
Research Article
Austin Food Sci. 2016; 1(5): 1022.
Polyphenolic Reductants in Cane Sugar
Uchimiya M¹*, Hiradateb S¹ and Chouc CC¹
¹USDA-ARS Southern Regional Research Center, USA
²National Institute for Agro-Environmental Sciences, Japan
³College of Food and Bio-engineering, South China University of Technologies, China
*Corresponding author: Uchimiya M, USDA-ARS Southern Regional Research Center Lee Boulevard, USA
Received: July 13, 2016; Accepted: September 28, 2016; Published: October 03, 2016
Abstract
Limited information is available to understand the chemical structures of cane sugar extracts responsible for their redox reactivity. This study employed Fremy’s salt to test the hypothesis that hydroquinone/catechol-semiquinonequinone redox cycle is responsible for the redox reactivity of sugarcane extracts; grape seed was employed as a quebracho tannin reference compound. Polyphenols were analyzed by the liquid 1H and 13C NMR before and after the selective oxidation by the Fremy’s salt. Antioxidant having greater amounts of polyaromatic phenols (sugarcane extract from Taiwan < sugarcane extract from Philippines << grape seed) exhibited greater ability to reduce Fe (III) and contained higher amounts of terminal (vanillin) and linking (acid-butanol) proanthocyanidins units. Sugarcane extract from Taiwan was characterized by the aliphatic fluorophores having low Ex/Em wavelengths and was enriched with ionizable functional groups. Small amount of polyphenols in cane sugar (compared to grape seed) engaging in one-electron transfer reaction could provide beneficial antioxidant properties to quench radicals.
Keywords: Proanthocyanidin; Colorimetric method; Transition metals; Tannin; Antioxidant
Introduction
Naturally-occurring (poly) phenolic antioxidant structures range from polyflavanols (procyanidins), anthocyanins, to phenolic acids [1,2]. The ability of (poly) phenols to form stable semiquinone radical [3] enables one-electron transfer reactions to quench radicals [1], rendering their antioxidant activity. Polyphenolic structures with aromatic rings attached to the α-β conjugated system of the quinine (fully oxidized structure) are (i) able to form resonancestabilized semiquinone radicals, (ii) less susceptible towards nucleophilic (Michael) addition, (iii) have low reduction potential, (iv) more likely to engage in reversible electron transfer [4,5] and are therefore expected to be the stronger antioxidant. Fremy’s salt and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) [6] are the inorganic and organic free radicals frequently employed to investigate the antioxidant activity of polyphenols. For example, the ability to quench DPPH decreased in the following order: hydroquinone >> ascorbic acid > vanillin > cinnamic acid ≈ citric acid ≈ salicylic acid [7]. Because of its polyaromatic structure, procyanidin extracted from sorghum showed 15-30 times greater peroxyl radical-quenching ability than phenols and Trolox [8].
Limited report is available to understand the chemical structures responsible for the redox reactivity of sugarcane extracts. Liquid chromatography-mass spectrometry analysis of commercial brown sugar showed phenolic acids such as vanillin, syringic acid and benzoic acid in a few mg kg-1 range; catechin and flavonoids were not detected [9]. However, others observed flavonoids in raw sugar [10]. Desugared sugarcane extract [11] showed higher solubility in water (73%) than n-butanol (22%) and ethyl acetate (5%). The n-butanol extract was composed of 12 phenolic glucosides and exhibited the highest tyrosinase inhibition [11]. Sugarcane molasses and kokuto (evaporated sugarcane juice without centrifugation) contained phenolic acids, phenyl glycosides, phenyl propanoid glycosides and phenyl propanoids [12]. The DPPH assay has been used to test antioxidant activity of phenolic structures in cane brown sugar [9]. The objective of this study was to understand the role of polyphenols in the electron-donating ability of sugarcane extracts; grape seed was employed as a proanthocyanidins reference material. Liquidphase 1H and 13C NMR Fremy’s salt oxidation was employed to first determine the existence of polyphenols in cane sugar. The NMR results were compared with the following quantitative chemical assays: proanthocyanidins contents by acid-butanol, flavonoids by vanillin, radical quenching by DPPH, Fe (III) reduction and UV/ visible and fluorescence spectrophotometry.
Materials and Methods
Distilled, Deionized Water (DDW) with a resistivity of 18 MOcm (APS Water Services, Van Nuys, CA) was used in all procedures. All chemical reagents were obtained from Sigma-Aldrich (Milwaukee, WI) with the highest purity available. Good’s buffers were used to set pH: sodium acetate (pKa 4.8 for 3.0 = pH < 5.5), 2-(N-Morpholino) ethanesulfonic acid monohydrate (MES; pKa 6.2 for 5.5 = pH < 6.5) and 3-(N-Morpholino)-propanesulfonic acid (MOPS; pKa 7.2 for 6.5 = pH < 8.0).
Naturally-occurring antioxidant samples
Sugarcane extracts from Taiwan (hereby denoted Taiwan) and Philippines (hereby denoted Philippines) were manufactured by heating cane juice at 90-100°C for 40min. The products were then spray-dried at > 90°C. Grape seed extracts were purchased off the market shelf and contained dicalcium phosphate and cellulose bulking agent. Each antioxidant (0.05g) was dissolved in 20mL acetone-water (70:30 vol%), a recommended solvent to minimize the decomposition of condensed tannins [13], or methanol in amber glass vials with Teflon lined screw cap (40mL nominal volume, Thermo Fisher Scientific, Waltham, MA) by rotating end-over-end at 70rpm for 5 d; sucrose crystals visibly settled in Taiwan and Philippines stock solutions. Because n-butanol was used to extract phenolic antioxidants from sugarcane extracts [11], separate stock solutions were obtained by dissolving 0.05g sample in 35mL n-butanol. All stock solutions were filtered (0.45μm Millipore Millex-GS; Millipore, Billerica, MA) and immediately analyzed.
Ferrozine, acid-butanol, vanillin and DPPH assays
Electron-donating capacity of each sample was investigated by (i) reduction of FeIII by acetone-water (70:30 v/v) extracts under acidic pH and (ii) quantification of FeII product using ferrozine colorimetric assay [14]. Acidic pH was utilized to (i) make FeIII reduction by dihydroxybenzene thermodynamically favorable [15], (ii) minimize oxidation of FeII product by O2 [16] and (iii) minimize FeII complexation by phenolic components of extracts [17]. Acetone-water extract (0.2mL) and FeCl3 stock solution (1mM in 0.5M HCl) were added to DDW to yield 50 μM FeCl3 in 5mL total volume. Reactors were allowed to stand for 30min and then 0.2mL of resulting solution was added to 5mL ferrozine stock solution (3-(2-Pyridyl)-5, 6-diphenyl-1, 2,4-triazine-p, p-disulfonic acid monosodium salt hydrate; 0.1 gL-1 in 0.5 M MOPS buffer at pH 7) [14]. Absorbance at 562nm [14] was recorded using diode-array UV/ visible spectrophotometer (HP8452A, Hewlett-Packard, Palo Alto, CA) with DDW as the blank immediately. Five-point calibration was obtained using 1.0 M FeCl2 stock solution prepared in 1.0 mM HCl daily to minimize autoxidation. Control experiments indicated negligible FeII loss by complexation and autoxidation.
Acid-butanol assay utilizes oxidative cleavage of proanthocyanidins at 4,8 linkages to form red anthocyanidin pigment having λmax of 550nm [18]. Briefly, 6mL of n-butanol+conc. HCl (95:5 vol%) and 1mL acetone-water extract were added to a culture tube. After adding 0.2mL of 2% (w/v) NH4FeIII(SO4)2 (in 2M HCl), the reactor was vortexed and then placed in boiling water bath for 50min. Full spectrum (280-650nm) was taken before and after boiling and absorbance was recorded at 550nm. Blank spectra were obtained for each extract before boiling. Calibration was obtained by repeating above-described procedures for 1-5mg L-1 delphinidin chloride.
Vanillin assay for meta-substituted flavanoids was conducted following the literature protocol [19]. Briefly, 1mL of methanol extracts was reacted with 5mL of working reagent (2.5mL of 1% vanillin+2.5mL of 8% HCl) in 30°C water bath for 20min and the absorbance was recorded at 500nm. Above-described procedure was repeated to obtain (1) blanks (1mL of sample and 5mL of 4% HCl) and (2) five point calibration (1mL of catechin standards+5mL of working reagent).
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) is a persistent free radical stabilized by p conjugation and sterically [7] and is quenched by hydroquinone as well as (poly) phenolic extracts of sorghum seeds [1]. The DPPH radical quenching assay followed the literature protocol [20]. Briefly, 4mL of DPPH dissolved in methanol (0.01g L-1) was added to 4mL of methanol extracts at successive dilutions to achieve 20-60% inhibition. Five point calibrations were obtained using trolox to determine the trolox equivalence (in μg trolox g-1 sample).
Vanillin and DPPH assays were performed in replicate and were statistically analyzed by one-way ANOVA using MATLAB version 8.5.0.197613 (R2015a) (Math works, Natick, MA) with PLS toolbox version 8.0.1 (Eigenvector Research, Manson, WA).
Fluorescence excitation-emission (EEM) spectrophotometry
Fluorescence EEM of each sample was prepared in 3 different solvents with dilutions to minimize the inner-filtering: (1) 5g L-1 acetone-water extracts diluted (1:20) in pH 5 acetate buffer (20mM); n-butanol (0.05g in 35mL without dilution); and 0.16g L-1 methanol extracts diluted (1:11) in methanol. Fluorescence EEM of each extract was obtained using F-7000 spectrofluorometer (Hitachi, San Jose, CA) set to 220-400nm excitation and 280-600nm emission wavelengths in 3nm intervals; 5nm excitation and emission slits; 0.5s response times; and 2400nm min-1 scan speed. The blank EEM of background solution solvents were obtained daily and subtracted from each sample to remove background EEM and the lower intensity Raman scattering [21].
Solid state 13C cross polarization and magic angle spinning (CPMAS) NMR
The 13C signals of finely ground powder in KEL-F spinning tube (6mm diameter, JEOL, Tokyo, Japan) were recorded at 75.45MHz with a magic angle spinning of 6kHz, 1 ms of contact time and 3 s of pulse interval with 100Hz broadening factor for the Fourier transform. The chemical shifts were interpreted with respect to tetramethylsilane (TMS) and adamantane external reference (29.50ppm). Resulting 13C NMR spectra were interpreted based on the chemical shift regions (in ppm): aliphatic C (0-45), O/N alkyl C (45-110), aromatic C (110-160) and carbonyl C (160-190).
Oxidation of antioxidants by Fremy’s salt
Following the literature protocol [22], few mg of the antioxidant sample was dissolved in 10mL D2O. Fremy’s salt (potassium nitrosodisulfonate, 50mg) was then added and the solution was magnetically stirred for 6h at 25°C. The oxidized samples were filtered (0.45μm) and then immediately analyzed by the solution-phase 1H and 13C NMR using ECA-600 FT NMR spectrometer (JEOL, Tokyo) with a 5mm probe. Spectra were recorded at 242.85MHz using a pulse width of 10.00μs (90°), an acquisition time of 0.4522 s and broadband proton decoupling at 30°C. The pulse delay time of 2s was employed. Each spectrum was scanned 30,000 times and a broadening factor of 5.00Hz was used in the Fourier transform procedure. Chemical shifts (ppm) were determined with respect to TMS (0ppm); HOD (4.7ppm in 1H) from D2O and methanol stabilizer of Fremy’s salt (3.3ppm in 1H, 49ppm in 13C) was visible in all oxidized samples.
Results and Discussion
Solid-state 13C CPMAS NMR
Figure 1 presents solid state 13C CPMAS NMR spectra of grape seed and evaporated sugarcane juice samples from Taiwan and Philippines. Aromatic C peaks (110-160ppm) are distinctively more visible in grape seed than Taiwan and Philippines. The spectrum of grape seed closely resembles quebracho (condensed) tannins [23] having catechol B-ring, catechin and fisetindin units, as illustrated by the structure of quebracho tetramer in (Figure 1). Quebracho proanthocyanidins contain a fingerprint peak at 118ppm that is attributable to 2-, 5’- and 6’-C positions of the catechol B-ring (red numberings in quebracho tetramer structure, (Figure 1) [23].
Additional aromatic C peaks in the 110-160ppm region of grape seed result from other C positions in the catechol B-ring as well as the A-ring of the catechin starter.
Previous solid state 13C NMR analysis of commercial (food-grade) grape seed showed similar enrichment of aromatic C, compared to peanut shell; walnut shell, peach stone and Eucalyptus bark [24]. However, FTIR of grape seed contained sharper aliphatic C-H peaks (near 3000cm-1) than other biomass, suggesting components other than proanthocyanidins [24]. Grape seed samples in the present study contained cellulose bulking agent. In (Figure 1), C1-C6 of cellulose appears within the O/N-alkyl region (50-100ppm) and overlap with catechin C of proanthocyanidins. As labeled on the grape seed spectrum, additional peaks from hemicelluloses and lignin [25] could overlap with proanthocyanidins. In particular, lignin gives broad peaks at 100-200ppm [26]. In summary, grape seed has the polyphenolic structural attributes of condensed tannins [23].
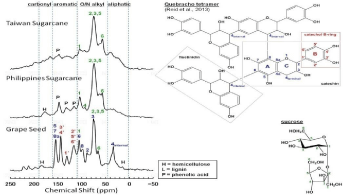
Figure 1: Solid-state 13C NMR spectra of grape seed and evaporated sugarcane juice. Grape seed closely resembles condensed tannins, while sugarcane extracts
are primarily composed of cellulose with minor contributions of lignin and hemicelluloses.
Compared to the grape seed, evaporated sugarcane juice from Taiwan and Philippines lacked phenolic peaks and resembled 13C CPMAS-TOSS NMR spectrum of sugarcane bagasse [27]. Especially for Taiwan, peaks primarily exist in the O/N alkyl region and are representative of C-1, C-4, C-2,3,5 and C-6 of cellulose [25]. Untreated sugarcane bagasse had a spectrum similar to pure cellulose with intense amorphous/crystalline cellulose peaks at 50-120ppm [26] with minor contributions from lignin and hemicelluloses [27]. Peaks in aromatic regions of Philippine sugarcane (Figure 1) are attributable to phenolic acids.
Acid-butanol assay [18] indicated the highest (3.3 wt% delphinidin chloride equivalent) proanthocyanidins in grape seed (Table 1), in agreement with the 13C NMR trends in (Figure 1). Only terminal units react in vanillin, while all except the terminal unit reacts in acid-butanol [28]. Acid butanol assay breaks interflavan bond to release flavanoid units and form anthocyanidin pigments. Vanillin (aromatic aldehyde) forms red adduct with the meta-substituted flavonoids. Based on vanillin and acid-butanol assays (Table 1), grape seed contained greater amount of terminal (vanillin) as well as linking (acid-butanol) units of proanthocyanidins.
Sample
absorbance (360nm)
λmax (210nm)
methanol
acid-butanol assay
μg g-1 delphinidin CI
Ferrozine assay
Fe (II) formed (μM)
Vanillina
[catechin]
Equiv. mg g1
DPPHb
[trolox]
Equiv. μg g-1
acetone-water
Pure
butanol
pH 5
pH 7
pH 8.5
Acetone-water
Acetone water
butanol
Grape seed
0.04
0.06
0.10
0.47
0.97
33,165
35
37
320±27 A
542±22 A
Philippines
0.41
0.43
0.51
1.60
0.75
1,307
36
21
0 B
11±5 B
Taiwan
0.44
0.46
0.59
0.44
0.50
843
39
9
0 B
139±7 C
Table 1: Characteristics of grape seed and sugarcane (from Philippines and Taiwan) extracts: DPPH radical quenching (in μg trolox g-1 seed), acid-butanol assay (in μg delphinidin g-1 seed), and reduction of 50 μM Fe (III), vanillin assay (in mg catehin g-1 seed). UV absorbance (360nm) is given for acetone-water extracts adjusted to pH 5, 7, and 8.5 and for pure butanol extract, and at λmax in methanol (210nm). Mean±s.d. for duplicatea and triplicateb assays; different letters within columns indicate statistical difference (Turkey, P < 0.05).
Solution-phase 13C and 1H NMR analyses of antioxidants before and after oxidation by Fremy’s salt
In order to understand the chemical functionality responsible for the redox reactivity of sugarcane extracts, solution-phase 13C and 1H NMR analyses were undertaken before and after oxidation using Fremy’s salt. Fremy’s salt is widely employed to oxidize hydroquinones and catechus to quinines [6].
Figure 2 presents liquid 1H NMR spectra of grape seed (pink), Taiwan (blue) and Philippines (green) before and after (black) oxidation using the Fremy’s salt. (Figure 2) shows the following chemical shift regions: aliphatic protons of CH3, CH2 and CH groups (1.0-3.0ppm), alkoxy (mostly methoxy and ethoxy) groups and carbohydrates (4.0-6.0ppm) and phenolic protons (>6ppm) [29].
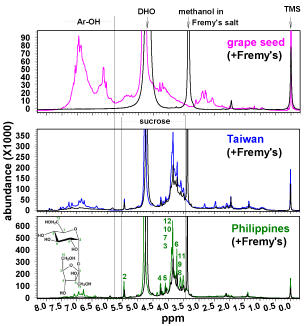
Figure 2: Liquid 1H NMR spectra of grape seed and evaporated sugarcane
juice before and after oxidation by Fremy are salt.
The alkoxy region of Taiwan and Philippine sugarcane extracts were dominated by sucrose [30] that was not oxidized by Fremy’s salt. Phenolic groups (>6ppm) were the primary functionality oxidized by Fremy’s salt (black spectra in (Figure 2) in each sample. In agreement with the solid-state 13C NMR (Figure 1), grape seed has extensive polyphenolic peaks in (Figure 2). In conclusion, the phenolic peaks were distinctively removed after the oxidation by Fremy’s salt in all samples in (Figure 2).
Figure 3 presents solution-phase 13C NMR spectra of grape seed (pink), Taiwan (blue) and Philippines (green) before and after (black) oxidation using the Fremy’s salt. In agreement with the solutionphase 1H NMR (Figure 2), Taiwan and Philippines are dominated by the sucrose C1-12 (green numberings for Philippines before oxidation, (Figure 3), bottom) at 60-110ppm. The sucrose peaks [31] remained visible in Taiwan and Philippines after the oxidation. Much like 1H NMR (Figure 2), grape seed is dominated by polyphenolic structures in (Figure 3) (>110ppm) [32,33]. The phenolic peak in Figure 3 presents solution-phase 13C NMR spectra of grape seed (pink), Taiwan (blue) and Philippines (green) before and after (black) oxidation using the Fremy’s salt. In agreement with the solutionphase 1H NMR (Figure 2), Taiwan and Philippines are dominated by the sucrose C1-12 (green numberings for Philippines before oxidation, (Figure 3), bottom) at 60-110ppm. The sucrose peaks [31] remained visible in Taiwan and Philippines after the oxidation. Much like 1H NMR (Figure 2), grape seed is dominated by polyphenolic structures in (Figure 3) (>110ppm) [32,33]. The phenolic peak in all 3 samples disappeared by Fremy’s salt. In conclusion, (Figures 2,3) indicate that only polyphenols were oxidized by Fremy’s salt; sugar peaks remained in Taiwan and Philippines after oxidation. Similar trends were observed for the oxidation by Fe (III) (Table 1): for butanol extracts, the electron-donating ability increased in the following order: Taiwan < Philippines < grape seed.
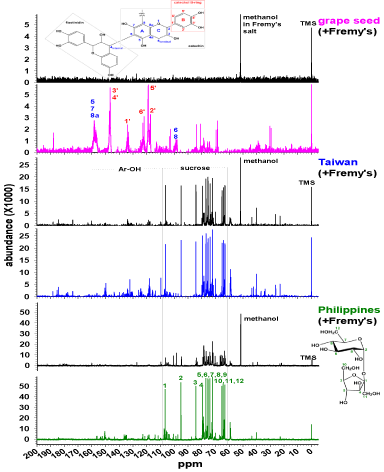
Figure 3: Liquid 1H NMR spectra of grape seed and evaporated sugarcane
juice before and after oxidation by Fremy are salt.
Conclusion
In conclusion, Fremy’s salt results agreed with the trend in (Table 1) for the following assays: acid-butanol, ferrozine (for the butanol extract), vanillin and the absorbance of methanol extracts at 210nm. (Table 1) presents UV (360nm) absorbance of acetone-water extracts at pH 5, 7 and 8.5. At each pH, the following sample-dependent trend was observed: grape seed <<< Philippines < Taiwan. For each sample, the following pH-dependent trend was observed: pH5 < pH7 << pH8.5. The pH dependence indicates the deprotonation of phenolic acids and proanthocyanidins (Figure 1). As shown in (Figure 4), pHdependent changes in UV/visible spectra (Figures 4d-4f) are greatest In conclusion, Fremy’s salt results agreed with the trend in (Table 1) for the following assays: acid-butanol, ferrozine (for the butanol extract), vanillin and the absorbance of methanol extracts at 210nm. (Table 1) presents UV (360nm) absorbance of acetone-water extracts at pH 5, 7 and 8.5. At each pH, the following sample-dependent trend was observed: grape seed <<< Philippines < Taiwan. For each sample, the following pH-dependent trend was observed: pH5 < pH7 << pH8.5. The pH dependence indicates the deprotonation of phenolic acids and proanthocyanidins (Figure 1). As shown in (Figure 4), pHdependent changes in UV/visible spectra (Figures 4d-4f) are greatest
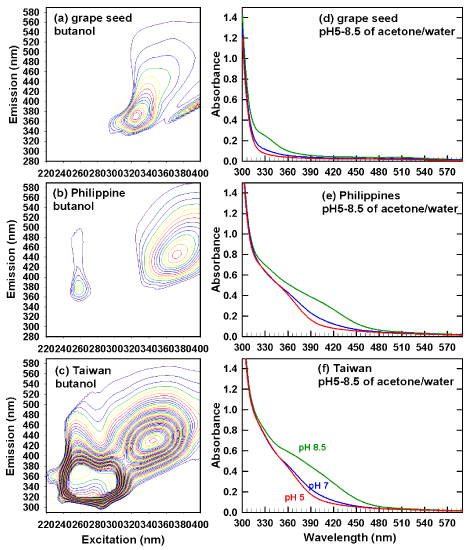
Figure 4: Fluorescence EEM in butanol (without dilution, a-c), and UV/v is
spectra of acetone/water extracts set to pH3-8.5.
References
- Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem. 2003; 51: 6657-6662.
- Poaty B, Dumarçay S, Gerardin P, Perrin D. Modification of grape seed and wood tannins to lipophilic antioxidant derivatives. Ind Crop Prod. 2010; 31: 509-515.
- Hotta H, Sakamoto H, Nagano S, Osakai T, Tsujino Y. Unusually large numbers of electrons for the oxidation of polyphenolic antioxidants. Biochim Biophys Acta Gen. 2001; 1526: 159-167.
- Patai S, Rappoport S. The Chemistry of the quinonoid compounds. New York Wiley. 1988.
- Clark WM. Oxidation-Reduction Potentials of Organic Systems. Baltimore MD: The Williams & Wilkins Company. 1960.
- Gardner PT, McPhail DB, Duthie GG. Electrons spin resonance spectroscopic assessment of the antioxidant potential of teas in aqueous and organic media. J Sci Food Agric.1998; 76: 257-262.
- Tang Y, He F, Yu M, Wang S, Li Y, Zhu D. Radical scavenging mediating reversible fluorescence quenching of an anionic conjugated polymer: Highly sensitive probe for antioxidants. Chem Mater. 2006; 18: 3605-3610.
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agr Food Chem. 1998; 46: 1887-1892.
- Payet B, Sing ASC, Smadja J. Assessment of antioxidant activity of cane brown sugars by ABTS and DPPH radical scavenging assays: Determination of their polyphenolic and volatile constituents. J Agr Food Chem. 2005; 53: 10074-10079.
- Mabry TJ, Liu YL, Pearce J, Dellamonica G, Chopin J, Markham KR, et al. New flavonoids from sugarcane (Saccharum). J Nat Prod. 1984; 47: 127-130.
- Chung YM, Wang HC, El-Shazly M, Leu YL, Cheng MC, Lee CL, et al. Antioxidant and tyrosinase inhibitory constituents from a desugared sugar cane extract, a byproduct of sugar production. J Agr Food Chem. 2011; 59: 9219-92125.
- Okabe T, Toda T, Inafuku M, Wada K, Iwasaki H, Oku H. Antiatherosclerotic function of Kokuto, Okinawan noncentrifugal cane sugar. J Agr Food Chem. 2009; 57: 69-75.
- Grabber JH, Zeller WE, Mueller-Harvey I. Acetone enhances the direct analysis of procyanidin-and prodelphinidin-based condensed tannins in lotus species by the butanol-HCl-iron assay. J Agr Food Chem. 2013; 61: 2669-2678.
- Stookey LL. Ferrozine-A new spectrophotometric reagent for iron. Anal Chem. 1970; 42: 779-781.
- Uchimiya M, Stone AT. Redox reactions between iron and quinines: Thermodynamic constraints. Geochim Cosmochim Ac. 2006; 70: 1388-1401.
- Kanzaki Y, Murakami T. Rate law of Fe (II) oxidation under low O2 conditions. Geochim Cosmochim Ac. 2013; 123: 338-350.
- Martell AE, Smith RM, Motekaitis RJ. Critically Selected Stability Constants of Metal Complexes Database. Version 8.0 ed National Institute of Standards and Technology. 2004.
- Porter LJ, Hrstich LN, Chan BG. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry. 1985; 25: 223-230.
- Price ML, Van Scoyoc S, Butler LG. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum-grain. J Agr Food Chem. 1978; 26: 1214-1218.
- Berger JM, Rana RJ, Javeed H, Javeed I, Schulien SL. Radical quenching of 1,1-diphenyl-2-picrylhydrazyl: A spectrometric determination of antioxidant behavior. J Chem Educ. 200; 85: 408-410.
- Christensen JH, Hansen AB, Mortensen J, Andersen O. Characterization and matching of oil samples using fluorescence spectroscopy and parallel factor analysis. Anal Chem. 2005; 77: 2210-2217.
- Harjivan SG, Wanke R, Ferreira Da Silva JL, Marques MM, Antunes AMM. The phenolic metabolites of the anti-HIV drug efavirenz: Evidence for distinct reactivities upon oxidation with Frémy's salt. Eur J Med Chem. 2014; 74: 7-11.
- Reid DG, Bonnet SL, Kemp G, Van Der Westhuizen JH. Analysis of commercial proanthocyanidins. Part 4: Solid state 13C NMR as a tool for in situ analysis of proanthocyanidin tannins, in heartwood and bark of quebracho and acacia and related species. Phytochemistry. 2013; 94: 243-248.
- Ferreira CIA, Calisto V, Santos SM, Cuerda-Correa EM, Otero M, Nadais H, et al. Application of pyrolysed agricultural biowastes as adsorbents for fish anaesthetic (MS-222) removal from water. J Anal Appl Pyrol. 2015; 112: 313-324.
- Hallac BB, Sannigrahi P, Pu Y, Ray M, Murphy RJ, Ragauskas AJ. Biomass characterization of Buddleja davidii: A potential feedstock for biofuel production. J Agr Food Chem. 2009; 57: 1275-1281.
- Rezende CA, De Lima M, Maziero P, Deazevedo E, Garcia W, Polikarpov I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4. 2011.
- Chandel AK, Antunes FAF, Anjos V, Bell MJV, Rodrigues LN, Polikarpov I, et al. Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels. 2014; 7: 63.
- Butler LG. Relative degree of polymerization of sorghum tannin during seed development and maturation. J Agr Food Chem. 1982; 30: 1090-1094.
- Bruice PY. Organic Chemistry. Second edn. New Jersey: Prentice Hall. 1998.
- Herve du Penhoat C, Imberty A, Roques N, Michon V, Mentech J, Descotes G, et al. Conformational behavior of sucrose and its deoxy analogue in water as determined by NMR and molecular modeling. J Am Chem Soc. 1991; 113: 3720-3727.
- Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009.
- Eberhardt TL, Young RA. Conifer seed cone proanthocyanidin polymers: Characterization by 13C NMR spectroscopy and determination of antifungal activities. J Agr Food Chem. 1994; 42: 1704-1708.
- Ling ZQ, Xie BJ, Yang EL. Isolation, characterization and determination of antioxidative activity of oligomeric procyanidins from the seedpod of Nelumbo nucifera Gaertn. J Agr Food Chem. 2005; 53: 2441-2445.