
Research Article
Austin Food Sci. 2016; 1(4): 1016.
Effects of Storage Conditions on Antioxidant Capacity of Olive Oil Produced in Mills without Auto-Control Systems
Haouhay NE1*, Sanchez CS1, Asehraou A2, Mir MV1 and De la Serrana HLG1
1Department of Nutrition and Food Science, University of Granada, Spain
2Laboratory of Biochemistry and Biotechnology, University Mohamed Premier, Morocco
*Corresponding author: Nassira El haouhay, Department of Nutrition and Food Science, Faculty of Pharmacy, University of Granada, Granada, Spain
Received: June 10, 2016; Accepted: July 14, 2016; Published: July 21, 2016
Abstract
This study was carried out to evaluate the effects of storage time of olive fruits, extraction system (used in traditional mills in Morocco), and storage conditions such as packaging type, on the quality of olive oil. Olive oil was obtained from Moroccan Picholine variety olive fruits stored at different periods, and extracted in granite mill and wood mill. Olive oil was stored in PET and dark glass bottles. Acidity, peroxide index, K232 and K270 increased during storage. While, phenolic contents and antioxidant capacity decreased significantly. Glass container appears to be the most appropriate for protection of these oils. However, type of mills was not significant in their classification. Principle components analysis showed that variable with the highest level of impact was the acidity. Discriminate functions and cluster analysis indicated that storage time of olive fruits was the most important in the evaluation of samples, followed by oil storage time.
Keywords: Olive fruit; Oil; Mills; Storage; Antioxidant capacity
Introduction
Olive oil obtained from olive tree fruit (Olea europaea L.) constitutes one of the components of the Mediterranean diet. Its worldwide growing interest is promoted by its beneficial effects on human health, mainly due to a high content of unsaturated fatty acids and antioxidant components [1]. Components of olive oil, mainly polyphenols are one of the most significant types of antioxidants. They decrease reactive species production and play an important role in preventing oxidative stress [2]. In addition to their beneficial health effects, phenolic compounds represent an important contribution to the oxidative stability of virgin olive oil against auto-oxidation [3].
To evaluate the validity of oils, there are different parameters. In fact, free fatty acids content is one of the most frequently determined quality indices; it can reflect the quality of olives and the procedures of oil extraction. Another parameter, of important causes of loss of olive oil quality is oxidation or autooxidation, which takes place mainly during the production and storage of oil [4], affecting its compounds and characteristics [5].
Furthermore, antioxidant compounds are also considered as important contributors to quality properties of olive oil. Hence, polyphenols content and antioxidant activity have usually been measured to evaluate the effect of antioxidants in delaying the extent of oxidation [5], and also to determine the stability of vegetable oils [6]. Antioxidant compounds of olive oil are influenced by several inherent factors related mainly to the variety, maturity index and harvesting procedure of olive fruits, and their handling, transportation and storage prior to milling [7] and also linked mostly the extraction system [8].
Storage conditions (time, light, oxygen and packaging) are considered critical variables influencing the quality and shelf life of olive oil [9], PET and Glass bottles are used in olive oils packaging, in fact, the major function of packaging materials is related to their barrier properties against oxygen ingress, auto-oxidation and photooxidation [10], considered as the main oxidation mechanisms during processing and storage of edible oils [11].
Production and consumption of olive oil in Morocco are increased during the few last years, thanks to the strategy set “Green Morocco Plan” pursued by Moroccan government. Nevertheless, in this country 98 % of olive oil sector is represented by traditional mills [12], so called, “maasras”. In these mills, olives are usually stored at ambient temperature before milling, and then ground into paste using roller of stone, in this case mill is called “granite mill”, or roller of wood and mill is called “wood mill”. In the first type, press is made of metal, while in the second, press is made of wood. Olive oil, in these traditional mills, is obtained without practice of quality control systems. In fact, in a previous work, we demonstrated that olives and there corresponding oils produced in these conditions are subject to various microbial contaminations [13], which may lead to the production of olive oil with lower and/or instable quality during storage.
The main objective of this work was to study combined effects; storage time of olives, type of container and storage time of olive oil on quality parameters and antioxidant components of olive oil from Moroccan picholine variety, obtained in two types of traditional mills, in the Eastern Region of Morocco. Olive oil extraction was performed in the same conditions practiced in this type of mills, in this region, in when; general steps of olive oil production have been previously described [13].
Materials and Methods
Sampling
Olive fruits and olive oils samples of the Moroccan Picholine variety were collected from traditional mills in the Eastern Region of Morocco, during the 2011 harvest. The olives were classified into three groups according to the storage periods before milling: 7 days, 15 days and 30 days. Olives were stored in plastic bags at ambient temperature of around 11°C and with a relative humidity of around 71%. Once the storage time of each group was completed, olives were distributed between two types of traditional mills: granite mill (with roller of granite and press made of metal) and wood mill (with roller and press made of wood), situated in the same area. From olive groups, stored at 7, 15 and 30 days were obtained oil groups A, B and C respectively. From granite mill was taken a total of 138 samples: 78 samples stored in Polyethylene Terephtalate (PET) bottles (26/ group) and 60 samples stored in amber glass bottles (20/ group). From wood mill were taken 72 samples: 36 samples in PET (12/ group) and 36 samples in amber glass bottles (12/ group). Samples were kept in darkness at laboratory ambient temperature (17-23°C) until analysis after 3 and 6 months of storage.
Reagents
Chloroform, acetic acid, diethyl ether, ethanol, cyclohexane, potassium hydroxide, sodium thiosulfate, potassium iodide, sodium acetate 3-hydrate, anhydrous sodium carbonate, methanol, ethanol, acetic acid glacial, hydrochloric acid (37%), ferric chloride 6-hydrate and potassium persulfate were supplied by Panreac (Barcelona,Spain), and Folin–Ciocalteu phenol reagent by Merck (Darmstadt, Germany). Gallic acid, 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox) standards, 2,2-azinobis-(3-ethylbensothiazoline)-6- sulfonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and N,N-dimethyl-p-phenylenediamine dihydrochloride (DMPD) were supplied by Sigma–Aldrich (Milan, Italy). The 2,4,6-tri(2-pyridyl)-striazine (TPTZ) for the FRAP method was also from Fluka Chemicals (Buchs, Switzerland) and water was obtained from a Milli-Q purification system (Millipore, Bedford, MA).
Analytical indices
Free acidity reported as percentage of oleic acid (%), peroxide value expressed as milliequivalents of active oxygen per kg of oil (meq O2/kg), and UV spectrophotometric indices. (K232, K270) were assessed according to the official methods described in Regulation EC 2568/91 of the Commission of the European Union (EEC, 2013).All the parameters were determined in triplicate.
Extraction conditions
The extracts of minor compounds of olive oil were obtained using methanol /water (80:20, v/v) in agreement with data reported in the literature [14,15]. The procedure was based on that recommended in previous work [16], with some modifications: 10 g of oil diluted in methanol to 80% (p/p) was homogenized by shaking for 60 min, the tubes were then centrifuged at 8000 rpm for 15 min, the volume of supernatant was adjusted to 25 ml with methanol to 80 % and the mixture was recovered in 5 ml aliquots and stored at 21°C, for no more than 2 months.
Total phenol contents
Total phenolic contents were determined using the Folin- Ciocalteu colorimetric method described by Singleton and Rossi [17] and modified in our laboratory; we added 2.5 ml of deionized water and 500 μl of Folin–Ciocalteu reagent, to 100 μl of methanolic oil extract. The mixture was allowed to stand for 5 min, and then 2 ml of a 10% aqueous Na2CO3 solution were added. The final volume was adjusted to 10 ml. Samples were allowed to stand for 90 min at room temperature before measurement at 700 nm versus the blank using a Boeco S-22 UV–VIS spectrophotometer (Hamburg, Germany). The amount of total phenolics was expressed as gallic acid equivalents per gram of fresh weight (μg gallic acid/g of olive oil) using the calibration curve of gallic acid.
Antioxidant capacity
The antioxidant capacity of the samples was measured in Lambda 25 UV/Vis spectrophotometer (Perkin-Elmer R, Madrid, Spain). The techniques used were those by Brand-Williams, et al. [18] with N,N0- dimethyl-pphenylenediamine (DPPH), the Antioxidant Equivalent Capacity (ABTS) of [19,20] based on the reducing ability of ferric iron (FRAP), and that by Fogliano, et al. [21] with DMPD method. DPPH, ABTS and DMPD methods are based on the formation of a colored radical. Post-addition tests were used, with formation of the radical in the absence of the sample until a stable signal was reached. In the FRAP assay, excess FeIII was used, and the rate-limiting factor of FeII-TPTZ, and hence color formation is the reducing ability of the sample. Resulting change in absorbance (discoloration of the radical in DPPH ABTS and DMPD, or blue color developing in the FRAP test), which was proportional to the antioxidant activity of the samples analyzed. Results were expressed as μmol trolox/g oil.
Statistical analysis
The results obtained were expressed as mean and standard deviations. To determine the influence of storage time, among other variables studied, when the variables fulfilled the parametric conditions, One-Way Analysis of Variance (ANOVA) was used, and when the variables were non-parametric, the Kruskal–Wallis test was used. Fisher’s least significant difference procedure was used to discriminate between the means of the variables when necessary. The Kolmogorov–Smirnov test and the Bartlett test, as well as the Wilcoxon test, were used to test the normal distribution of variables and the homogeneity of variances. Statistical analysis was performed using SPSS 20.0 (IBM® SPSS® Statistics 20.0) and Statgraphics1 Plus 4.1 software. Differences of p < 0.05 were considered significant.
Results and Discussion
Quality indices
Quality parameters; degree of acidity, peroxide index and absorbance in Ultraviolet light absorbance (K232 and K270) of olive oil samples obtained from olive fruits of Moroccan Picholine variety, stored at different times (7, 15 and 30 days), produced in two types of Moroccan traditional mills (granite and wood mill), recovered in two type of container (PET and glass bottles) and analyzed at 3 and 6 months of storage are summarized in (Table 1).
Mill/container
Group of oils
Acidity (% oleic acid)a
Peroxide index (meq O2/kg)a
K232a
K270a
3 months
6 months
3 months
6 months
3 months
6 months
3 months
6 months
Ganite:
PET
A
0.64 ± 0.21 cG*
0.77 ± 0.24 bD*
3.35 ± 1.04 aG
7.56 ± 3.34 cI
1.95 ± 0.35 bG
2.47 ± 0.55 dG
0.11 ± 0.03 aG*
0.21 ± 0.05 cD
B
1.36 ± 0.39 cH
1.51 ± 0.40 bE*
3.71 ± 1.78 aG*
8.43 ± 3.09 cD
2.13 ± 0.16 bI*
2.52 ± 0.39 dH*
0.13 ± 0.04 aH*
0.22 ± 0.06 cD*
C
4.19 ± 3.88 aI
4.70 ± 4.07 d F*
7.26 ± 5.27 bH
13.79 ± 8.26 dE
2.60 ± 1.02 aD*
3.33 ± 1.68 cD*
0.21 ± 0.12 bI*
0.32 ± 0.12 dF*
Glass
A
0.61 ± 0.11 aG
0.77 ± 0.11 dD
3.34 ± 1,05 aG
7.14 ± 2.62 cI
1.66 ± 0.43 aH
2.46 ± 0.32 dG
0.11 ± 0.04 aG
0.20 ± 0.04 cE
B
1.33 ± 0.22 bH
1.68 ± 0.39 eE
3.61 ± 0.83 aG
8.23 ± 1.85 dD
2.02 ± 0.52 bI
2.86 ± 0.44 eI
0.13 ± 0.02 aH*
0.21 ± 0.02 cE*
C
4.15 ± 0.83 cI*
4.88 ± 0.80 f F*
7.17 ± 2.80 bH*
13.32 ± 4.99 eF*
2.49 ± 0.66 cE
3.21 ± 0.70 fE
0.21 ± 0.01 bI
0.31 ± 0.6 dJ
Wood:
PET
A
0.90 ± 0.26 aG*
1.60 ± 0.92 dG*
3.49 ± 0.73 aG
7.04 ±1.56 cG
1.87 ± 0.03 aG
2.66 ± 0.23 dG
0.18 ± 0.03 aG*
0.23 ± 0.03 dG
B
1.80 ± 0.65 bI
3.73 ± 1.02 eI*
5.37 ± 1.30 bH*
9.76 ± 2.45 dH
2.69 ± 0.38 bI*
3.76 ± 0.47 eI*
0.24 ± 0.02 bI*
0.31 ± 0.05 eI*
C
4.26 ± 0.84 cD
5.61 ± 1.35 f E*
6.72 ± 1.58 cD
13.70 ± 3.13 eD
4.23 ± 0.80 cE*
5.53 ± 1.01 fE*
0.35 ± 0.06 cE*
0.47 ± 0.07 fE*
Glass
A
0.62 ± 0.20 aH
0.95 ± 0.37 d H
3.17 ± 1.32 aG
5.96 ± 2.68 dG
1.50 ± 0.22 aH
1.90 ± 0.27 dH
0.13 ± 0.05 aH
0.17 ± 0.04 cH
B
1.44 ± 0.41 bI
2.09 ± 0.76 eD
3.87 ±1.18 bI
7.14 ± 2.14 eH
2.18 ± 0.14 bD
2.69 ± 0.24 eD
0.19 ± 0.03 bD*
0.24 ± 0.03 dD*
C
2.75 ± 0.89 cE*
3.47 ± 0.75 fF*
4.34 ± 1.19 cE*
9.27 ± 2.36 fE*
2.88 ± 0.54 cF
3.58 ± 0.53 fF
0.20 ± 0.05 bF
0.31 ± 0.08 eF
Note: Granite (PET (n=78), glass (n=60)); wood (PET (n=36), glass (n=36)). A: oil obtained from olives stored for 7 days, B: oil obtained from olives stored for 15 days and C: oil obtained from olives stored for 30 days. a Means ± SD of three replicates. Means followed by the same small letter are not significantly different (p > 0.05), comparing, between the same type of parameter, of the same type of container and of the same type of mill. Means followed by the same big letter are not significantly different (p > 0.05), comparing, between two type of container of the same type of mill. Means followed by the same symbol:* are significantly different (p < 0.05), comparing, between the same container of two type of mill.
Table 1: Quality parameters of olive oils, obtained from Moroccan Picholine variety in traditional mills (granite and wood), and stored for 3 and 6 months.
The values of free fatty acids content, expressed as percentage of oleic acid, showed an increase during storage, with the lowest increment corresponding to the oils of group A (obtained from olive fruits stored during 7 days), packaged in glass bottles and analyzed at 3 months of storage, with small differences between mills (granite and wood). The highest increase in acidity was found in the oils of group C (obtained from olive fruits stored during 30 days), stored in PET plastic and determined at 6 months of olive oil storage. These results are in agreement with those of Jabeur, et al. [7] who reported the increase of oil acidity during olive storage. Considering only the values of free fatty acids, 100% of the oils of group C were classified as lampante at 3 months of storage, according to the to the European Regulation (EEC, 2013).
Peroxide value expressed in milliequivalents of active oxygen per kilogram of oil (meq O2/kg), increased significantly at 6 month of storage, higher values were shown in oils stored in PET than those in glass, although no statistically significant differences were found (p > 0.05). It can be seen that at the end of the storage period (6 months) none of the oils analyzed exceeded the maximum Peroxide Value (PV) for extra-virgin olive oil category (20 meq O2/kg) established by the European Regulation [22].
K232, the specific extinction coefficient showed low values in oils stored in glass than in oils in PET at 3 months of storage. Most of the oils with K232 values higher than the established legal limits (2.5) were obtained in samples of group B (obtained from olives stored during 15 days) and group C, both stored in PET, although no significant difference between all groups was observed. At 3 and 6 months of oil storage statistically significant variations (p < 0.05) were shown between groups A and C.
K270 showed an increase with storage in all samples. Although, the oils of groups A and B were found to be within the permitted legal limits, significant changes were shown between groups A and C at 3 and 6 months of storage. And, oils stored in PET showed higher values of 270 than oils in glass. In previous studies [23] found that peroxide value, K232 and K270 increased during oil storage of Jordanian olive varieties. Similar results were also reported by Ben-Hassine, et al. [24] for two olive varieties Chemlali (Tunisia) and Coratina (Italy).
According to the European Regulation [22], at 3 months of storage, 33.33% of oil stored in glass was classified as extra virgin, whereas for oils in PET only 22.80% fell into this olive oil category. Therefore, most of the studied oils (72.38% of the total of 210 studied samples) were classified as lampante at 6 months of storage, and they needed to be refined in order to make them acceptable for consumption.
Total phenol content
As shown in (Table 2), a general decline was observed in phenol contents in all samples during storage (p < 0.05). The largest decrease in total phenol contents was observed in the oils stored in PET. The smallest decrease was in the oils of group A (oils obtained from olives stored during 7 days), followed by the oils of group B (oils obtained from olives stored during 15 days), and strong fall was shown in oils of group C (oils obtained from olives stored for 30 days). Significant differences (p < 0.5) were observed between samples of A and C at 3 and 6 months of storage. Regarding the type of mill, there was no important effect on phenol content changes. This result is consistent with previous research in that, polyphenol amounts decrease with olive storage [7] and oil storage [24]. The losses of phenols could be a result of the increase of oxidation and auto-oxidation mostly occurring during olive fruits storage, confirmed by the data of group C. In fact, olive fruits were stored at ambient temperature, exposed at free air and light. However detrimental effects of factors such as light, oxygen and time on phenol compounds of olive oil were reported by several authors [8,25]
Mill / container
Group of oils
TPC(μg/g gallic acid)a
3 months
6 months
Ganite:
PET
A
100.42 ± 38.79 aM*
75.03 ± 33.32 bG*
B
78.37 ± 22.15 cP*
56.17 ± 22.68 dI*
C
71.39 ± 25.50 cE*
48.70 ± 21.67 dK *
Glass
A
136.55 ± 30.39 aN
118.50 ± 35.00 dH
B
110.81 ± 30.08 bD
98.18 ± 30.29 bJ
C
92.71 ± 24.37 cF
79.87 ± 27.83 cL
Wood:
PET
A
134.57 ± 40.29 aM*
110.53 ± 29.09 dE*
B
105.10 ± 42.97 bN*
73.48 ± 50.01 bF*
C
66.05 ± 17.41 cC*
43.23 ± 18.42 cG*
Glass
A
150.63 ± 34.55 aM
130.72 ± 26.13 aE
B
128.34 ± 36.55 abN
105.57 ± 29.82 bF
C
98.25 ± 24.44 dD
81.11 ± 20.89 dH
Note: Granite (PET (n=78), glass (n=60)); wood (PET (n=36), glass (n=36)). A: oil obtained from olives stored for 7 days, B: oil obtained from olives stored for 15 days and C: oil obtained from olives stored for 30 days. A Means ± SD of three replicates. -Means followed by the same small letter are not significantly different (p > 0.05), comparing, between the same type of variable, of the same type of container and of the same type of mill. Means followed by the same big letter are not significantly different (p > 0.05), comparing, between two type of container of the same type of mill. Means followed by the same symbol:* are significantly different (p < 0.05), comparing, between the same container of two type of mill.
Table 2: Total Phenol Content (TPC) of olive oils obtained from Moroccan Picholine variety in traditional mills (granite and wood), and stored for 3 and 6 months.
Furthermore, in these oils as well as in their corresponding olive fruits, we have found microorganisms; mesophilic and psychrotrophic bacteria, moulds and yeasts [13], indicated in hydrolysis and degradation of phenol compounds [26,27].
However, process of hydrolysis stimulated by photosensitised oxidation, autooxidation or microorganisms increases the degradation of phenols, causing the loss of health benefits of olive oil compounds [25]. In the other hand, the reduction of phenol content can accelerate the susceptibility to oxidation and autooxidation, inflowing the antioxidant compounds of olive oil.
Antioxidant capacity
The results of antioxidant capacity determined by DPPH, ABTS, FRAP and DMPD were summarized in (Table 3).
Mill / container
Group of oils
DPPH (microM/g)a
ABTS (microM/g)a
FRAP (microM/g)a
DMPD (microM/g)a
3 months
6 months
3 months
6 months
3 months
6 months
3 months
6 months
Ganite:
PET
A
0.36 ± 0.26 aN*
0.23 ± 0.15 bG*
0.71 ± 0.37 aN
0.23 ± 0.09 bD*
11.56 ± 6.82 aN
5.45 ± 3.14 bD
11.18 ± 3.13 aN
7.61 ± 4.09 bE
B
0.21 ± 0.11 cR*
0.14 ± 0.07 dI*
0.42 ± 0.06 cP
0.18 ± 0.02 dF*
11.28 ± 3.54 aP
4.45 ± 2.29 cF*
0.88 ± 0.41 cP*
0.57 ± 0.63 dG*
C
0.16 ± 0.10 cE*
0.13 ± 0.17 dK*
0.41 ± 0.21 cR*
0.17 ± 0.06 dH
11.18 ± 6.54 aR*
4.29 ± 2.48 cH
0.43 ± 0.23 cD*
0.51 ± 0.53 d I
Glass
A
0.89 ± 0.30 aP
0.73 ± 0.29 aH
0.74 ± 0.25 aN
0.33 ± 0.15 dE*
11.95 ± 2.38 aN
6.84 ± 1.48 bE*
12.64 ± 5.55 aN
8.56 ± 3.71 cF
B
0.58 ± 0.30 bD
0.47 ± 0.32 bJ
0.52 ± 0.21 bcP
0.25 ± 0.08 dG
11.45 ± 1.98 aP
5.07 ± 1.07 cG*
1.07 ± 0.39 bR
0.82 ± 0.45 dH
C
0.47 ± 0.23 bF
0.30 ± 0.13 cL
0.42 ± 0.19 cR
0.21 ± 0.06 eH
11.22 ± 1.59 aR*
4.90 ± 1.44 cI*
0.75 ± 0.30 bD
0.65 ± 0.24 dJ
Wood:
PET
A
0.73 ± 0.26 aN*
0.43 ± 0.21 dE*
0.48 ± 0.25 aN
0.35 ± 0.14 dD*
10.75 ± 1.06 aN
4.30 ± 1.19 cN
10.31 ± 3.33 aN
7.28 ± 3.66 cD
B
0.52 ± 0.25 bP*
0.30 ± 0.09 bF*
0.35 ± 0.12 bP
0.26 ± 0.08 debE*
9.45 ± 1.27 bR
3.03 ± 0.79 dP*
1.06 ± 0.58 bP*
0.76 ± 0.48 bE*
C
0.32 ± 0.13 cR*
0.23 ± 0.06 cG*
0.23 ± 0.07 cR*
0.18 ± 0.034 eF
8.91 ± 1.39 bE*
2.90 ± 0.95 dR
0.68 ± 0.95 bR*
0.51 ± 0.26 bF
Glass
A
0.89 ± 0.31 aN
0.60 ± 0.26 dE
0.56 ± 0.25 aN
0.44 ± 0.15 aD*
11.64 ± 0,97 aP
4.42 ± 1.06 cN*
10.74 ± 1.25 aN
8.86 ± 2.07 bD
B
0.71 ± 0.37 abP
0.47 ± 0.29 eF
0.49 ± 0.17 aP
0.34 ± 0.19 caE
10.21 ± 1,55 bD
3.44 ± 0.84 dP*
1.13 ± 0.59 cP
0.79 ± 0.47 cE
C
0.51 ± 0.20 bD
0.38 ± 0.18 bH
0.30 ± 0.11 dR
0.22 ± 0.05 dG
9.89 ± 0,88 bE*
3.19 ± 0.82 dR*
0.72 ± 0.27 cR
0.56 ± 0.22 cF
Note: Granite (PET (n=78), glass (n=60)); wood (PET (n=36), glass (n=36)). A: oil obtained from olives stored for 7 days, B: oil obtained from olives stored for 15 days and C: oil obtained from olives stored for 30 days. a Means ± SD of three replicates. Means followed by the same small letter are not significantly different (p > 0.05), comparing, between the same type of variable, of the same type of container and of the same type of mill. Means followed by the same big letter are not significantly different (p > 0.05), comparing, between two type of container of the same type of mill. Means followed by the same symbol: * are significantly different (p < 0.05), comparing, between the same container of two type of mill.
Table 3: Evolution of antioxidant capacity determined by four methods (DPPH, ABTS, FRAP and DMPD) of olive oils obtained from Moroccan Picholine variety in traditional mills (granite and wood), and stored for 3 and 6 months.
The evaluation of antioxidant capacity measured by the four methods showed reduction levels in all samples during storage, indicating a considerable deference between group A (oils obtained from olives stored during 7 days) and group C (oils obtained from olives stored during 30 days). At 6 months of storage level of antioxidant activity determined by DPPH fell around 18 % to 43% in all oil samples; more reduction was observed in oils in PET than in glass. Highest losses were again more significant, approximately 68% in oils stored in PET, using the ABTS method. The decrease in antioxidant capacity evaluated by FRAP was related to the storage time, although, no important differences were observed between all groups of samples, which may indicate that the major antioxidant compounds of oils, capable to reduce Fe3+ to Fe2+ have been highly degraded during the first week of olive storage. Significant differences (p < 0.05) were showed at 6 months of oil storage; showing decreases around 43% and 68%. Furthermore, oils in glass had values higher than oils in PET bottles, although without significant differences (p > 0.05) between all groups. The results obtained of the total antioxidant capacity measured by the DMPD showed a statistically significant differences (p < 0.05) between the group A and two groups B and C, indicating a strong reduction of antioxidant compounds, caused by the prolonged time of olive storage (more than 15 days). From 3 months to 6 months of oil storage, this reduction of antioxidant capacity determined by DMPD method ranged between 13.33% and 35.22%. Moreover, a great degradation was also detected in oils packaged in PET. Excessive reduction of antioxidant capacity in PET bottles indicates that plastic container increases the risk of oxidation of olive oil components, due to the non null permeability of this material [10].
Concerning the type of mills, the antioxidant capacity values determined by DPPH, DMPD and FRAP methods varied little, which can indicate that components capable to reduce the radicals of DPPH and DMPD or the Ferric cation were influenced by similar conditions in these mills, although results of ABTS assay showed that oils produced in granite mill had less reduction of antioxidant capacity (22% to 30%) than oils extracted in granite mill (50% to 68%), indicating that a portion of antioxidant component capable to reduce the radical of ABTS was lost during oil extraction (highly showed in wood mill), may be due to a long exposition of olive paste to oxidative reactions.
Such decrease of antioxidant capacity could be related with the degradation of antioxidant compounds. In fact, the presence of microorganisms in these oils and in their corresponding olives, as indicated previously [13], might have a strong influence in their antioxidant compounds. Furthermore, yeast strains isolated from commercial extra virgin olive oil [28] have been reported to comprise several enzymes involved in the reduction of olive oil total phenolic compounds and o-diphenols [29]. Such compounds are indicated as strong antioxidants (Mancebo-Campos et al., 2014). Additionally, the prolonged time of olive storage in uncontrolled conditions and the exposition of olive paste at light and oxygen could increase the production of free radicals. However, activation of the main olive oxidoreductase enzymes involved in phenolic oxidation, like polyphenol oxidase and peroxidase, were indicated during time of the milling step [30], where they caused a significant decrease in the phenolic content, particularly in the antioxidant compounds [30], leading a loss of oxidative stability of olive oil [30,31].
Correlations
The four methods DPPH, ABTS, FRAP and DMPD can be used for determination of antioxidant capacity of hydrophilic fraction, which corresponded to a total phenolic compounds [32]. In this study, the correlation between the antioxidant capacity measured by DPPH, ABTS, DMPD and the total phenol content was significant (p < 0.05) and positive (r = 0.61, 0.17, 0.07 and 0.23, respectively), whereas, between FRAP and the total phenol content was positive but no significant (p > 0.05).
Higher correlations of antioxidant capacity determined by DPPH with phenol contents have been previously reported in olive oil (r = 0.995) [5].
The correlation found between ABTS, measuring antioxidant capacity, and total phenol contents was low, whereas some of the previous studies reported that the correlation between total phenols and antioxidant capacity measured by ABTS was very high in olive oil of Turkish Halhali variety [33]. DMPD assay has been also investigated for antioxidant activity and total phenolic content measurements in olive oil [34]. The FRAP assay is reported as less suited for samples with lipophilic antioxidants compared to the DPPH assay [35], this might explain the lack of significant difference in the correlation between the antioxidant capacities measured by FRAP and total contents of phenol compounds.
The correlations of the antioxidant capacity measured by the different methods were significant (p < 0.05) between DPPH and ABTS (r = 0.29), DPPH and FRAP (r = 0.21), DPPH and DMPD (r = 0.38), ABTS and FRAP (r = 0.53), ABTS and DMPD (r = 0.46) and between FRAP and DMPD (r = 0.21). However, values of correlation between these methods were lowers; hence, these four tests tend to be used together in the study of the antioxidant capacity of this olive oil, the first to determine the capacity to capture free radicals and the second to evaluate the reductive capacity of a sample [36].
Multivariate statistical analysis
Principal Component Analysis (PCA): The classification pattern obtained according to studied variables was shown in Figure 1. The main results that can be drawn from this PCA analysis showed that variability found in the samples can be seen in component, which explains the 68.38% of variability. The linear combination found for this main component is defined by the equation:
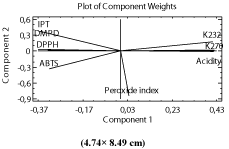
Figure 1: Plot of PCA applied to the studied variables in olive oils of Moroccan
Picholine variety, produced in traditional mills in Morocco.
0,0368645*Peroxide index + 0,416062*K270 + 0,412759*K232 + 0,419971*Acidity - 0,364409* TPC - 0,369983*DMPD - 0,320522*ABTS - 0,326148*DPPH
The effects of each variable, as observed in (Figure 1) indicated that variables related to the quality parameters; free fatty acid, peroxide index, K232 and K270 had a higher impact on the explanation of variability than the other variables; TPC and antioxidant capacity. Clearly, our analyses found that, free fatty acid showed the highest value of impact, regardless of time and storage material of the olive oil. In fact, we have demonstrated in these oils and in their corresponding olives presence of microorganisms; mesophilic and psychrotrophic bacteria, moulds and yeasts (El haouhay et al., 2014), capable to produce lipase releasing free fatty acids by hydrolyzing triglycerides [37,38]. In their turn the free fatty acids were also indicated in the acceleration of oxidation and auto-oxidation [39]; which together with factors such as oxygen and light may explain the relative high impact of the other quality parameters, peroxide index, K232 and K270.
Discriminant Analysis (DA): This procedure is designed to develop an ensemble of discriminating functions which can help predict groups of samples (A, B and C) based on the values of all quantitative variables. The 2 discriminating functions (Figure 2) with P-values less than 0.05 are statistically significant.
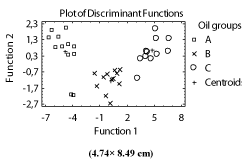
Figure 2: Classification of olive oil samples according to storage time of olive
fruits. (A: 7, B: 15 and C: 30 days).
The function 1 has separated completely the group A (obtained from olives stored during 7 days) from both groups B and C (obtained from olives stored during 15 and 30 days, respectively). According to the studied variables, components of oils extracted from olives stored during long time (more than 7 days) were found highly altered. Clearly, the prolonged storage of olives had a significant and important influence on the quality of their corresponding oils.
Hierarchical Cluster Analysis (HCA): It has created 1 cluster from the 24 observations supplied, according to type of mill (G: granite and W: wood), type of container (PET and Glass), time of olive storage (A (7 days), B (15 days) and C (30 days)) and time of oil storage (3: 3 months and 6: 6 months). The distance between groups of samples was evaluated using Centroid Method Euclidean, and the group formation was represented graphically in a dendrogram, which indicates the different groups at a normalized or rescaled distance of each kind of samples from the others, when read from right to left or left to right. For this study the oils were classified by storage time, type of storage container, and type of mill.
As shown in the (Figure 3), two clusters were formed; one consisting of the oils of group A, and the other formed by oils of groups B and C. Furthermore, mostly, for each group of samples, the oils stored during 3 months were found in the first order followed by the oils stored during 6 months. These results confirm that the quality of oils obtained from olives with reduced time of storage (7 days) was less affected or degraded than the quality of oils produced from olives with longer time of storage. Equally, the increase of time of oil storage had a negative influence in the quality of these oils. As observed in the dendogram the type of mill (granite or Wood) as well as the type of container (PET or glass) was not significant in the classification of these oils when the Centroid Method Euclidean was used.
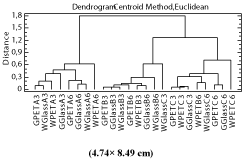
Figure 3: Dendrogram plot of olive oils according to storage time of olive
fruits (A: 7, B: 15 and C: 30 days) and olive oils (3/ 6 months), type of mills
(W/G: Wood/Granite), and container type (Glass/PET).
Conclusion
During olive and oil storage, quality parameters had a significant increase, while, total phenol content and total antioxidant capacity had an important reduction. The variable with the highest level of impact was acidity, which is linked directly to enzymatic degradation of triglycerides, followed by the oxidation and auto-oxidation, demonstrated by peroxide index, K232 and K270. Furthermore, the storage time of olive fruits was the most important in the classification of samples, followed by storage time of olive oils. The material of the container also had an important influence on the characteristics of samples; glass appears to be the most appropriate for protection of olive oils. However, type of mills (granite or wood) was not significant in the evaluation of quality of these oils. In fact, to protect quality and antioxidant capacity of olive oil, factors capable to accelerate hydrolysis and oxidation or auto-oxidation should be avoided during all stages of processing, mainly the extended storage of fruits in uncontrolled conditions. The time of milling should be reduced, and after extraction olive oil should be filtered and preferably conserved in dark glass bottles. So, it is necessary to improve conditions of olive oil production in Moroccan traditional mills, by implanting and practicing the systems to guarantee food quality and safety such as Hazard Analysis and Critical Control Point.
References
- Lamy S, Ben Saad A, Zgheib A, Annabi B. Olive oil compounds inhibit the paracrine regulation of TNF-a-induced endothelial cell migration through reduced glioblastoma cell cyclooxygenase-2 expression. J Nutr Biochem. 2016; 27: 136–145.
- Martin-Pelaez S, Covas MI, Fito M, Kusar A, Pravst I. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Mol Nutr Food Res. 2013; 57: 760–771.
- Gharby S, Harhara H, Matthaus B, Bouzoubaa Z, Charrouf Z. The chemical parameters and oxidative resistance to heat treatment of refined and extra virgin Moroccan Picholine olive oil. Journal of Taibah University for science. 2015; 10: 100-106.
- Mohammadi A, Jafari SM, Esfanjani AF, Akhavan S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chemistry. 2016; 190: 513–519.
- Mancebo-Campos V, Salvador M.D, Fregapane G. Antioxidant capacity of individual and combined virgin olive oil minor compounds evaluated at mild temperature (25 and 40°C) as compared to accelerated and antiradical assays. Food Chemistry. 2014; 150: 374–381.
- Yang Y, Song X, Sui X, Qi B, Wang Z, Li Y, et al. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Industrial Crops and Products. 2016; 80: 141–147.
- Jabeur H, Zribi A, Abdelhedi R, Bouaziz M. Effect of olive storage conditions on Chemlali olive oil quality and the effective role of fatty acids alkyl esters in checking olive oils authenticity. Food Chem. 2015; 169: 289–296.
- Catania P, Vallone M, Farid A, De Pasquale C. Effect of O2 control and monitoring on the nutraceutical properties of extra virgin olive oils. Journal of Food Engineering. 2016; 169: 179-188.
- Lozano-Sanchez J, Bendini A, Quirantes-Pine R, Cerretani L, Segura-Carretero A, Fernandez- Gutierrez, A. Monitoring the bioactive compounds status of extra-virgin olive oil and storage by-products over the shelf life. Food Control. 2013; 30: 606–615.
- Bacigalupi C, Maurey A, Boutroy N, Peyron S, Avallone S, Chalier P. Changes in nutritional value of a multi-vitamins fortified juice packed in glass and standard PET bottles. Food Control. 2016; 60: 256-262.
- Silva SF, Anjos CA, Cavalcanti RN, Celeghini RM. Evaluation of extra virgin olive oil stability by artificial neural network. Food Chem. 2015; 179: 35–43.
- Ministere de l’Agriculture et de la Peche Maritime. Veille economique-Secteur oleicole. Direction de la Strategie et des Statistiques. Note strategique n°95. 2013.
- El haouhay N, Samaniego-Senchez C, Asehraou A, Villalon-Mir M, Lopez-García de la Serrana H. Microbiological characterization of Picholine variety olives and analysis of olive oil produced in traditional oil mills in Morocco. CyTA – Journal of Food, 2014; 918178: 1–9.
- Montedoro G, Cantarelli C. Phenolic substances present in olive oils. Rivista Italiana delle Sostanze Grasse. 1969; 46: 115–124.
- Vazquez Roncero A, Janer del Valle ML. Polyphenol evolution during the pickling process of green olives. II. Changes in total polyphenols. Grasas y Aceites (Sevilla, Spain), 1978; 29: 23–27.
- Montedoro GF, Servili M, Baldioli M, Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. Journal of Agricultural and Food Chemistry. 1992; 40: 1571–1576.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture, 1965; 16: 144-158.
- Brand-Williams W, Cuvelier M. E, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Science and Technology. 1995; 28: 25–30.
- Pellegrini N, Re R, Yang M, Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2-20-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decolorization assay. Methods in Enzymology. 1999; 299: 379–389.
- Benzie IF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power’’: The FRAP assay. Analytical Biochemistry. 1996; 239: 70–76.
- Fogliano V, Verde V, Randazzo G, Ritieni A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J Agric Food Chem. 1999; 47: 1035-1040.
- EEC. Characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Commission implementing regulation (EU) 299/2013 of March 26, 2013 amending regulation EEC 2568/91. Official journal of the European Communities. 2013; 90: 52–70.
- Rababah TM, Feng H, Yang W, Eriefej K, Al-omoush, M. Effects of type of packaging material on physicochemical and sensory properties of olive oil. International Journal of Agricultural Biological Engineering. 2011; 4: 66–72.
- Ben-Hassine K, Taamalli A, Ferchichi S, Mlaouah, A, Benincasa C, Romano E, et al. Physicochemical and sensory characteristics of virgin olive oils in relation to cultivar, extraction system and storage conditions. Food Research International. 2013; 54: 1915–1925.
- Cicerale S, Conlan XA, Barnett, NW, Keast RSJ. Storage of extra virgin olive oil and its effect on the biological activity and concentration of oleocanthal. Food Research International. 2013; 50: 597-602.
- Ciafardini G, Zullo BA. Microbiological activity in stored olive oil. Int J Food Microbiol. 2002; 75: 111 –118.
- Fang F, Han H, Zhao Q, Xu C, Zhang L. Bioaugmentation of Biological Contact Oxidation Reactor (BCOR) with phenol-degrading bacteria for Coal Gasification Wastewater (CGW) treatment. Bioresour Technol. 2013; 150: 314–320.
- Zullo BA, Cioccia G, Ciafardini G. Distribution of dimorphic yeast species in commercial extra virgin olive oil. Food Microbiol. 2010; 27: 1035-1042.
- Zullo BA, Cioccia G, Ciafardini G. Effects of some oil-born yeasts on the sensory characteristics of Italian virgin olive oil during its storage. Food Microbiol. 2013; 36: 70–78.
- García-Rodríguez R, Romero-Segura C, Sanz C, Perez AG. Modulating oxidoreductase activity modifies the phenolic content of virgin olive oil. Food Chem. 2015; 171: 364–369.
- Hachicha Hbaieb R, Kotti F, Garcia-Rodriguez R, Gargouri M, Sanz C, Perez AG. Monitoring endogenous enzymes during olive fruit ripening and storage: Correlation with virgin olive oil phenolic profiles. Food Chem. 2015; 174: 240–247.
- Fernandez-Orozco R, Roca M, Gandul-Rojas B, Gallardo-Guerrero L. DPPH-scavenging capacity of chloroplastic pigments and phenolic compounds of olive fruits (cv. Arbequina) during ripening. Journal of Food Composition and Analysis. 2011; 24: 858–864.
- Kesen S, Kelebek H, Selli S. Characterization of the volatile, phenolic andantioxidant properties of monovarietal olive oil obtained from cv. Halhali. Journal of the American Oil Chemists’ Society. 2013; 90: 1685–1696.
- Lara-Ortega FJ, Sainz-Gonzalo FJ, Gilbert-López B, García-Reyes, JF, Molina-Díaz, A. Multicommuted flow injection method for fast hotometric determination of phenolic compounds in commercial virgin olive oil samples. Talanta. 2016; 147: 531–536.
- Ozgen M, Reese RN, Tulio AZ, Scheerens JC, Miller AR. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric Reducing Antioxidant Power (FRAP) and 2,20-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem. 2016; 54: 1151-1157.
- Dini I, Tenore GC, Dini, A. Effect of industrial and domestic processing on antioxidant properties of pumpkin pulp. LWT – Food Science and Technology. 2013; 53: 382–385.
- Ertugrul S, Donmez G, Takac S. Isolation of lipase producing Bacillus sp. from olive mill wastewater and improving its enzyme activity. Journal of Hazardous Materials. 2007; 149: 720–724.
- Ciafardini G, Zullo BA. Effect of lipolytic activity of Candida adriatica, Candida diddensiae and Yamadazyma terventina on the acidity of extra-virgin olive oil with a different polyphenol and water content. Food Microbiol. 2015; 47: 12-20.
- Paradiso VM, Gomes T, Nasti R, Caponio F, Summo C. Effects of free fatty acids on the oxidative processes in purified olive oil. Food Research International. 2010; 43: 1389–1394.