
Mini Review
Austin Food Sci. 2016; 1(2): 1007.
Review of Traditional and Non-Traditional Medicinal Genetic Resources in the USDA, ARS, PGRCU Collection Evaluated for Flavonoid Concentrations and Anthocyanin Indexes
Morris JB*, Wang ML and Tonnis B
Department of Agriculture, Plant Genetic Resources Conservation Unit, USA
*Corresponding author: Morris JB, United States Department of Agriculture, Agricultural Research Service, Plant Genetic Resources Conservation Unit, USA
Received: March 11, 2016; Accepted: April 04, 2016; Published: April 07, 2016
Abstract
Non-traditional medicinal species include velvetleaf (Abutilon theophrasti Medik.), Desmodium, Termanus labialis (L.f.) Spreng. and the traditional roselle (Hibiscus sabdariffa L.). Flavonoids and anthocyanins have been shown to have anti-cancer activities in humans. Fruit and leaves from velvetleaf, seeds from D. discolor Vogel, D. incanum (G. Mey) D.C., D. intortum (Mill.) Urb., D. E. Mey., D. tortuosum (Sw.) D.C., T. labialis, and calyces from roselle accessions in the USDA, ARS, PGRCU collection could be sources of myricetin, quercetin, kaempferol, isorhamnetin, luteolin, apigenin, and anthocyanins. The objectives of this review article are to report medicinal plant progress for flavonoid and anthocyanin index variability among these species which can be used to develop superior cultivars for use as nutraceuticals, functional foods, and phytopharmaceuticals.
Keywords: Flavonoid; Anthocyanin index; Medicinal plant; Velvetleaf; Roselle; Legumes
Introduction
Many species in the USDA, ARS, Plant Genetic Resources Conservation Unit (PGRCU) germplasm collection contain novel flavonoid traits for use as medicinal or functional food plants [1-7]. Additional species conserved in the PGRCU collection have potential use as medicinal plants including velvetleaf (Abutilon theophrasti Medik.) which has been used non-traditionally to alleviate ephemeral fever in animals [8]. Other potential non-traditional medicinal species for livestock could include Desmodium intortum (Mill.) Urb. D. sandwicense E. Mey., D. incanum (G. Mey.) DC., D. discolor (Vogel), and D. tortuosum (Sw.) DC. Desmodium intortum reduces the worm parasite (Haemonchus contortus) in goats [9], D. sandwicense is cold tolerant [10], D. incanum dominates natural pastures in Brazil [11], D. discolor provides hay with good animal palatability [12], and D. tortuosum is sold as wild bird feed. The legume, Teramnus labialis (L.f.) Spreng. is used as a pulse food crop by humans in southern India [13] and roselle calyces (Hibiscus sabdariffa L.) are traditionally used in health teas [1].
Dietary supplements including herbal medicinal plant sales increased to about $6 billion in 2013 [14]. Flavonoids such as quercetin, kaempferol, myricetin, isorhamnetin, luteolin, apigenin, and anthocyanins have potential for use as new medicines from plants. The flavonoid, quercetin is apoptotic to human breast cancer cells [15], and kaempferol reduces cancer cell growth and seems to protect normal cells [16]. Myricetin in combination with chlorogenic acid and quercetin lowers blood glucose levels in type 2 diabetes [17]. Isorhamnetin is more cytotoxic to gastric cancer when combined with chemotherapy medicines [18]. Luteolin in combination with other chemicals is apoptotic to lung cancer and carcinoma cells of the head and neck cancer cell lines [19], and apigenin is effective against breast cancer cells [20]. Anthocyanins have been shown to have chemopreventive effects [21]. Therefore it was very important to report this review because theseflavonoidsfrom medicinal species representing several countries of origin in the PGRCU collection. In addition, leaf anthocyanin indexes needed to be evaluated from the majority of the velvetleaf collection. An anthocyanin index value predicts estimated and non-destructive anthocyanin content in plants [22]. Evaluations from one traditional and several nontraditional species in the PGRCU germplasm collection including velvetleaf [2], D. discolor, D. incanum, D. intortum, D. sandwicense, D. tortuosum [6], roselle, (H. sabdariffa) [1], and T. labialis [7] for flavonoid variability using reverse-phase HPLC will be discussed. Velvetleaves were also evaluated for Anthocyanin indexes using an anthocyanin meter as described in [2]. There is little information in the literature regarding additional research work for the specific flavonoids discussed in this paper.
Velvetleaf
Forty two velevetleaf (2009) accessions (Table 1) were grown in the field at Griffin, GA for 1 year (2009) and evaluated for anthocyanin indexes using an ACM-200 plus anthocyanin index meter (Opti- Sciences, Hudson, NH). Quercetin, kaempferol, and myricetin concentrations were quantified using revers-phase HPLC [2] from 26 accessions. Leaves and fruit were collected from each accession after about 8 weeks of growth and stored at -20°C until analysis. About 0.3 g of mature ground velvetleaf and fruit tissue was used for flavonoid reverse-phase HPLC evaluations. Additional methods and research results were reported in [2]. Significant variation for flavonoids and anthocyanin indexes among velvetleaf accessions occurred. Overall, quercetin and kaempferol production from velvetleaves were superior to myricetin, quercetin, and kaempferol production from velvetleaf fruit based on country of origin (Figure 1), adapted from data in [2]. We found mean concentrations of quercetin (4 mg g-1, *P ≤ 0.05) produced from velvetleaves of the Japanese accessions (PI 499255 and PI 499213) were higher than many accessions from China, Asia, Europe, former Soviet Union, Africa, and the United States (Figure 1). Mean kaempferol production (2 mg g-1, *P ≤ 0.05) from velvetleaves originating from Africa exceeded those velvetleaves from many of the other countries also. Mean anthocyanin indexes of velvetleaves from China, Asia, the former Soviet Union, and Japan averaging 9 (*P ≤ 0.05) were higher than those indexes from velvetleaves originating from Europe, Africa, and the United States (averaging 8, *P ≤ 0.05) (Figure 2), adapted from data in [2]. Tian et al. 2012 identified quercetin and luteolin in velvetleaf exocarps. They also found naringenin in leaves and exocarps as well as rutin in roots, stems, leaves, seeds, and exocarps [23]. However they did not identify myricetin in any velvetleaf organ. Matlawska and Sikorska [24] observed the identification of kaempferol, myricetin, and quercetin glycosides in velvetleaf flowers. Velvetleaf seed coats were reported to consist of delphinidin, cyanidin, quercetin, myricetin, (+)-catechin, and (-)-epicatechin with anti-fungal and allelopathic potential [24,25].
Species
Number of accessions
Country of origin
Abutilon theophrasti
8
China
4
Middle Asia, India
14
Europe
12
Former Soviet Union
1
Africa
1
United States
2
Japan
Desmodium discolor
2
Brazil
1
India
Desmodium incanum
2
Brazil
1
Florida, U.S.A.
1
Uruguay
Desmodium intortum
1
Brazil
1
Spain
Desmodium sandwicense
10
Australia
Desmodium tortuosum
1
Australia
1
India
1
Tanzania
1
Trinidad and Tobago
2
Virgin Islands, U.S.A.
Hibiscus sabdariffa
8
St. Croix, Virgin Islands
Teramnus labialis
4
Dominican Republic
3
Kenya
3
S. Africa
5
Virgin Islands, U.S.A.
Table 1: Species, number of accessions, and country of origin used for flavonoid evaluations.
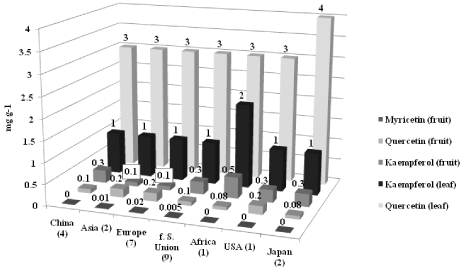
Figure 1: Mean flavonoid concentrations (*P ≤ 0.05) among 26 Abutilon
theophrasti accessions grown in the field at Griffin, GA in 2009 based on
origin [2].
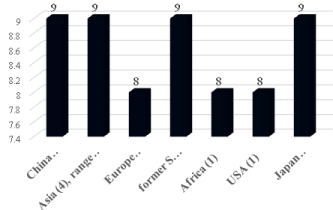
Figure 2: Mean anthocyanin indexes (*P ≤ 0.05) of leaves from 42 Abutilon
theophrasti accessions grown in the field during 2009 at Griffin, GA based
on origin [2].
Desmodium species
Twenty-five Desmodium accessions including 3 D. discolor, 2 D. intortum, 10 D. sandwicense, 6 D. tortuosum, and 4 D. incanum controlswere grown in the field at Griffin, GA for two years (2010- 2011). Mature pods were harvested from each Desmodium accession between 3 to 6 months after transplanting. The pods were dried at 21°C and 25% relative humidity for 1 week and then threshed. Seeds from each accession were evaluated for flavonoid concentrations including quercetin, isorhamnetin, luteolin, kaempferol, and apigenin using reverse-phase HPLC [6]. Additional methods and research details were reported in [6].We found mean flavonoid concentrations were significantly higher (*P = 0.05, **P = 0.01, and ***P = 0.0001) than all of the D. incanum control accessions (Figure 3). (Figure 3) (Adapted from data in [6]) shows that the most quercetin was produced from the D. intortum accessions (averaging 838 μg g-1, ***P = 0.0001) and followed closely by the D. sandwicense accessions (averaging 708 μg g-1, ***P = 0.0001) when compared to all of the D. incanum control accessions (averaging 18 μg g-1) during 2010. However, the most isorhamnetin was produced from the D. tortuosum (averaging 718 μg g-1, ***P = 0.0001) and D. discolor (averaging 596 μg g-1, ***P = 0.0001) accessions when compared to the controls (0 μg g-1) during 2010. The D. tortuosum accessions produced the most luteolin concentrations (averaging 441 μg g1, ***P = 0.0001) when compared to the controls (0 μg g-1). More kaempferol (averaging 176 μg g-1, ***P = 0.0001) was produced in the D. intortum accessions and more apigenin (averaging 112 μg g-1, **P = 0.01 and ***P = 0.0001) was produced in the D. tortuosum accessions when compared to the controls (averaging 26 μg g-1).
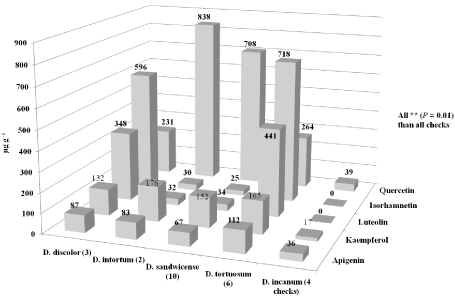
Figure 3: Mean flavonoid concentrations (significantly higher than all of the
checks, *P = 0.05, **P = 0.01, and ***P = 0.0001) in seeds from 21 field grown
(Griffin, GA) Desmodium accessions (2010) [6].
During 2011, both D. intortum and D. sandwicense accessions produced the most quercetin (averaging 719 and 589 μg g-1, ***P = 0.0001, respectively) when compared to the D. incanum controls (averaging 17 μg g-1) (Figure 4) which were similar to the 2010 results. Similar results were observed for isorhamnetin. However, the D. discolor accessions produced a higher average of isorhamnetin (676 μg g-1, ***P = 0.0001) followed by the D. tortuosum accessions (averaging 649 μg g-1, ***P = 0.0001) when compared to the controls (0 μg g-1) during 2011. The D. discolor accessions produced the most luteolin concentrations (413 μg g-1, ***P = 0.0001) when compared to the controls (0 μg g-1). Similar concentrations of kaempferol were produced from all Desmodium species (ranging from 143 - 158 μg g-1, **P = 0.01 and ***P = 0.0001) when compared to the controls (averaging 17μg g-1). Similar concentrations of apigenin were also produced in the Desmodium accessions (ranging from 55 - 110 μg g-1, *P = 0.05, **P = 0.01, and ***P = 0.0001). However, the D.discolor accessions produced slightly more apigenin (averaging 110 μg g-1, **P = 0.01) than the controls (averaging 33 μg g-1). Several other closely related Desmodium species are used in traditional Chinese medicine [26]. They identified luteolin in above ground plant parts from D. sambuense; apigenin in the whole plant and roots of D. styracifolium; kaempferol and quercetin in plant parts of D. syracifolium and D. gangeticum.
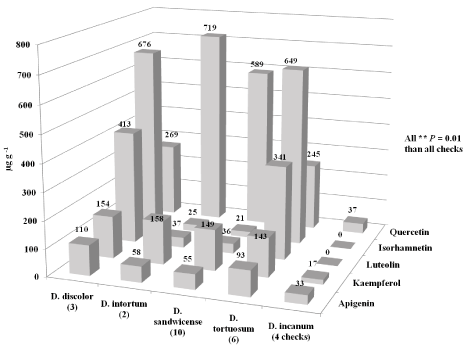
Figure 4: Mean flavonoid concentrations (significantly higher than all of the
checks, *P = 0.05, **P = 0.01, and ***P = 0.0001) in seeds from 21 field grown
(Griffin, GA) Desmodium accessions (2011) [6].
Hibiscus sabdariffa
Eight photo-period sensitive roselle accessions originating from Saint Croix, Virgin Islands were grown in 27.5 cm x 27.5 cm plastic pots containing potting soil in the greenhouse during 2013 at Griffin, GA. Mature calyces were harvested from each roselle accession 5 to 6 months after planting. Freeze dried roselle calyces from eight accessions were ground to a fine powder and used for flavonoid analysis in a reverse-phase HPLC. A similar protocol in the paper, [1] was used. However, an XDB-phenyl column was used during the HPLC procedure. Additional modifications were: the mobile phase was 4:1 acetonitrile/methanol (B) and 0.1% formic acid in sterile water (A); the flow rate was 0.75 ml/min at a gradient of 5% B for 1 min. followed by 40% B for 20 min.; sample injection rate was 10 μl; and UV detection was monitored with a 370 nm DAD. The accessions 30 and 31 produced significantly more myricetin (1925 μg g-1 and 1436 μg g-1, respectively, ***P < 0.0001) and quercetin (68 μg g-1 and 67 μg g-1, respectively, ***P < 0.0001) in their calyces than all other roselle accessions (Figure 5). However, Ramirez-Rodrigues et al. 2011 found roselle calyces ranged in quercetin-3-rutinoside content from 8450 to 9380 μg g-1. Accessions 31 and 35 produced more kaempferol (62 μg g-1 and 59 μg g-1, respectively, ***P < 0.0001) than all other accessions [27].
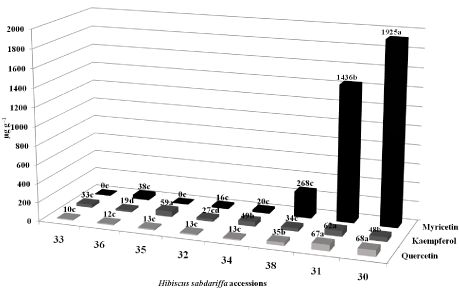
Figure 5: Mean flavonol concentrations differed significantly (P < 0.0001) in
calyces of 8 H. sabdariffa accessions from St. Croix, VI grown in the Griffin,
GA greenhouse in 2013.
Teramnus labialis
Fifteen photo-period sensitive T. labialis accessions including 4 from the Dominican Republic, 5 from the Virgin Islands, 3 each from S. Africa and Kenya were grown in 27.5 cm x 27.5 cm plastic pots containing potting soil in the greenhouse during 2010-2011 at Griffin, GA. Mature pods were harvested from each T. labialis accession about 5 to 8 months after planting, dried at 21oC, 25% RH for 1 week, and threshed. Seeds from each accession were evaluated for quercetin, kaempferol, and isorhamnetin concentrations using reverse-phase HPLC [7]. Additional methods and research details were reported in [7]. There was more quercetin detected in the S. African (2 mg g-1, ***P < 0.0001) and Kenyan accessions (1.5 mg g-1, ***P < 0.0001) (Figure 6). However, very low amounts of kaempferol and isorhamnetin were found in all of the accessions. Flavonoid compound content (~2.5 mg g-1) in entire plants of T. labialis was reported by Vasagam et al. [28] and were similar to those content values we found in T. labialis seeds. However the flavonoid compounds found by Vasagam et al. 2012 were not identified [28].
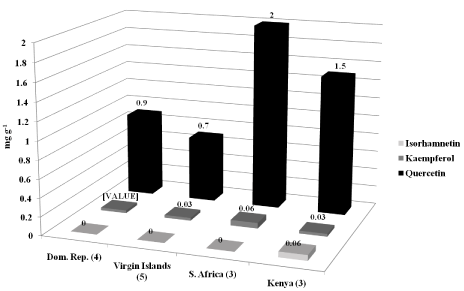
Figure 6: Mean flavonol concentrations differed significantly (P < 0.0001) in
seeds from 15 T. labialis accessions grown in the greenhouse at Griffin, GA
from 2010-2011 based on origin [7].
The objectives of this review article are to report medicinal plant progress for flavonoid and anthocyanin index variability among these species which can be used to develop superior cultivars for use as nutraceuticals, functional foods, and phyto-pharmaceuticals.
Conclusion
Since roselle calyces [1] and T. labialis seeds [13] are edible and contain valuable flavonoids, they have functional food potential because they are currently used in various food products and health teas. However, further conformational studies should be conducted. The Academy of Nutrition and Dietetics defines a functional food as a food that provides additional health benefits that may reduce disease risk and/or promote good health. Roselle accessions from Saint Croix, Virgin Islands and T. labialis accessions from Kenya and S. Africa consist of high quality genotypes containing elevated concentrations of myricetin and quercetin. Flavonoids from these species may contribute antioxidants, prevent cancer, and lower cholesterol when consumed by humans. The A. theophrasti and Desmodium species may provide animals and humans with natural constituents such as flavonoids when eaten. These traditional and non-traditional medicinal species in the USDA, ARS, PGRCU germplasm collection consists of enough genetic variability to implement a breeding program for the development of new cultivars or germplasm with high flavonoid concentrations.
References
- Morris JB, Wang ML, Thomas T. Quercetin, Kaempferol, Myricetin, and Fatty Acid content among several Hibiscus sabdariffa accession calyces based on maturity in a greenhouse. Chikamatsu T, Hida Y. In: Quercetin: Dietary Sources, Functions and Health Benefits. Nova Science Publishers. 2012; 269-282.
- Morris JB, Wang ML. Anthocyanin indexes, Quercetin, Kaempferol and Myricetin concentration in leaves and fruit of Abutilon theophrasti Medik. genetic resources. Plt Gen Res Chara and Util. 2013; 11: 87-89.
- Morris JB, Wang ML, Grusak MA, Tonnis B. Fatty acid, Flavonol, and Mineral composition variability among seven Macrotyloma uniflorum (Lam.). Verdc. Accessions. Agri. 2013; 3: 157-169.
- Morris JB, Grusak MA, Wang, ML, Tonnis B. Mineral, flavonoid, and fatty acid concentrations in ten diverse Lablab purpureus (L.) Sweet accessions. Hai-Xue K, In: Phytochemicals: Occurrence in Nature, Health Effects and Antioxidant Properties. Nova Publishers. 2013; 219-224.
- Morris JB, Wang ML, Tonnis B. Variability for phenotype, anthocyanin indexes, and flavonoids in accessions from a close relative of soybean, Neonotonia wightii (Wight & Arn JA Lackey). El-Shemy Hany A, In: Soybean-Bio-active Compounds. In Tech. 2013; 375-386.
- Morris JB, Wang ML, Tonnis B. Desmodium genetic resources for improving flavonoid concentrations, oil content and fatty acid compositions. Plt Gen Res Char and Util. 2014; 12: 120-128.
- Morris JB, Tonnis B, Wang ML. Flavonol content, oil% and fatty acid composition variability in seeds of Teramnus labialis and T. uncinatus accessions with nutraceutical potential. J of Diet Suppl. 2014; 11: 294-303.
- Khan MA, Hanif W. Ethno veterinary medicinal uses of plants from Samahni Valley Dist. Bhimber, (Azad Kashmir) Pakistan. Asian J Plt Sci. 2006; 5: 390-396.
- Debela E, Tolera A, Eik LO, Salte R. Condensed tannins from Sesbania sesban and Desmodium intortum as a means of Haemonchus contortus control in goats. Trop Anim Health Prod. 2012; 44: 1939-1944.
- Whiteman PC. Seasonal changes in growth and nodulation of perennial tropical pasture legumes in the field. I. The influence of planting date and grazing and cutting on Desmodium uncinatum and Phaseolus atropurpureus. Aust J Agri Res. 1970; 21: 195-206.
- Tanure S, Potter B, Augusto A, Lobato JFP. Natural and improved natural pastures on the reproductive performance of first-calf beef cows. Rev Brasli De Zootec. 2011; 40: 690-699.
- Boultwood JN. Two valuable perennial legumes - horse marmalade (Desmodium discolor) and kuru vine (D. intortum). Rhod Agri J. 1964; 61: 70-72.
- Viswanathan MB, Thangadurai D, Vendan KT, Ramesh N. Chemical analysis and nutritional assessment of Teramnus labialis (L.) Spreng. (Fabaceae). Plant Foods Hum Nutr. 1999; 54: 345-352.
- Lindstrom A, Ooyen C, Lynch ME, Blumenthal M, Kawa K. Sales of herbal dietary supplements increase by 7.9% in 2013, marking a decade of rising sales: turmeric supplements climb to top ranking in natural channel. Herbal Gram. 2014; 103: 52-56.
- Chen FP, Chien MH. Phytoestrogens induce apoptosis through a mitochondria/caspase pathway in human breast cancer cells. Climacteric. 2014; 17: 385-392.
- Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013; 138: 2099-2107.
- Ahrens MJ, Thompson DL. Effect of emulin on blood glucose in type 2 diabetics. J Med Food. 2013; 16: 211-215.
- Ramachandran L, Manu KA, Shanmugam MK, Li F, Siveen KS, Vali S, et al. Isorhamnetin inhibits proliferation and invasion andinducesapoptosis through the modulation of peroxisome proliferator-activated receptor y activation pathway in gastric cancer. J Biol Chem. 2012; 287: 38028-38040.
- Amin AR, Wang D, Zhang H, Peng S, Shin HJ, Brandes JC, et al. Enhanced anti-tumor activity by the combination of the natural compounds (-)-epigallocatechin-3-gallate and luteolin: potential role of p53. J Biol Chem. 2010; 285: 34557-34565.
- Mafuvadze B, Benakanakere I, Hyder SM. Apigenin blocks induction of vascular endothelial growth factor mRNA and protein in progestin-treated human breast cancer cells. Menopause. 2010; 17: 1055-1063.
- Thomasset S, Teller N, Cai H, Marko D, Berry DP, Steward WP, et al. Do anthocyanins and anthocyanidins, cancer chemopreventive pigments in the diet, merit development as potential drugs? Cancer Chemo and Pharmacol. 2009; 64: 201-211.
- Van Den Berg A, Perkins TD. Nondestructive estimation of anthocyanin content in Autumn sugar maple leaves. Hort Sci. ; 40: 685-686.
- Tian C, Wang M, Shen C, Zhao C. Accuracy mass screening and identification of phenolic compounds from the five parts of Abutilon theophrasti Medic. by reverse-phase high-performance liquid chromatography-electrospray ionization-quadrupoles-time of flight-mass spectrometry. J Sep Sci. 2012; 35: 763-772.
- Matlawska I, Sikorska M. Flavonoids from Abutilon theophrasti flowers. Acta Pol Pharm. 2005; 62: 135-139.
- Paszkowski WL, Kremer RJ. Biological activity and tentative identification of flavonoid components in velvetleaf (Abutilon theophrasti Medik.) seed coats. J Chem Ecol. 1988; 14: 1573-1582.
- Ma X, Zheng C, Hu C, Rahman K, Qin L. The genus Desmodium (Fabaceae)-traditional uses in Chinese medicine, phytochemistry and pharmacology. J Ethnopharm. 2011; 138: 314-332.
- Ramirez-Rodriques MM, Plaza ML, Azeredo A, Balaban MO, Marshall MR. Physiochemical phytochemical properties of cold and hot water extraction from Hibiscus sabdariffa. J Food Sci. 2011; 76: 428-435.
- Vasagam GA, Muthu AK, Manavalan R. An in-vitro free radical scavenging activity of methanolic extracts of whole plant of Teramnus labialis (Linn.). Int J Pharm Chem Sci. 2012; 1: 924-929.