
Special Article - Food Chemistry
Austin Food Sci. 2016; 1(3): 1013.
Biochemical Properties of Insoluble Fraction of Ultrasonicated Chicken Actomyosin Redissolved in High Molarity Sodium Chloride
Saleem R, Hasnain A and Ahmad R*
Department of Zoology, Aligarh Muslim University, India
*Corresponding author: Riaz Ahmad, Section of Genetics, Department of Zoology, Faculty of Life Sciences, Aligarh Muslim University, India
Received: May 03, 2016; Accepted: June 14, 2016; Published: June 15, 2016
Abstract
Low frequency (20 kHz) sonication of chicken Actomyosin (AM) solution for 5 to 30 min caused quantitative reduction in the insoluble protein fractions. The insolubility of sonicated AM depended on the sonication time as well as the NaCl molarity (0.2M to 0.5M) of the dilutions. The protein contents in the insoluble pellets were lowest at 0.5M NaCl and highest at 0.2M NaCl dilutions. As judged by the protein content changes, the reduction in the pellets of sonicated AM was outstanding and supported the earlier findings on the solubility enhancing effect of low frequency sonication on AM. In the SDS-PAGE profiles of redissolved pellets, the most obvious reduction occurred in the intensity of Myosin Heavy Chain (MyHC). The two noteworthy biochemical characteristics of various pellets of sonicated AM were: the dissolution in 0.6M NaCl and the retention of functional properties. Myosin existed in rather active state at 0.3M NaCl, since the specific Ca2+- and K+(EDTA)-ATPase activities which are integrity indicators of myosin molecule showed maxima at 0.3M NaCl. The specific Mg2+-ATPase activity, the indicator of actin-myosin interaction, reached the maximum at 0.4M NaCl. Compared to Mg2+-ATPase activity, the decline in the specific Ca2+- and K+(EDTA)-ATPase activities (30-60%) was exceptionally higher. This suggested that the ATP hydrolysis site of myosin was more susceptible to 20 kHz sonication than the actin-myosin interaction. Thus, AM in the insoluble fractions represented a state of conformational plasticity that was unlike the irreversible aggregation by heat denaturation. Therefore, the biochemical attributes of insoluble fractions of sonicated AM are at par with the thermal processing requirements; wherein the solubility and functionality, both play crucial roles.
Keywords: Chicken actomyosin; Insoluble fraction; Conformational plasticity; Myosin heavy chain; Protein solubility; SDS-PAGE; Ultrasonication
Introduction
Ultrasonication is gaining increasing attention in meat industry, as it can help decontaminate meat besides assisting in meat processing, determining its composition and formulating new products [1-4]. The low frequency ultrasonication can alter structural properties of raw meat which enhance tenderness, a trait of high consumer acceptability [5-7]. As for the chicken muscle, post mortem tenderization can be achieved by sonic exposure. The proposed mechanism includes weakening of actin-myosin interaction and an increase in the length of sarcomeres, which increases susceptibility of myofibrillar proteins to intrinsic calpains [8]. While tenderness mostly depends on the structural alterations in myofibrillar proteins, thermal processing proceeds by way of solubilizing these proteins. The sonication helps in both of these events i.e. in tenderization of meat as well as solubility enhancement of myofibrillar proteins [9-12]. Therefore, expansion of basic understanding of the effects of sonication on structural proteins of myofibrils and underlying interactions has wide implications.
We have been investigating the effect of low frequency (20 kHz) ultrasonication on chicken actomyosin with a focus on the duration of sonic exposure and solubility of the products at various NaCl dilutions. Saleem et al. [13] showed that the sonication increased solubility of the low ionic strength soluble constituents of AM if suspended in low ionic buffer, either with or without 0.1M NaCl. However, low frequency sonication of high ionic strength (0.6M NaCl) solution of AM caused marked changes in tertiary structure and exposed more of the thiols [14-16]. As for the solubility, we have shown that sonic exposure for 10-12 min can solubilize out about 61.55% of soluble AM in 0.2M NaCl [17]. The same study also showed that conformational changes in myosin, along with the actin-myosin interaction, determine the biochemistry of sonicated soluble AM.
Although sonication decreased various specific ATPase activities of unpartitioned AM solution, thermal gels formed by it showed better water holding properties because of the distinct improvement in three-dimensional network of the gels [14]. Generally, the denaturation by temperature and other physical factors inflict a loss in water holding capacity. However, we have recently shown that sonicated AM can be fractionated/partitioned in to ‘soluble’ and ‘insoluble fractions’. Unlike unpartitioned AM, such soluble fractions demonstrated activation in various ATPase activities [17,18]. Besides, an outstanding increase in protein solubility at 0.2M was also observed. Therefore, the biochemical characteristics of the residual (insoluble) fraction of the partitioned AM deserved investigations.
The present reports deals with the ‘insoluble fractions’ of sonicated AM obtained at various dilutions of NaCl.
Materials and Methods
Chemicals
The following chemicals were purchased from authorized dealers of Sigma-Aldrich chemicals Pvt. Ltd. in India: acrylamide, bisacrylamide, Phenyl Methane Sulfonylfluoride (PMSF), Adenosine 5'-Triphosphate-Disodium salt (ATP), ammonium persulfate and Tetramethylethylene Diamine (TEMED). The source of analytical grade potassium chloride, bovine serum albumin and Tris buffer and 1-Amino-2-Naphthol-4-Sulphonic Acid (ANSA) and other reagents was SRL, India.
Preparation of actomyosin
Actomyosin was prepared from breast muscle (Pectoralis major) of 3-months-old broiler chicken. Following excision, muscle was immediately immersed in ice bath to stop glycolysis. The procedure of extraction frequently used in this laboratory was followed [19,20]. Briefly, prior to overnight extraction in the buffer (0.6M KCl in 25mM Tris-maleate buffer of pH 7.0, containing 2mM PMSF), minced muscle was washed 3 times with 50mM phosphate buffer (pH 7.2). Thick viscous solution of Natural Actomyosin (NAM) was recovered after centrifugation at 10K rpm (4°C) for 15 min. Actomyosin was precipitated out by 10 fold dilution with chilled distilled water and collected by centrifugation at 5K rpm for 15 min (4°C). The precipitate thus collected was saved and dissolved in 0.6M NaCl in 20 mM Trismaleate buffer, pH 7.2 (dissolving buffer). The traces of free myosin were removed by precipitating 0.6M AM solution to 0.2M. The step was repeated once more and the precipitated AM was solubilized in dissolving buffer. The AM solution was then passed through one more cycle of 10-times dilution. After finally dissolving the washed AM precipitate in 0.6M NaCl (with 20mM Tris-maleate), the AM solution was overnight dialyzed against the same buffered NaCl. Any insoluble residue was removed by centrifugation at 10K rpm.
Protein estimation
Protein in all of the samples was estimated according to the protocol of Lowry et al. [21]. Bovine serum albumin, in known quantities, was used as the standard. Optical density was recorded at 660 nm on BioSyn UV-Visible spectrophotometer. All of the expressed values are the average of three readings from each protein sample.
Ultrasonic treatment
AM solution (10 ml) in 0.6M NaCl with the protein concentration of 2.5-3 mg/ml was exposed to low frequency (20 kHz, 50 W) ultrasonic waves for 30 min. Immersion probe type sonicator (Ralco, India Ltd.) was used throughout the study. The immersion probe was 12 mm in diameter. The probe of sonicator was immersed in the middle of AM container and was held in a central position. During ultrasonication procedure, sample tubes were constantly kept under ice bath and to offset heating, each ultrasonic burst of 15 seconds was followed by a regular 10 second of cooling lag. Five aliquots of equal volume (2.0 ml) were collected at the intervals of 5, 10, 15, 20 and 30 min.
Preparation of insoluble fractions of sonicated AM at various NaCl dilutions of sonicated AM
Aliquots of sonicated AM solution in 0.6M NaCl were collected at specific time intervals, as described above. Each aliquot was diluted with cold distilled water to bring down the molarity to 0.1M, 0.2M, 0.3M, 0.4M and 0.5M NaCl. Partitioning into soluble and insoluble fractions was accomplished by centrifuging various AM dilutions at 10K rpm for 15 min. The supernatants (soluble fraction) were removed and the pellets (insoluble fraction of actomyosin) were saved for investigating various biochemical parameters. Each pellet could be dissolved again in 0.6M NaCl (20mM Tris-maleate) and such solutions were subjected to further analysis. Solubility at the given dilution was expressed as the ratio of protein content in pellets at a given dilution to total protein using the following formula:
Protein contents (%) = Cs/Ct × 100
Where Cs is the protein concentration (mg/ml) in the pellet at a given dilution and, Ct the total protein content.
Assay of ATPase activity
ATPase activities of control actomyosin samples and different redissolved pellets were assayed following the standard protocol [22]. Ca2+ or Mg2+ ion activated ATPase was assayed independently at 20°C in the final concentrations of 50mM KCl, 20mM Tris-maleate of pH 7.0 and 5 mM CaCl2 (or Mg2+). K+(EDTA)-ATPase was an exception as for this assay AM pellets were dissolved in 0.6M KCl [15]. K+(EDTA)- ATPase was assayed in final concentration of 0.6M KCl. In the assay mixtures, the reaction was started by adding 1mM ATP and quenched by adding perchloric acid (15%) at 2 and 4 min. Inorganic phosphate liberated during hydrolysis of ATP was estimated by the method of Fiske and Subbarow [23]. Data was plotted taking average of 2 min and 4 min readings.
Electrophoretic profiling of redissolved pellets of sonicated AM and documentation
With some minor modifications reported previously [19], vertical slab SDS-PAGE protocol of Laemmli [24] was employed to monitor polypeptide abundance, relative intensities and stoichiometry of individual polypeptides in insoluble fraction of ultrasonicated AM. Vertical slab gels, 10% in polyacrylamide (10×10×0.1 cm) were run in electrophoretic assembly (Genetix Asia Pvt Ltd.) initially at 5mA until samples entered separating gel and for the remaining duration gels were run at 15mA. The gels were fixed in a mix of methanol (40%) and acetic acid (10%) for 2 hrs at 25°C that was also the washing solution to remove SDS. The presence of methanol is necessary to fix myosin light chains [25]. Gels were stained with Coomassie Brilliant Blue R-250 (CBBR-250) (0.25% w/v) in 45% methanol and 10% acetic acid. Destaining was performed by overnight incubation of gels in 7% acetic acid. Stained gels with clear background were photographed or directly scanned by normal document scanner (HP Deskjet F370) with computer back up.
Quantitative assessment of SDS-PAGE profiles using Integrated Density Values (IDV)
Data from both, digital photographs and the direct gel-scans, were compared and selected lanes were subjected to densitometric analysis using Scion Imaging (Scion Corporation; Beta release: 4.0) software. Gel Pro software (Cybernetics, USA) was employed for Integrated Density Value (IDV) analysis of selected bands. IDV of MyHC and actin bands were used to calculate expected actin IDV using the formula:
Expected IDV of actin = IDVMt × (IDVA/IDVM)
Where Mt is IDV of MyHC in the under analysed test lane; IDVA/ IDVM is the Actin: MyHC ratio of unsonicated AM in dissolving buffer (Standard reference or Srf) that was run in lane-1 of each gel. Srf is the unsonicated AM solution in 0.6M NaCl containing 20mM Tris-maleate of pH 7.0.
Statistical analysis
Data on various biochemical properties were obtained on AM prepared from 25 birds. Results are based on average values of the duplicate determinations on different actomyosin preparations. Student’s t-test was performed to demonstrate the significant difference between the control and sonicated samples of AM and the values were taken significant at 5% level of significance.
Results and Discussion
Sonication-time and salt-concentration dependence of insoluble fraction
The protein contents in various redissolved pellets, obtained after the maximum sonic exposure for 30 min at 20 kHz, have been compared with their corresponding controls in (Figure 1). The control was normal (unsonicated) AM solution in 0.6M NaCl, which is a well established parameter of its total solubility [26]. The decline in the solubility has an inverse correlation with the decreasing NaCl concentration for both, the different dilutions of sonicated AM and their respective controls. In other words, lesser the salt concentration more was the quantity of insoluble or precipitated AM. This is actually demonstrated by the protein values of the control (unsonicated) and the sonicated AM at the dilutions from 0.2-0.5M NaCl (Figure 1).
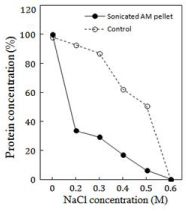
Figure 1: The profiles of the decrease in the total protein concentration
of various pellets (continuous line) sedimented after 0.2M to 0.5 M NaCl
dilutions of AM that was sonicated for 30 min. The pellets were redissolved in
0.6 M NaCl. The values are expressed as per cent of protein (2.5-3 mg/ml) in
unsonicated AM (Srf) taken as 100%. The same applies to per cent values of
controls (broken line) at similar dilutions.
The insolubility curves of protein in various pellets and those of the controls followed similar trends (Figure 1). However, the protein contents in the sonicated AM pellets had extraordinarily low values. Even at the highest NaCl dilution of 0.5M NaCl, the protein was ~45 mg/ml less than the corresponding control (P<0.05).
The observed decrease in the protein contents of various pellets of sonicated AM supports our earlier observations on the exceptional enhancement in the solubility of sonicated AM at <0.2M NaCl dilutions [17]. In other words, a remarkable quantity of proteins which sedimented as the pellets in the control AM dilutions (Figure1) was solubilized by sonication to be the part of different soluble fractions of the sonicated AM.
Sonication-time and salt-concentration dependence of various ATPase activities
The plots in Figure 2 demonstrate that during the entire course of sonication each of the ATPase activity declined from 0.2 to 0.5M NaCl in a sonication time-dependent manner, although the magnitudes of decline showed fluctuations. The figure further shows the salt concentrations where Mg2+, Ca2+ or K+ activated ATPases are maximal (shown by the arrow).
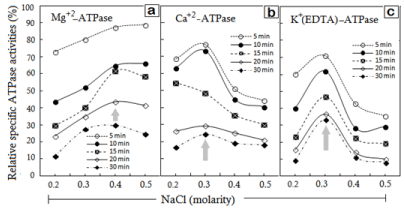
Figure 2: NaCl-concentration dependence of various specific ATPase
activities in redissolved pellets plotted as the function of timings of AM
sonication. Different timings of sonication indicated in individual frames (a, b
and c) of specified ATPase.
The specific Mg2+-ATPase declined by 10% of the corresponding controls in the pellets that were obtained at the 0.4-0.5M NaCl dilutions of AM, sonicated for the maximum duration of 30 min (Figure 2a). As for the time dependence, there was an orderly decline of Mg2+-ATPase from 5 to 30 min, while the salt-dependent slopes showed a descent from 0.4M to 0.2M NaCl. More notably, the peak specific Mg2+-ATPase activity was observed at 0.4M NaCl (shown by the arrow), while the activity maxima of Ca2+- and K+(EDTA)- ATPases were at 0.3 M NaCl (Figures 2b & 2c). As the slopes of activity curves display, either of these two ATPase activities declined if the NaCl concentration was increased beyond the peak NaCl molarity of 0.3M NaCl or decreased below 0.3M NaCl (P<0.05) (Figures 2a-2c). Because the activity maximum of Mg2+-ATPase is 0.4M NaCl, it is the centre-point of the flanking slopes in this case. It is noteworthy that the specific Ca2+- and K+(EDTA)-ATPase activities displayed more sensitivity to sonication, since an exposure for just 5 min caused ~25- 30% decline in the peak activity at 0.3M NaCl (Figures 2b & 2c). The Mg2+-ATPase activity was relatively less sensitive.
The behaviour of various ATPase activities in any of the redissolved pellets was similar to the trend observed for the decline in activities of ‘unpartitioned’ sonicated AM solution [14]. In other words, the gradual decline in ATPase activities coincided with the increasing sonication-time. Just opposite to it, instead of inactivation of Mg2+, Ca2+ and K+(EDTA)-ATPase activities, substantial activation was observed in ‘sonication-solubilized fractions’ [17]. Therefore despite retaining functional properties, the inactivation of ATPase activities in various pellets suggests conformational differences between ‘sonication-solubilized’ and ‘sonication insolublized’ AM fractions.
While the Mg+-ATPase which is a measure of actin-myosin interaction displayed less sensitivity to sonic exposure, myosinintegrity indictors Ca2+ and K+ activated ATPase activities were remarkably sensitive [27-28]. It is thus apparent that ATP hydrolysis site in MyHC is more accessible or susceptible to sonication.
Visible changes in the relative intensities of constituent polypeptides of AM
SDS-PAGE profiles in Figure 3 demonstrate polypeptides and their relative abundance in the AM preparations used in this study. The profiles are in agreement with those typically documented for chicken skeletal muscle AM preparations [13-17]. For the sake of discussion to follow, Myosin Heavy Chain (MyHC), actin, regulatory proteins (tropomyosin and troponins) and Myosin Light Chains (LC 1-3) are considered important here.
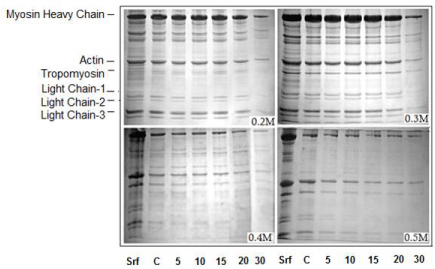
Figure 3: SDS-PAGE profiles of redissolved pellets of sonicated AM (Srf)
showing overall reduction in MyHC. The depletion in regulatory proteins and
myosin LCs is outstanding in 0.4M and 0.5M NaCl pellets.
From left to right, the lanes are: lane-1, Srf or standard reference (unsonicated
and unpartitioned AM solution in 0.6M NaCl); lane-2 C, pellet of unsonicated
AM at corresponding dilution of NaCl (control). Lanes 5, 10, 15, 20 and 30
indicate sonication time at which the AM aliquots were drawn, diluted and the
pellet recovered.
Sonication-induced changes evident in the relative intensities of various polypeptides in the SDS-PAGE profiles of AM are: (i) a remarkable sonication-time dependent decrease in the intensity/ density of MyHC (200kDa) band which is maximum at 30th min; (ii) a salt-concentration dependent decrease which is most prominent at 0.4-0.5M NaCl; (iii) a solubility, sonication-time as well as saltconcentration dependent reduction in actin (46 kDa) and, (iv) the gradual but most visible reductions in the relative intensities of regulatory proteins and Light Chains (LCs); particularly at 0.4M and 0.5M NaCl.
In our previous study on the ‘soluble fractions’ of sonicated AM, the low intensity sonication was shown to solubilize out >61% of proteins at 0.2M within 10-12 min [17]. According to those observations, an exceptional enhancement in the solubility corresponded with an obvious increase in MyHC that was also time- and salt-concentration dependent. The present study supports those observations, since the decrease in protein concentration of insoluble fraction (pellet) is in agreement with the decreasing amount of MyHC (Figures 3 & 4). To reassert, all of the observed changes are concomitant and depend on sonicated time and salt-concentration.
In SDS-PAGE profiles of redissolved pellets, the changes in the polypeptides of molecular weight lesser than that of actin were most obvious (Figure 3). Corresponding to sonication-time and salt-dilutions, there was a gradual disappearance of tropomyosin, troponins and all of the myosin light chains. The increase in the relative intensities of LCs noticed in SDS-PAGE profiles of the sonication-solubilized fractions was rather innocuous [15]. However, the visible reduction in the intensity of myosin light chains in electrophoretic profiles of redissolved pellets strongly supports those earlier observations.
Implications of the dynamics of IDV of MyHC and actin bands
NaCl-dilution dependent decrease in ID values of MyHC band of various pellets is presented in (Figure 4). Lane-1 in each gel represents SDS-PAGE profiles of the undiluted AM solution or Srf (Figure 3). As per the formula given under Materials and Methods, the calculated IDV ratio of actin: MyHC (A:M) in the Srf lane was =0.62 (P<0.05). The A: M ratio of Srf was used for further assessment whether the actin contents in a particular lane show deviations from this expected relationship. In fact, in the pellets obtained at 0.2M NaCl dilution, IDV of actin were lesser than the expected values and also showed a progressive decrease corresponding to increasing sonication time (Figure 4a). At 0.3M NaCl (Figure 4b), the A: M ratio was close to that of the Srf. However, the actual (observed) IDV of actin showed a decrease in the pellets from 0.4M and 0.5M NaCl dilutions (Figure 4c). It is well established that actin is soluble in low ionic strength or water only if it exists dissociated from myosin/MyHC. Therefore, instead of remaining with the insoluble pellet, any dissociated actin will go into the soluble fraction. By comparing deviations in the ‘calculated’ and ‘observed’ A: M ratio of the ‘soluble’ with ‘insoluble’ fractions of sonicated AM solution, it can be determined which of the two fractions contained disproportionate quantities of actin to MyHC.
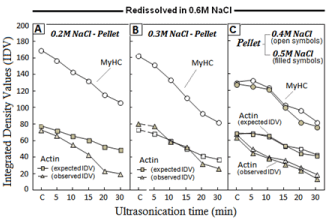
Figure 4: Sonication-time and NaCl-concentration dependence of Integrated
Density Values (IDV) of various polypeptides resolved in SDS-PAGE profiles
of various pellets.
Reductions in the relative IDV of MyHC and deviations from the observed
and expected values of actin are evident in the pellets from 0.2M, 0.4M and
0.5M NaCl and; a balanced relationship at 0.3M NaCl. Calculation details of
actin: MyHC ratios under Materials and Methods.
In our previous report on the ‘soluble’ fractions of partitioned sonicated AM, we had already shown that the free actin was in excess of standard A: M binding ratio in the soluble fraction at 0.2M NaCl [15]. As argued in the preceding paragraph, after passage of more actin to the ‘soluble fraction’, less of the actin should be found in the residual pellet, which is factually evident from the Figure-4. Similarly, actin contents in the pellets from 0.4M and 0.5M NaCl dilutions were low (<expected values), because the ‘soluble fraction’ at these NaCl dilutions had actin in unexpectedly high amount [17].
The ‘decrease’ in MyHC and the actin bands of redissolved ‘insoluble pellets’ (Figure 4) corresponded to the ‘increase’ in these polypeptides of the ‘soluble fractions’ [17], and represented a ‘fitin’ trend. In general, the decrease of protein in the insoluble pellet simply means the passage of more protein into solubilized fraction. However, appreciable retention of functional properties is the unique characteristic of various insoluble fractions. Since the pellets of sonicated AM could be dissolved in 0.6M or even NaCl of lesser molarity, the insolubility caused by sonication of AM happens to be reversible. This is just opposite to the behaviour of thermally denatured AM, where aggregates are irreversibly insolublized [29-33] and suggest a different conformational state of AM constituents in the pellets.
As already pointed out, the present investigations demonstrate a similarity between the pattern of decrease in ATPase activities of the redissolved pellets with that of unpartitioned sonicated AM [14]. In contrast, subsequent to an initial decline in various ATPase activities by 5th min of sonication, the ‘soluble fraction’ of sonicated AM had shown a substantial activation [17]. With respect to solubility, the behaviour of AM was similar to myofibrils [12] and to some extent power ultrasonication curing effect on meat [34]. It can thus be inferred that the reversibility of the sonication-insolublized fractions and the retention of functionality impart a kind of conformational plasticity to actomyosin system, which is important for thermal processing. To some extent, the effect of sonication appears analogous to the effect of high pressures on muscle proteins [35].
Conclusion
Low frequency (20 kHz) ultrasonication brought about changes in actomyosin (AM) solution which depend on sonication-time and salt-concentrations. As compared to the controls, less protein sedimented as insoluble pellets in the dilutions from 0.2M to 0.5M NaCl. The most apparent reason of the reduction in the insoluble fraction is passage of more proteins into the soluble fraction, caused by sonication-induced enhancement in AM solubility. The timedependent decline in various ATPase activities of redissolved pellets was in accordance with the trend reported for total (unpartitioned) AM. However, sonication discriminated between sites of actin-myosin interaction and ATP hydrolysis within the MyHC. Polypeptides of molecular weight lesser than actin and the myosin LCs tend to disappear in the pellets from 0.4M and 0.5M NaCl dilutions. Unlike thermally denatured AM aggregates, the sonication-induced insolubility is reversible. The sedimented proteins remain more likely participants in meat processing events, since various pellets redissolve in 0.6M salt and retain considerable functional activity. The sonication insolublized proteins of AM are among unique cases of conformational plasticity.
Acknowledgement
The authors are thankful to the University Grants Commission (UGC), New Delhi for providing financial assistance to AH/ RA for carrying out this work. Authors are grateful to the Aligarh Muslim University and the Chairman, Department of Zoology for providing laboratory facilities. Thanks are due to Prof. SMA Abidi for ultrasonication facility.
References
- Bhaskaracharya RK, Kentish S, Ashokkumar M. Selected applications of ultrasonics in food processing. Food Eng Rev. 2009; 1: 31-49.
- Weiss J, Gibis M, Schuh V, Salminen H. Advances in ingredient and processing systems for meat and meat products. Meat Sci. 2010; 86: 196-213.
- Chen L, Chen J, Ren J, Zhao M. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J Agric Food Chem. 2011; 59: 2600-2609.
- Barbut S. Convenience breaded poultry meat products-New developments. Trends Food Sci Tech. 2012; 26: 14-20.
- Dolatowski ZJ, Stadnik J, Stasiak D. Applications of ultrasound in food technology. Acta Sci Pol Technol. 2007; 6: 89-99.
- Jayasooriya SD, Torley PJ, D’Arcy BR, Bhandari BR. Effect of high power and ageing other physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 2007; 75: 628-639.
- Alaa El-Din, Carne AB, Ha M, Franks P. Physical interventions to manipulate texture and tenderness of fresh meat: a review. Int J Food Prop. 2014; 17: 433-453.
- Li S, Xu X, Zhou G. The roles of the actin-myosin interaction and proteolysis in tenderization during the aging of chicken muscle. Poultry Sci. 2011; 91: 150-160.
- Xiong YL, Brekke CJ. Protein extractability and thermally induced gelation properties of myofibrils isolated from pre-and post rigor chicken muscles. J Food Sci. 1991; 56: 210-215.
- Matthews SJ, Ross NW, Lall SP, Gill TA. Astaxanthin binding protein in Atlantic salmon. Comp Biochem Physiol B Biochem Mol Biol. 2006; 144: 206-214.
- Wang Y, Wang R, Chang Y, Gao Y, Li Z, Xue C. Preparation and thermo-reversible gelling properties of protein isolate from defatted Antarctic krill (Euphausia superba) byproducts. Food Chem. 2015; 188: 170-176.
- Ito Y, Toki S, Omori T, Ide H, Tatsumi R, Wakamatsu JI, et al. Physicochemical properties of water-soluble myofibrillar proteins prepared from chicken breast muscle. Anim Sci J. 2004; 75: 59-65.
- Saleem R, Hasnain A, Ahmad R. Changes in some biochemical indices of stability of broiler chicken actomyosin at different levels of sodium bicarbonate in presence and absence of sodium chloride. Int J Food Prop. 2015; 18: 1373-1384.
- Saleem R, Ahmad R. Effect of low frequency ultrasonication on biochemical and structural properties of chicken actomyosin. Food Chem. 2016; 205: 43-51.
- Saleem R, Hasnain A, Ahmad R. Solubilisation of muscle proteins from chicken breast muscle by ultrasonic radiations in physiological ionic medium. Cog Food Agr. 2015; 1: 1046716.
- Ahmad R, Hasnain A. Ultrasonication of chicken natural actomyosin: Effect on ATPase activity, turbidity and SDS-PAGE profiles at different protein concentrations. Am J Biochem Mol Biol. 2013; 3: 240-247.
- Hasnain A, Saleem R, Ahmad R. Biochemical variations in salt soluble fractions of ultrasonicated actomyosin isolated from broiler breast-muscle. Int J Food Prop. 2016.
- Puolanne E, Halonen M. Theoretical aspects of water-holding in meat. Meat Sci. 2010; 86: 151-165.
- Ahmad R, Hasnain A. Correlation between biochemical properties and adaptive diversity of skeletal muscle myofibrils and myosin of some air-breathing teleost. Ind J Biochem Biophys. 2006; 43: 217-225.
- Ahmad R, Hasnain A. Peptide mapping of polymorphic myosin heavy chain isoforms in different muscle types of some freshwater teleosts. Fish Physiol Biochem. 2012; 39: 721-731.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265-275.
- Hasnain A, Samejima K, Takahashi K, Yasui T. Molecular adaptability of carp myosin: A study of some physico-chemical properties and their comparison with those of rabbit myosin. Archi Phys Biochem. 1979; 87: 643-662.
- Fiske C H, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925; 66: 375-400.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227: 680-685.
- Saleem R, Hasnain A, Ahmad R. Effect of pre-stain methanol washing on the sensitivity of CBBR-250 staining in SDS-polyacrylamide gels. Sample Preparation. 2015; 49-54.
- Skaara T, Regenstein JM. The structure and properties of myofibrillar proteins in beef, poultry, and fish. Journal of Muscle Foods. 1990; 1: 269-291.
- Rayment I. The structural basis of the myosin ATPase activity. J Biol Chem. 1996; 271: 15850-15853.
- Iwamoto H. Influence of ionic strength on the actomyosin reaction steps in contracting skeletal muscle fibers. Biophys J. 2000; 78: 3138-3149.
- Xiong YL, Brekke CJ, Changes in protein solubility and gelation properties of chicken myofibrils during storage. J Food Sci. 1989; 54: 1141-1146.
- Samejima K, Lee NH, Ishioroshi M, Asghar A. Protein extractability and thermal gel formability of myofibrils isolated from skeletal and cardiac muscles at different post-mortem periods. Journal of the Science of Food and Agriculture. 1992; 58: 385-393.
- Van Laack RL, Lane JL. Denaturation of myofibrillar proteins from chickens as affected by pH, temperature, and adenosine triphosphate concentration. Poult Sci. 2000; 79: 105-109.
- Wattanachant S, Benjakul S, Ledward DA. Effect of heat treatment on changes in texture, structure and properties of Thai indigenous chicken muscle. Food Chem. 2005; 93: 337-348.
- Nahar MK, Zakaria Z, Hashim U. Protein solubility behaviour of fresh and frozen chicken meat in slaughtering and non slaughtering condition. Int Food Res J. 2014; 21: 135-137.
- McDonnell CK, Allen P, Morin C, Lyng JG. The effect of ultrasonic salting on protein and water-protein interactions in meat. Food Chem. 2014; 147: 245-251.
- Ma H, Zhou G, Ledward DA, Yu X, Pan R. Effect of combined high pressure and thermal treatment on myofibrillar proteins solubilization of beef muscle. Int J Mol Sci. 2011; 12: 3034-3041.