycaemic agents, benzodiazepines, antipsychotics, Proton Pump Inhibitors (PPIs) and dopamine agonists. Blood and urine samples were collected at 8.00 am, after 10 hours’ night fasting, for later determinations of osmolarityin blood and urine and copeptin in blood.
Copeptin – a 39-amino acid-long peptide derived from the C-terminus of pre-pro-hormone of AVP, neurophysin II, and copeptin, whichis co-released with AVP from the hypothalamus – was measured as a reliable AVP surrogate, because AVP is difficult to quantify due to its very short half-life. Frozen plasma ethylenediaminetetraacetic acid (EDTA) samples were quantitatively analysed using a commercial immune luminometric sandwich assay (Kryptor, BRAHMS, Berlin, Germany), which has a Coefficient of Variation (CV) of 10%.
Osmolality– which is not influenced by the size, weight, or electric charge of particles, but by the number of osmolytes per unit of solvent – was defined as the number of moles of osmotically active particles per kg of solution, and was expressed in milliosmoles per L of water (as 1L of water weighs 1kg). Plasma and urine sampleosmolarities were analysed using the Micro-osmometer Osmo1 (Advanced Instruments). Measurement was by freezing point depression osmometry, based on the principle that each mole of dissolved solute decreases the freezing point of a liquid by 1.86°C. Results were converted to mOsm/L H2O. Plasma osmolarity was categorized as <280 mOsm/L (hypoosmolarity), 280-295 mOsm/L (normal or isoosmolarity), or >295 mOsm/L (hyperosmolarity).
Peripheral R-AVP, considered as an early state of nephrogenic diabetes insipidus, was defined as relatively elevated AVP levels accompanied by low urineosmolarity (even with preserved plasma osmolarity). There exist a linear relationship between urinary osmolarity and plasmatic copeptin levels, so that, under normal conditions, urinary osmolarity is expected to increase in proportion to the increase in plasma copeptin levels. The urinary osmolarity (in mOsm/L)/copeptin (in pmol/L) ratio (u-osm/C ratio) reflects the renal ability to concentrate urine, with a low ratio indicating a poor ability to concentrate urine despite relatively high copeptin (or AVP) levels. In order to establish a cut-off point that defines a state of R-AVP, the u-osm/C ratio value above which the correlation (r) between urinary osmolarity and copeptin is stabilized in maximum levels has been sought (Figure 1). Therefore, R-AVP was consideredwhen u-osm/C ratio was ≤35. According to this cut-off point, copeptin levels above 12 pmol/L (considered as high) would correspond to urinary osmolarity levels above 450mOsm/L.
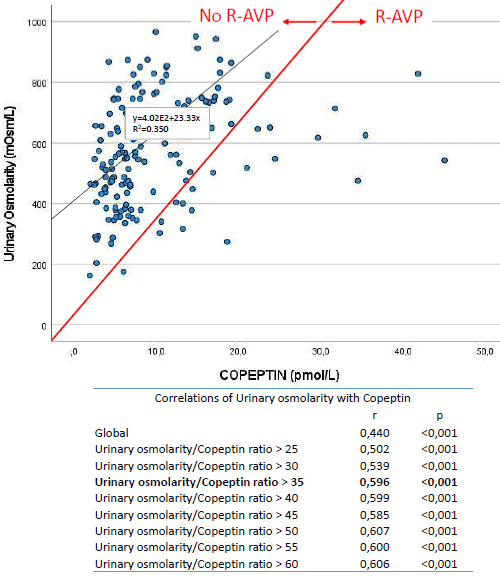
Figure 1: Relationship between urinary osmolarity and copeptine.
Statistical Analysis
Since this was mainly an exploratory descriptive study, no a priori power analysis was performed. Continuous variables were described using mean, Standard Deviation (SD), median, and minimum and maximum values, and categorical variables were described using percentages. To evaluate factors associated with plasma and urineosmolarities, used were the t-test or the Mann- Whitney U-test for dichotomous variables, and Pearson or Spearman correlation coefficients for numerical variables. R-AVP prevalence and the corresponding 95% confidence interval (CI) were calculated, and used to evaluate associated variables were the chi-square test or Fisher’s exact test for categorical variables, and the Mann-Whitney U-test or the t-test for independent numerical variables. The odds ratio (OR) and the corresponding 95% CI was calculated as a measure of association using bivariate logistic regression analysis. All analyses were performed for the overall sample, by sex, and by age groups (70- 79 years and ≥80 years). Statistical significance was set at p<.05.
Results
A total of 237 subjects (52.7% women) was recruited, with a mean (SD) age of 75.7 (4.7) years. The main comorbidities were arterial hypertension (64.6%), arthritis (54.8%), dyslipidaemia (48.2%), depression (17.9%), peripheral vascular disease (17.1 %), and diabetes (15.7%). The mean number of different drugs consumed per subject was 4.68 (3.3). Blood and urine samples were obtained from 192 subjects (81% of the sample), who constitute the sample for this analysis.
Plasma and Urine Osmolarities by Sex and Age
Table 1 summarizes details of plasma and urineosmolarity for the overall sample and broken down by sex and age. Mean (SD) plasma osmolarity was 285.8(5.0) mOsm/L, and mean (SD) urine osmolarity was 590.0(178.0) mOsm/L. While no significant differences were observed for plasma osmolarity between the sexes and the age groups, significant differences were observed for urineosmolarity for men vs women (668 vs 524 mOsm/L; p<0.001) and for subjects in the younger (70-79 years) vs older (≥80 years) age groups (603 vs 535 mOsm/L; p=0.038). Assessment of the correlation between age and both plasma and urine osmolarities showed the following correlation coefficients (r); for plasma osmolarity in men an r=0.207 (p=0.053) and in women an r=0.056 (p=0.570), and for urine osmolarity in men an r=-0.071 (p=0.513) and in women an r=-0.138 (p=0.162).
Plasmaosmolarity (mOsm/L)
Mean
Median
SD
Min.
Max.
<280
280-295
>295
All (N=193)
286
286
5
257
304
8.3 %
89.6 %
2.1%
Women (N=105)
285
285
5
264
295
10.5 %
89.5%
0%
Men (N=88)
286
286
6
257
304
5.7 %
89.8%
4.5%
All 70-79 y (N=158)
286
285
5
257
304
7.6 %
91.8%
0.6%
All =80 y (N=35)
287
287
7
264
299
11.4%
80.0%
8.6%
Women 70-79 y (N=85)
285
285
4
278
295
9.4%
90.6%
0%
Women =80 y (N=20)
285
286
7
264
295
15.0%
85.0%
0%
Men 70-79 y (N=73)
286
286
5
257
304
5.5%
93.2%
1.4%
Men =80 y (N=15)
289
287
6
279
299
6.7%
73.3%
20.0%
Urineosmolarity (mOsm/L)
Mean
Median
SD
Min.
Max.
<300
300-900
>900
All (N=192)
590
604
178
162
966
5.2%
92.2%
2.6%
Women (N=104)
524
512
167
162
966
6.7%
91.3%
1,9%
Men (N=88)
668
694
159
268
915
3.4%
93.2%
3.4%
70-79 y (N=156)
603
622
178
162
966
5.1%
92.3%
2.6%
>80 y (N=36)
535
481
171
175
912
5.6%
91.7%
2.8%
Women 70-79 y (N=83)
536
537
166
162
966
7.2%
91.6%
1.2%
Women =80 y (N=21)
477
453
165
175
912
4.8%
90.5%
4.8%
Men 70-79 y (N=73)
679
704
160
268
951
2.7%
93.2%
4.1%
Men =80 y (N=15)
616
649
148
282
864
6.7%
93.3%
0%
Differences in plasma osmolarity category percentages were significantly associated with age (p=0.008), age in men (p=0.006), and sex (p<0.047). Differences in mean urine osmolarity were statistically significant forthe sexes (p<0.001), for the sexes aged 70-80 years (<0.001), for the sexes aged>80 years (p=0.004), and for the older vs younger age groups (p=0.038).
Table 1: Plasmaand urineosmolarities (as continuous variables) in a community-dwelling population aged 70 years and older by sex and age.
Table 1 also reports prevalence rates for plasma and urine hyperosmolarity and hypoosmolarity for the overall sample, by sex, and by age group. Plasma hyperosmolarity was present in 4.5% of men and 0% of women, and plasmahypoosmolarity in 5.7% of men and 10.5% of women. Prevalence of plasma hyperosmolarity was a significant 14 times greater in the older age group than in the younger age group.
Plasma and Urineosmolarity Associations with Clinical Factors and Drugs
Plasma osmolarity was associated with diabetes (287.7 vs 285.5 mOsm/L in diabetics vs non-diabetics; p=0.024) and was correlated with plasma levels of urea (r=0.527; p<0.001), creatinine (r=0.269; p<0.001), and glycated haemoglobin (HbAC1) (r=0.251; p<0.001). Plasma osmolarity was associated with use (vs non-use) of oral hypoglycaemic agents (287 vs 286 mOsm/L; p=0.044) and PPIs (287 vs 285 mOsm/L; p=0.025). Plasma hyperosmolarity (>290 mOsm/L) was associated with use (vs non-use) of loop diuretics (33.3% vs 1.1%; p<0.001), ARBs (7.3% vs 0.7%; p=0.025), and PPIs (5.6% vs 0%; p=0.023).
Urine osmolarity was associated with diabetes (606.9 vs 583.1 mOsm/L in diabetics vs non-diabetics; p=0.024), depression (530.3 vs 601.5 mOsm/L in depressed vs non-depressed subjects; p=0.031), cancer (653.9 mOsm/l vs 577.3 in cancer vs non-cancer subjects; p=0.045), and the number of medications (r=-0.165; p=0.026). No specific drug showed a significant association with urineosmolarity, while plasma and urine osmolarities were correlated (r=0.325; p<0.001).
Copeptin Levels and Peripheral R-AVPby Sex and Age
Table 2 summarizes mean copeptin levels, mean u-Osm/C ratios, and R-AVP prevalence rates for the overall sample, by sex, and by age group. Copeptin levels were significantly higher in men than in women, but there were no significant differences between age groups. u-Osm/C ratios were significantly higher in women than in men (87.2 vs 71.1, respectively; p=0.045), and also in the younger than in the older age group (84.2 vs 63.0, respectively; p=0.020). Although R-AVP prevalence in the older group nearly doubled that in the younger group, this difference did not reach statistical significance. Assessment of Copeptin level and plasma osmolarity relationship showed an r=0.40 (p<0.001) and a linear regression coefficient (beta) of 0.23 (p<0.001) for the whole sample; for the younger and older age groups, the corresponding coefficients were r=0.36 (p<0.001) and beta=0.18 (p=0.001), and r=0.55 (p=0.002) and beta=0.47 (p=0.003), respectively.
All
Women
Men
p
70-79 y
=80 y
p
Copeptin, mean (SD)pmol/L
10.0
(7.3)8.4
(6.5)12.2
(7.7)<0.001
9.8
(7.3)10.7
(7.5)0.528
u-Osm/C ratio, mean (SD)
80.4 (46.2)
87.2 (50.3)
71.1 (38.4)
0.045
84.2 (47.8)
63.0 (33.4)
0.020
R-AVP prevalence (95% CI)
14.1% (8.8-19.4)
12.8% (6.0-19.6)
15.9% (7.3-24.5)
0.565
12.7% (7.1-18.3)
20.7% (6.0-35.4)
0.252
Table 2: Copeptin levels and R-AVP in a community-dwelling population aged 70 years and older by sex and age.
R-AVP Association with Plasma Osmolarity
Plasmaosmolarity was greater in subjects with vs without R-AVP (287.6 mOsm/L vs 285.4 mOsm/L; p=0.023), while prevalence of plasma hyperosmolarity (>295 mOsm/L) for those same groups was 8.7% and 0.7% (p=0.053), respectively. Linear regression analysis showed an R-AVP effect on plasma osmolarity, reflected in a beta of 2.13(p=0.069); however, this effect was neither significant after adjusting for creatinine levels and loop diuretic use. In men, plasma hyperosmolarity was associated with R-AVP with accrued OR of 12.4 (p=0.048). Plasma osmolarity also showed a negative correlation with the u-Osm/C ratio (rs=-0.257; p<0.001).
U-Osm/C Ratio and R-AVP Associations with Clinical Factors and Drugs
A part from age and sex groups, the capacity to concentrate urine measured as u-Osm/C ratio was associated with gastric ulcer (82.7 vs 51.8 if not present vs present; p=0.031), chronic renal failure (83.1 vs 55.9 if not present vs present; p=0.042),and loop diuretic use (82.9 vs 36.4 in non-users and users; p=0.005). U-Osm/C ratio was also correlated with insulin resistance measured by HOMA index (rs=- 0.174; p=0.027), IL-6 (rs=-0.173; p=0.027), urea (rs=-0.164; p=0.036), creatinine (rs=-0.215; p=0.006), and Na (rs=-0.158; p=0.044).
R-AVP was related with loop diuretic use (11.9% vs 66.7% in non-users vs users; p<0.001), IL-6 (4.4 vs 7.1 pg/mL in non R-AVP vs R-AVP; p=0.030), and creatinine (0.8 vs 0.9 mg/dLin non R-AVP vs R-AVP; p=0.048). Differences in R-AVP prevalence approached, but did not reach, statistical significance for patients with vs without diabetes (24.1% vs 11.7%; p=0.133), with vs without chronic renal failure (25.0% vs 13.4%; p=0.311), or with vs without a previous stroke (30.8% vs 12.5%; p=0.088), or for users vs non-users of oral hypoglycaemic agents (24.1% vs 11.7%; p=0.133).
Discussion
Ourmain findings regarding plasma and urine osmolarity levels and R-AVP in a community-dwelling population aged 70 years and older indicate that: (a) plasma hyperosmolarity (>295 mOsm/L) is present in 4.5% of men and 0% of women, and increases to 20% in men in the ≥80 year old group; (b) the ability to concentrate urine decreases with age and is lower in men; (c) R-AVP prevalence of 12% in the 70-79 year old group increases to 20% in the ≥80 year old group; (d) R-AVP greatly increases the risk of plasma hyperosmolarity; and (e) ability to concentrate urine is associated with insulin resistance, renal failure and inflammation, while R-AVP is also associated with loop diuretic use.
The fact that men showed slightly higher levels of plasma and urineosmolarity than women, that plasma osmolarity in men, but not in women, increased with age (from 80 years), that men presented poorer capacity to concentrate urine, and that hyperosmotic stress (>295 mOsm/L) was only observed in men, raise the question of whether those differences are intrinsically due to sex, or conversely, to other sex-related clinical conditions acting as possible confounders. The fact that sex differences in plasma osmolarity disappeared after adjusting for creatinine levels suggests not an effect of sex but of renal function. However, sex differences have been reported for baseline plasma osmolality and for the plasma osmolarity threshold for AVP release during hyperosmotic saline infusion [20]. The fact that oestrogens, progesterone, and testosterone modulate the osmotic regulation of AVP release, as reported in other studies [21,22], may explain plasma osmolarity differences between men and women. It has also been reported that the increasing sensitivity of collecting ducts to AVP is greater in women than in men after a period of water deprivation [23]. That result agrees with our study suggesting lower R-AVP in women, and may explain the lower plasma osmolarity levels and the lack of hyperosmotic stress observed in women. Nonetheless, there is a need for further studies to determine issues such as whether the detrimental health effects of altered plasma osmolarity levels are the same for men and women, which comorbidities or drugs may act as confounders, and what mechanisms may account for sex differences in body fluid regulation.
As for urine osmolarity, the observation of lower levels in both men and women in the older age group agrees with previous scientific literature reporting a diminished capacity to concentrate urine with age [16]. This fact inspired our hypothesis that a low capacity to concentrate urine is due to peripheral (renal) R-AVP. While the men in our study had higher copeptin levels, coherent with their higher plasma osmolarity, we observed no significant age group differences in copeptin levels. Those results suggest preserved AVP release with advanced age, reinforcing the idea that peripheral R-AVP is responsible for poor urine concentration – an idea supported by the higher AVP levels reported for elderly compared to younger adults by Johnson et al. [24]. We have proposed, as an indicator of renal capacity to concentrate urine, the u-Osm/C ratio after 10 hours of absolute fasting, and have proposed a ratio cut-off consistent with reference values for healthy adults that also maximize the correlation between urinary osmolarity and copeptin. This indicator is acceptable to patients and professionals, as it does not cause significant patient discomfort, is easily and quickly calculated, is a relatively inexpensive measure, and behaves consistently in increasing as plasma osmolarity increases. We suggest that it could be useful in identifying the onset of age-related water dysregulation, most especially after the age of 80.
Our results show that hyperosmotic stress prevalence in aged subjects with R-AVP is a statistically significant 12 times greater than in aged subjects without R-AVP, suggesting a major peripheral R-AVP role in water dysregulation. AVP acts, at the renal level, primarily through expression of aquaporin 2 (AQP-2), which promotes water reabsorption, and whose levels may be altered in the elderly, especially from the age of 80 years. A study with rats showed that aquaporin2 expression was down regulated by 80% and markedly redistributed into the intracellular compartment in inner medulla of senescent rats, but not in renal cortex, and suggested that age-related polyuria was associated with a down regulation of AQP2 expression [24]. We have observed that plasma osmolarity is associated with diabetes and impaired renal function, and that the effect of R-AVP disappears after adjusting for urea or creatinine levels. The relationship between impaired renal function and R-AVP in humans needs to be studied in more detail, and more research is also needed on AQP-2 expression in aged populations and on its association with copeptin, plasma and urine osmolarities, and R-AVP.
On the other hand, hydration status is known to be altered by the chronic consumption of certain drugs [26]. Aged adults are more exposed to pharmacological side effects due to physiological changes, comorbidities, and polypharmacy [27]. In this population, already at increased risk of dehydration, the consumption of certain drugs may aggravate dehydration status [28]. Drugs can alter hydration status through several mechanisms, including increased elimination of water through diarrhoea, urine, and sweat, reduced thirst, decreased appetite, and alterations to central thermoregulation [29]. Our study points to a relationship between plasma hyperosmolarity and treatments with loop diuretics, ARBs, and PPIs. Diuretics can cause dehydration due to excess urine production [30], with the increased volume contributing to volume depletion occurring in conjunction with electrolyte and acid-base disturbances [31]. The fact that diuretics are frequently used in elderly patients with cardiovascular disease places these at greater risk of dehydration [32]. As for ARBs, the inhibition of angiotensin II reduces the thirst sensation and, consequently, fluid intake [30], both related with the fundamental role played by the renin-angiotensin system in blood pressure and fluid homeostasis. Finally, regarding PPIs, diarrhoea has been described as a mechanism that alters hydration status [29]. More specifically, PPIs have been associated with the presence of microscopic colitis in several studies, especially in prolonged treatment and in combination with anti-inflammatory drugs [33]. The link between antidiabetic drugs and plasma osmolarity may be due to the underlying hyperglycaemia or to other related factors. Metformin, for instance, can cause both gastrointestinal disorders (nausea and diarrhoea), decreased appetite, and taste alterations that may contribute to alterations in hydration status [29,34]. In summary, since certain medications affect hydration status, there is a need to monitor their use by elderly subjects. Further studies are undoubtedly needed to determine the independent effect of the afore-mentioned drugs on hydration status and their impact on the health of aged adults in usual clinical practice [35].
The main limitations of this study include its cross-sectional design, which does not allow any causal relationship to be established between R-AVP and plasma osmolarity, and the relatively small sample size, which reflects poor statistical power regarding the detection of weak associations, risk factors with low prevalence, and possible multivariate analyses. Regarding the use of the u-Osm/C ratio as an indicator of renal capacity to concentrate urine and also as an indicator of R-AVP, although this parameter has not been previously or formally validated, it would seem to be a reasonable (and useful) indicator of R-AVP, given that R-AVP develops when urine osmolarity levels are relatively low for a given level of AVP, and given that it behaves consistently and as expected in relation to plasma osmolarity levels.The selection of the cut-off point at 35 can be considered a bit conservative since a copeptin level of 12 pmol/ L(considered as high) corresponds to a urinary osmolarity of 450 mOsm/L (very low for such copeptin levels). A cut-off point at 50 would also have been reasonable (it would correspond to a urinary osmolarity of 600 mOsm/L for the same mentioned copeptin level), so a u-Osm/C ratio between 35 and 50 could be considered as at risk of R-AVP. Further studies are required to assess clinical relevance of such cut-offs.
In summary, the results of our study show that, in a sample of community-dwelling aged population, urine concentration capacity decreases and plasma hyperosmolarity increases with age, most especially after the age of 80. Our findings also point to R-AVP as a clinical condition, affecting12% of 70-79-year-olds and rising to 20% of =80-year-olds, that is related to plasma osmolality and that increases hyperosmotic stress risk. Moreover, ability to concentrate urine and R-AVP are related with inflammation, impaired renal function, insulin resistance and loop diuretic use. More studies are required to assess reasons for low grade chronic dehydration or water imbalance in aged populations, as well as its potential impact on cell functioning and health.
Statements and Declarations
• The project was carried out according to ethical standards and current legislation.The local ethics committee approved the study protocol (code CEIm CSdM 65/19).
• All participants gave their informed consent by writing before inclusion.
• All authors declare that they have no conflict of interest in relation to this study.
• This study was funded by a grant from the Spanish Ministry of Health-Instituto de Salud Carlos III (ISCIII), reference code PI19/00500.
References
- Bhave G, Neilson EG. Body fluid dynamics: back to the future. Journal of the American Society of Nephrology: JASN. 2011; 22: 2166-2181.
- Barrett KE, Barman SM, Boitano S, Brooks H. Hypothalamic regulation of hormonal functions. In Ganong’s Review of Medical Physiology, 23th ed.; McGraw-Hill Medic: New York, NY, USA, 2010; 273–288.
- Treschan TA, Peters J. The Vasopressin System: Physiology and Clinical Strategies. Anesthesiology. 2006; 105: 599-612.
- Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiological reviews. 2007; 87: 1441-1474.
- Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. Journal BioMolecular Concepts. 2012; 3: 345-364.
- Enhörning S,Melander O. The vasopressin system in the risk of diabetes and cardiorenal disease, and hydration as a potential lifestyle intervention. Ann Nutr Metab. 2018; 72: 21–27.
- Gosmanov AR, Schneider EG, Thomason DB. NKCC activity restores muscle water during hyperosmotic challenge independent of insulin, ERK, and p38 MAPK. American journal of physiology. Regulatory, integrative and comparative physiology. 2003; 284: R655-R665.
- Schliess F, Richter L, vom Dahl S, Häussinger D. Cell hydration and mTORdependent signaling. Acta Physiol. 2006; 187: 223–229.
- Yamada Y, Yoshida T, Yokoyama K, Watanabe Y, Miyake M, Yamagata, et al. The extracellular to intracellular water ratio in upper legs is negatively associated with skeletal muscle strength and gait speed in older people. J Gerontol A Biol Sci Med Sci. 2017; 72: 293–298.
- Serra-Prat M, Lorenzo I, Palomera E, Yébenes JC, Campins L, Cabré M. Intracellular Water Content in Lean Mass is Associated with Muscle Strength, Functional Capacity, and Frailty in Community-Dwelling Elderly Individuals. A Cross-Sectional Study. Nutrients. 2019; 11: 661.
- Stookey JD, Purser JL, Pieper CF, Cohen HJ. Plasma Hypertonicity: Another Marker of Frailty?. JAGS. 2004; 52: 1313-20.
- Hooper L, Bunn D, Jimoh FO, Fairweather-Tait SJ. Water-loss dehydration and aging. Mechanisms of Ageing and Development. 2014; 136-137: 50-58.
- Maughan RJ. Hydration, morbidity, and mortality in vulnerable populations. Nutrition reviews. 2012; 70: S152-S155.
- El-Sharkawy AM, Watson P, Neal KR, Ljungqvist O, Maughan RJ, Sahota O, et al. Hydration and outcome in older patients admitted to hospital (The HOOP prospective cohort study). Age and Ageing. 2015; 44: 943-947.
- Dmitrieva NI, Liu D, Wu CO, Boehm M. Middle age serum sodium levels in the upper part of normal range and risk of heart failure. European heart journal. 2022.
- Tamma G, GoswamiN,Reichmuth J, De Santo NG,Valenti G. Aquaporins, vasopressin, and aging: Current perspectives. Endocrinology. 2015; 156: 777–788.
- Cowen LE, Hodak SP, Verbalis JG. Age-associated abnormalities of water homeostasis. Endocrinology and metabolism clinics of North America. 2013; 42: 349-370.
- Stöllberger C, Lutz W,Finsterer J. Heat-related side-effects of neurological and non-neurological medication may increase heatwave fatalities. Eur J Neurol. 2009; 16: 879–882.
- Marwick C, Santiago VH, McCowan C, Broomhall J, Davey P. Community acquired infections in older patients admitted to hospital from care homes versus the community: cohort study of microbiology and outcomes. BMC Geriatrics. 2013; 13: 12 - 12.
- Stachenfeld NS, Splenser AE, Calzone WL, Taylor MP, Keefe DL. Sex differences in osmotic regulation of AVP and renal sodium handling. Journal of applied physiology. 2001; 91: 1893-1901.
- Stachenfeld NS, DiPietro L, Palter SF, and Nadel ER. Estrogen influences osmotic secretion of AVP and body waterbalance in postmenopausal women. Am J Physiol Regulatory Integrative Comp Physiol 1998; 274: R187–R195.
- Stachenfeld NS, Silva CS, Keefe DL, Kokoszka CA, andNadelER.Effects of oral contraceptives on body fluid regula-tion. J Appl Physiol. 1999; 87: 1016–1025.
- Hancock ML, Bichet DG, Eckert GJ, Bankir L, Wagner MA, Pratt JH. Race, sex, and the regulation of urine osmolality: observations made during water deprivation. American journal of physiology. Regulatory, integrative and comparative physiology. 2010; 299: R977-R980.
- Johnson AG, Crawford GA, Kelly D, Nguyen TV, Gyory AZ. Arginine Vasopressin and Osmolality in the Elderly. Journal of the American Geriatrics Society. 1994; 42: 399-404.
- Preisser L, Teillet L, Aliotti S, Gobin R, Berthonaud V, Chevalier J, et al. Downregulation of aquaporin-2 and -3 in aging kidney is independent of V2 vasopressin receptor. Am J Physiol Renal Physiol. 2000; 279: F144–F152.
- Puga AM, Lopez-Oliva S, Trives C, Partearroyo T, Varela-Moreiras G. Effects of Drugs and Excipients on Hydration Status. Nutrients. 2019; 11: 669.
- Jetha S. Polypharmacy, the Elderly, and Deprescribing. The Consultant pharmacist : the journal of the American Society of Consultant Pharmacists. 2015; 30: 527-532.
- Jéquier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. European Journal of Clinical Nutrition. 2010; 64: 115-123.
- Puga AM, Lopez-Oliva S, Trives C, Partearroyo T, Varela-Moreiras G. Effects of Drugs and Excipients on Hydration Status. Nutrients. 2019; 11: 669.
- KalischEllett LM, Pratt NL, Le Blanc VT, Westaway K, Roughead EE. Increased risk of hospital admission for dehydration or heat-related illness after initiation of medicines: a sequence symmetry analysis. J Clin Pharm Ther. 2016; 41: 503-507.
- Khow KSF, Lau SY, Li JY, Yong TY. Diuretic-associated electrolyte disorders in the elderly: risk factors, impact, management and prevention. Current drug safety. 2014; 9: 2-15.
- Levine M, LoVecchio F, Ruha A, Chu G, Roque P. Influence of Drug Use on Morbidity and Mortality in Heatstroke. Journal of Medical Toxicology. 2012; 8: 252-257.
- Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis - proposal for a scoring system and review of the literature. Aliment Pharmacol Ther. 2005; 22: 277-284.
- Bouchoucha M, Uzzan B, Cohen R. Metformin and digestive disorders. Diabetes & metabolism. 2011; 37: 90-96.
- Puga AM, Partearroyo T, Varela-Moreiras G. Hydration status, drug interactions, and determinants in a Spanish elderly population: a pilot study. Journal of Physiology and Biochemistry. 2017; 74: 139-151.
