
Research Article
Austin Food Sci. 2016; 1(5): 1023.
Comparative Study of the Physicochemical Characteristics of Oil from Transgenic Corn (Ajeeb YG) with its Non-Transgenic Counterpart
Shatta AA¹, Rayan AM¹*, El-Shamei ZS¹, Gab-Alla AA¹ and Moussa EA²
¹Food Technology Department, Suez Canal University, Egypt
²Departments of Anatomy and Embryology, Suez Canal University, Egypt
*Corresponding author: Rayan AM, Food Technology Department, Suez Canal University, Egypt
Received: August 08, 2016; Accepted: September 29, 2016; Published: October 05, 2016
Abstract
The objective of this study was to evaluate the physicochemical characteristics of crude oil from transgenic corn (Ajeeb YG) and it’s near isogenic (Ajeeb). The proximate composition of transgenic corn is substantially equivalent to that of the isogenic counterpart. Regarding the physicochemical characteristics, non-significant differences in refractive index and specific gravity values were observed. While acid value, phosphorous and carotenoids contents were differed significantly. In addition, non-significant differences in fatty acid components between oil from isogenic and transgenic corn except for linolenic acid. The oxidation parameters including peroxide and Cox (calculated oxidizability value) were recorded as 0.990-0.963 meq O2/kg and 4.50-4.63, respectively. The fatty acid composition showed the presence of palmitic acid (11.24-11.45%), stearic acid (1.616-1.516%), oleic acid (48.36-46.07%), linoleic acid (37.106-38.97%) and linolenic acid (0.916-0.716%) for isogenic and transgenic corn, respectively. The MUFA, PUFA and PUFA/SFA were (48.36- 46.07%), (38.02-39.69%) and (2.965-3.062), respectively. Therefore, oil from transgenic corn had properties similar to that of isogenic counterpart. For the safety of (GM) food and its products, there is still need for further studies.
Keywords: Transgenic corn; Ajeeb YG; Oil; Physicochemical characteristics; Fatty acid composition
Introduction
The nutritive and calorific values of seeds make them good sources of edible oils. Seed oils have extensive demands both for human consumption and for industrial applications [1] and also have been rated as the second most valuable commodity in the world trade today [2]. There is an increasing awareness of the importance of vegetable oils as sources of food, biofuel, health enhancing compounds, i.e., nutraceutical, as feedstock for industrial polymers and for many other products. Thus the world demand for vegetable oils is set to rise even more rapidly from year to year and this trend will impact on the price levels of oils. Oil World report in 2015 [3] indicated that vegetable oil for world production was not sufficient to satisfy global demand for food and for oleochemical industry as well as for the energy sector. Total global consumption of vegetable oils was 175.65 million tons in 2014/2015 while the total world production was 173.27 million tons [3].
Vegetable oils and fats have wide application in foods where they are used in frying, salad dressing, shortening of pasty, margarine, cooking and ice cream manufacture [4]. Corn oil is produced as a co-product of wet and dry corn milling. Corn oil production increased markedly in recent years because of increased volumes of corn being used in sweetener and starch production [5]. Corn oil is refined into high quality oil for the food industry and also as a vehicle in certain pharmaceutical formulations such as in suspensions and emulsions [6]. Corn oil has a high concentration of the essential, Poly Unsaturated Fatty Acids (PUFA) such as linoleic acid (46-60%), very little linolenic acid (1%) and high concentrations of tocopherol and carotenoid antioxidants [7]. Thus, presence of PUFA plays a pivotal role in improvement of human health through maintaining the body homeostasis and regulating serum lipid profile [8].
The genetically modified cultivars of several oilseed and oilproducing plants are currently under commercial production worldwide, e.g. soybean, maize and rapeseed. In 2002 approximately 35% of corn acreage in Untied States was pest-resistant or herbicide, genetically modified hybrids. Varieties with the oil content increased from 6.5 to 11% have also been developed to improve the energy density for livestock feeding [9]. The industry is interested in genetic manipulation to produce different fatty acid compositions from the standpoint of improved functionality or improved nutritional properties.
Genetic modification of oilseeds produces a wide variety of oils with different fatty acid composition. Oxidative stability, functionality and quality of vegetable oils can be affected by selective modification of the fatty acid composition, which consequently alters the distribution of minor bioactive components [10]. In the context of consumer safety, the European Union (EU) has recently implemented changes in its labeling regulation, laid down in Regulation (EU) No 1169/2011, which entered into force in December 2014. The new regulation formalizes the provision of food information to consumers, including details of the vegetable oil’s composition, its manufacturer and the storage and preparation methods used.
Corn/maize is the second most cultivated Genetically Modified Organism (GMO) in the world, which represents the staple constituent of many foods. Maize represents 25% of the total GM plant area under cultivation. In recent years, foods produced by genetic engineering technology have been on the world food market. The bio safety aspects, regulations and labeling of these foods are still contentious issues in most countries. Ajeeb-YG is genetically modified to produce the naturally occurring Bacillus Thuringiensis (Bt) protein, Cry1Ab, which gives it whole-plant, season-long protection against stalk borers such as the pink corn borer (Sesamia cretica), the purplelined corn borer (Chilo agamemnon) and the European corn borer (Ostrinia nubilalis).
The major problem facing oil production in Egypt is the wide gap between production and consumption. Egypt’s production covers less than 10% of the national consumption [11]. In addition, market profitability of new modified oilseeds depends heavily on rich sources of minor constituents [10]. The physicochemical examination of oils is mainly made from the standpoint of their edible as well as industrial uses. The quality of vegetable oils can be judged by the knowledge of their physical and chemical characteristics. Therefore, the objective of this study was to compare the physicochemical characteristics and fatty acid composition of genetically modified corn (Ajeeb YG) with isogenic counterpart (Ajeeb) and evaluate the effects of genetic modification on these characteristics.
Materials and Methods
Materials
The transgenic corn line (Ajeeb YG) containing the Cry1Ab gene and its isogenic counterpart (Ajeeb) were grown in a field trail under the same environmental conditions and field management. Samples were harvested on September, 2011 in Hehia, Sharkia Governorate, Egypt. Samples were stored at 4°C until use.
Methods of analysis
Proximate analysis of corn seed samples: The moisture, crude protein (N×6.25), fat, ash and fiber were determined in triplicate according to the Standard Association of Official Analytical Chemists (AOAC) procedures [12]. Carbohydrates were calculated by difference.
Corn oil
Oil extraction: Oil was extracted from the dried ground corn by blending with hexane overnight (twice). The hexane was separated from the oil by evaporating under vacuum using a rotary evaporator (Buchi 461, Switzerland).
Physical properties: Color attributes were carried out using the CIELAB (L*,a*,b*) color scale with a Minolta colorimeter (Konica Minolta Sensing, Inc. Osaka, Japan). Color was expressed by CIE L* (whiteness or brightness), a* (redness/ greenness) and the b* (yellowness/ blueness) coordinates [13]. The refractive index of extracted oil was measured according to Association of Official Analytical Chemists (AOAC) [12] using Abbe refractometer at 25°C while the specific gravity of oil samples were determined gravimetrically according to the method outlined by Association of Official Analytical Chemists (AOAC) [12].
Chemical properties: Acid value and free fatty acids were determined according to International Union of Pure and Applied Chemistry (IUPAC) [14]. Iodine absorption value, saponification number, unsaponifiable matter and peroxide value have been determined according to Association of Official Analytical Chemists (AOAC) [12]. The concentration of phosphorous in the corn oil samples were determined using the method of Zhukov and Vereshchagin [15]. The total carotenoid content was assayed according to the British standard method of analysis [16]. Oxidizability value (Cox) was calculated according to Farhoosh et al. [17], based on the percentage of C18 unsaturated fatty acids as follows:
Cox = [1(18:1%) + 10.3 (18:2%) + 21.6 (18:3%)] / 100
Fatty acid analysis: The oil was converted to methyl esters according to the method of O’Fallon et al [18]. The sample (40 μl of the oil) was placed into a screw-cap Pyrex tube to which 0.7 ml of 10 N KOH in water and 5.3 ml of MeOH were added. The tube was incubated in a 550C water bath for 1.5h. After cooling below room temperature in a cold tap water bath, 0.58 ml of 24 N H2SO4 in water was added. The tube was incubated again in a 550C water bath for 1.5 h. After Fatty Acid Methyl Esters (FAME) synthesis, the tube was cooled in a cold tap water-bath. Three milliliters of hexane was added and the tube was vortex-mixed for 5 min. The tube was centrifuged for 5 min and the hexane layer, containing the FAME, was analyzed. Analyses of FAME were carried out with a Hewlett Packard Gas Chromatograph (HP 6890 series), equipped with a flame ionization detector and a capillary column, HP5, (25 m; i.d. 0.32 μm; 0.17 μm film thickness). The column temperature was programmed from 170 to 2400C at a rate of 50C/min and the injector and detector temperatures were 260 and 2750C, respectively. Nitrogen was the carrier gas. The identification of the peaks was achieved by retention times and by comparing them with authentic standards (Sigma, USA) analyzed under the same conditions.
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Scientists (SPSS) version 16.0 (SPSS, Inc, Chicago, IL, USA). All analyses were performed in triplicate. Data were expressed as mean ± Standard Deviation (SD) and statistical significance was assigned at P = 0.05 level. An independent sample t-test was conducted to compare the means between the properties of isogenic and transgenic corn oil samples at the 0.05 significance level.
Results and Discussion
Proximate composition of tested corn seed cultivars
The proximate composition of the isogenic and transgenic corn kernels are presented in (Table 1). There were no significant differences between the isogenic and transgenic corn samples at (p=0.05). The moisture content was 11.09 and 10.66% for isogenic and transgenic samples, respectively while ash levels were 1.37 and 1.30% for the isogenic and transgenic corn, respectively. These results were similar to those obtained by [19-21] for different corn varieties. Protein, an essential growth promoting factor, is present in fairly high amount of 10.89 and 10.05% for the isogenic and transgenic corn, respectively. These results are in agreement with those reported by [20-22]. The crude fat content was 3.98 and 4.39% for the isogenic and transgenic corn, respectively. These results were higher than those reported by [23], but fell within the range reported for different corn cultivars [19-21]. The crude fiber content was 2.18 and 2.23% for the isogenic and transgenic corn, respectively. These results are in the literature range [19-21,24]. The carbohydrates content was 81.29 and 81.73% for the isogenic and transgenic corn, respectively. The gross energy value for isogenic and transgenic corn samples was 1689.45 and 1698.23 Kj/100g and this high-energy value is due to high protein and carbohydrates.
Component (%)
Corn samples
Isogenic (Ajeeb)
Transgenic (Ajeeb YG)
Moisture
11.09 ± 0.079a
10.66 ± 0.235a
Ash
1.37 ± 0.114a
1.30 ± 0.012a
Protein
10.89 ± 0.226a
10.05 ± 0.226a
Fat
3.98 ± 0.123a
4.39 ± 0.137a
Crude fiber
2.18 ± 0.076a
2.23 ± 0.057a
Carbohydrates
81.29 ± 0.174a
81.73 ± 0.384a
Gross energy (Kj/100g)
1689.45
1698.23
*The same letter in the same row is not significant different (P<0.05). *Results are mean ± SD (n= 3).
Table 1: Proximate composition of the isogenic and transgenic corn oil samples.
From the results, it can be concluded that the composition of transgenic corn (Ajeeb YG) is substantially equivalent to that of the isogenic (Ajeeb), as well as to corn varieties in commerce. In addition, all tested parameters fell within the range of conventional standard values reported by ILSI [25].
Physicochemical properties of corn oil
The knowledge of chemical and physical properties of edible oils is important having role in processing functionality, storage stability and nutritional behavior. Plant seeds are rich source of lipids [26]. Edible oils in general exhibit considerable deviations in their composition, thus it is difficult to define single values for chemical and physical properties of edible oils [27]. The physicochemical properties of oil from the isogenic and transgenic corn are shown in (Table 2).
Corn oil sample
Properties
Isogenic (Ajeeb)
Transgenic (Ajeeb YG)
Color
Yellow
Yellow
Refractive index
1.4663 ± 0.0015a
1.4600 ± 0.0061a
Specific gravity
0.8588 ± 0.0004a
0.8891 ± 0.0003a
Acid value (mg KOH/g)
2.386 ± 0.165b
2.468 ± 0.112a
Free fatty acid (%)
1.200 ± 0.084b
1.240 ± 0.056a
Iodine value (g I2/100g)
113.21 ± 0.515a
106.18 ± 1.18a
Saponification value (mg KOH/g)
181.66 ± 1.527a
182.66 ±1.527a
Unsaponifiable matter (%)
0.850 ± 0.02a
0.870 ± 0.03a
Peroxide value (meq peroxide/kg)
0.990 ± 0.01a
0.963 ±0.01a
Oxidizability value (Cox)
4.50 ± 0.72a
4.63 ± 0.89a
Phosphorous (%)
0.423 ± 0.015b
0.541 ±0.021a
Carotenoids (ppm)
55.11 ± 0.798b
69.58 ±3.60a
*The same letter in the same row is not significant different (P<0.05). *Results are mean ± SD (n= 3).
Table 2: Physicochemical properties of the isogenic and transgenic corn oil samples.
Physical properties
Color: Color of oil reflects the degree of refining and is an important criterion for its intended use in food formulations. The flavor of the oil is also key property, which is subjective to temperature, moisture, air in contact, light and presence of antioxidants [28].
Oil from isogenic and transgenic corn samples had yellow color (Table 2). The CIELab coordinates (L* and a*) values for isogenic and transgenic corn were not differed significantly. However, the b* value of the oil obtained from the isogenic corn was significantly higher (P<0.05) than transgenic corn oil sample. Moreover, corn oil from the two investigated samples showed L* and b* values (Figure 1) indicating that the color of the two oils was lighter and more yellow. Hsu and Yu [29] reported that the CIE Lab coordinates (L*, a* and b*) values of some vegetable oils, such as palm, soybean, sunflower, olive and corn were ranged from 63.4 to 69.5; 3.8 to 4.4 and 9.2 to 10.4, respectively. O’Brien [9] reported that crude oil has darker reddish amber color than other vegetable oils, which can usually be processed to light colored oil.
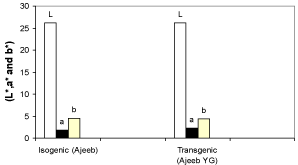
Figure 1: CIEL a,b coordinates (L*, a* and b*) of oil extracts from the isogenic
and transgenic corn samples.
Refractive index: As seen in (Table 2), the refractive indices of isogenic and transgenic corn oil were 1.4663 and 1.4600, respectively which not differed significantly. Refractive index of oil increased with increase in the number of double bonds (iodine value). In general, the refractive indices of oils relate to the degree of unsaturation in a linear way [27].
Specific gravity: The specific gravity is a good indicative of purity of oil; it is depend on the number of double bonds and the chain length of the fatty acids [28]. The specific gravities measured at room temperature for isogenic and transgenic corn samples were nearly the same being 0.8891 and 0.8588, respectively (Table 2). The results for specific gravity and refractive index are closely associated with those reported by Rudan-Tasic and Klofutar [27] for maize germ oil. CRA [30] also reported typical values for physical parameters of maize germ oil: color; pale yellow, specific gravity (0.922-0.928), refractive index (1.470-1.474) and flavor; slight corn, slight nutty/buttery.
Chemical Properties
Acid and free fatty acid values: The acid value is an indicator for edibility and freshness of oils. Humidity and temperature result in increased acid value due to hydrolysis of glycerides. Higher acid value gives an idea about increased susceptibility of oils to rancidity [31]. From (Table 2), the acid and free fatty acids values of the transgenic corn were significantly higher than the isogenic sample. The values were 2.386, 2.468 mg KOH/g, 1.200 and 1.240% for oil from isogenic and transgenic corn samples, respectively (Table 2). The Acid value of the seed oils studied fell within allowable limits for edible oils [32].
Iodine value: Iodine value is used to assess degree of instauration of fatty acids and indicator of oxidative stability. The higher iodine value represents the greater degree of instauration [33]. Iodine values of oil from the isogenic and transgenic corn cultivars were not differed significantly being 113.21 and 106.15g I2/100g (Table 2). Our results are similar to those obtained by Leibovitz and Ruckenstein [34].
Saponification number: Saponification value gives the idea of molecular weight of fatty acids present in oil; higher value corresponds to lower molecular weight of fatty acids [31]. Saponification numbers for isogenic and transgenic corn were insignificantly different (P<0.05), they were 181.66 and 182.66 mg KOH/g, respectively (Table 2). These results are in agreement with those reported by Sabah ELKheir et al. [35].
Unsaponifiable matter content: Unsaponified matter includes those substances frequently found dissolved in fats and oils which cannot be saponified by caustic alkali but are soluble in ordinary fat solvents [36]. Unsaponifiable matter contents for isogenic and transgenic corn oil samples were not significantly different (P<0.05) being 0.85 and 0.87%, respectively (Table 2). These results are similar to that obtained by Abdulkadir and Abubakar [37].
Peroxide (PV) and Oxidizability (Cox) values: The oxidative state of tested oil can be evaluated from the combined analysis of its PV and its cox index (Table 2). Peroxide value is an index of rancidity, thus the high peroxide value of oil indicates a poor resistance of the oil to peroxidation during storage [38]. Corn oils exhibited a good oxidative state as indicated by a low peroxide value (0.990-0.963 meq O2/kg of oil) for isogenic and transgenic corn, respectively (Table 2). The values were also lower than those of sunflower (7.9 meq/kg) and olive oil (10 meq/kg) [39]. The obtained results revealed that the peroxide values were insignificant (P<0.05) being 0.990 and 0.963 meq peroxide/kg, respectively (Table 2). Furthermore, no significant different between the PUFA/SFA ratio and the Cox value (p=0.05). This may be due to the relative stability of corn oil’s fatty acids against oxidation reactions.
Phosphorous content: The concentration of phosphorous in the corn oil samples was determined as the indicator of the level of phospholipids in oils (Table 2). The phosphorous content of isogenic corn (0.423%) was significantly lower (P<0.05) than that of the transgenic corn (0.541%). Feng et al. [5] reported that higher concentrations of phosphorous will result in more phospholipids present in the oils.
Carotenoids content: As seen in (Table 2), the carotenoids content of the isogenic corn oil was significantly lower (P<0.05) than that of transgenic corn, they were 55.11 and 69.58 ppm, respectively. Carotenoids play an important potential role in human health by acting as biological antioxidants protecting cells and tissues from the damaging effects of free radicals and singlet oxygen [40].
Fatty acids composition of corn oil samples: Fatty acids composition of corn oil samples are presented in (Table 3). Palmitic, stearic, oleic, linoleic and linolenic acids were identified. There were no significant differences in fatty acid composition between isogenic and transgenic corn oil except for linolenic acid (Table 3). The total saturated and unsaturated fatty acid composition of corn seeds oil, are 12.862, 12.962 and 86.386, 85.760%, respectively and the most abundant fatty acid is oleic acid. The major saturated fatty acids in corn seeds oil were palmitic acid (11.246-11.446%) while the major unsaturated fatty acids in two corn seeds oil were oleic (48.364- 46.074%) and linoleic (37.106-38.970) acids (Table 3). Our results fell within the literature range [41,42].
Fatty acid
Corn oil samples
Isogenic (Ajeeb)
Transgenic (Ajeeb YG)
Palmitic (16:0)
11.246 ± 0.312a
11.446 ± 0.382a
Stearic (18:0)
1.616 ± 0.028a
1.516 ± 0.028a
Oleic (18:1)
48.364 ± 1.198a
46.074 ± 1.526a
Linoleic (18:2)
37.106 ± 0.941a
38.970 ± 1.015a
Linolenic (18:3)
0.916 ± 0.028a
0.716 ± 0.028b
? unsaturated fatty acids
86.386
85.76
? saturated fatty acids
12.862
12.962
T. saturated /T. unsaturated
0.149
0.151
MUFA
48.364
46.074
PUFA
38.022
39.686
T. PUFA / T. SFA
2.956
3.062
*The same letter in the same row is not significant different (P<0.05%). *Results are mean ± SD (n= 3).
Table 3: Fatty acid composition of isogenic and transgenic corn oil samples (% of total lipid).
There were no chemical or biological differences between the fatty acid components of vegetable oils produced from GM or non-GM plants [43-45]. For example, lauric acid or oleic acid molecules from GM plants will be identical to lauric or oleic molecules derived from non-GM plants [46]. In the case of input trait-modified GM plants, their entire oil compositions will be indistinguishable from those produced by non-GM versions of the same crop. For example, oils from the most common GM crops grown at present (maize, soybean, rapeseed and cotton) have the same acyl compositions as oils from non-GM varieties of these four oilseed crops [47].
Several commercially important refined vegetable oils are derived from plants which are recognized as potent food allergens. Full refining of oils results in the almost complete removal from oils of protein, which is responsible for allergic reactions [48]. Allergenic reactions to proteins expressed in GM crops have been one of the prominent concerns among biotechnology critics and a concern of regulatory agencies [49]. Therefore, several studies are still needed to evaluate the safety of the small amount of protein or DNA, remained after refining, from the original GM crop in vegetable oil. Therefore, several studies are still needed to evaluate the safety of the small amount of protein or DNA, remained after refining, from the original GM crop in vegetable oil. Furthermore, GM food needs to be tested on a case-by-case basis bases.
References
- Kyari MZ. Extraction and characterization of seed oils. Int Agrophysics. 2008; 22: 139-142.
- Obasi NA, Ukadilonu J, Eze E, Akubugwo EI, Okorie UC. Proximate composition, extraction, characterization and comparative assement of coconut (Cocos nucifera) and melon (Colocynthis citrullus) seeds and seed oils. Pak J Biol Sci. 2012; 15: 1-9.
- USAD (United States Department of Agriculture). World Agricultural supply and demand estimation. WASDE-551. 2016.
- Othman OC, Ngassapa FN. Physicochemical characteristics of some imported edible vegetable oils and fat marketed in Dar es Salaam. Tanzania J Nat App Sci. 2010; 1: 138-147.
- Feng F, Myers DJ, Hojilla-Evangelista MP, Miller KA, Johnson LA, Singh SK. Quality of Corn Oil Obtained by Sequential Extraction Processing. Cereal Chem. 2002; 79: 707-709.
- Alvarez AMB, Rodriguez MLG. Lipids in pharmaceutical and cosmetic preparations. Grasas Aceites. 2000; 51: 74-96.
- Weber EJ, SA Watson, PE Ramstad. Corn: Chemistry and Technology. Am Assoc St Paul. 1987; 311-349.
- Ramaa CS, Shirode AR, Mundada AS, Kadam VJ. Nutraceuticals: an emerging era in the treatment and prevention of cardiovascular diseases. Curr Pharm Biotech. 2006; 7: 15-23.
- Brien RD. Fats and Oils: Formulating and Processing for Applications. Second Edition London CRC Press. 2004; 192-199.
- Abidi SL, List GR, Rennick KA. Effect of genetic modification on the distribution of minor constituents in canola oil. JAOCS. 1999; 76: 463-467.
- Taher HE, Abde-Twab YM, E-Sharihi REA. Dialell fingerprinting analysis of five sunflower parents. Egypt J Plant Breed. 2008; 12: 187-201.
- W. Hortuntzed. Association of Official Analytical Chemists (AOAC). Washington. 17th edn. 2000.
- Lopez J, Vega-Galvez A, Torres MJ, Lemus-Mondaca R, Quispe-Fuentes I, Scala KD. Effect of dehydration temperature on physico-chemical properties and antioxidant capacity of goldenberry (Physalis peruviana L). Chil J Agric Res. 2013; 73: 293-300.
- Dieffenbacher A, Pocklington WD. Standard Methods for the Analysis of Oils, Fats and Derivatives. International Union of Pure and Applied Chemistry. Oxford. 1987.
- Zhukov AV, Vereshchagin AG. Quantitative determination of phosphorus in soybean lipids. J Lipid Res. 1969; 10: 711-713.
- British Standard Methods of Analysis. BS 684 section 2.20. Determination of carotene in vegetable oils. 1977.
- Farhoosh R, Tavakoli J, Hadad Khodaparast MH. Chemical composition and oxidative stability of kernel oils from two current subs species of Pistacia atlantica in Iran. J Am Oil Chem Soc. 2008; 85: 723-729.
- Fallon JV, Busboom JR, Nelson ML, Gaskins CT. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J Anim Sci. 2007; 85: 1511-1521.
- Marzok MA. Detection of genetically modified soybeans and maize in Egypt as well as comparative nutritional safety investigations of isogenic and transgenic (Bt) maize in broiler nutrition: Broiler performance, degradation and metabolic fate of maize DNA in some tissues and organs. Berlin 2004; 2800.
- Wu X, Zhao R, Wang D, Bean SR, Seib PA, Tuinstra MR, et al. Effects of amylose, corn protein, and corn fiber contents on production of ethanol from starch-rich media. Cereal Chem. 2006; 83: 569-575.
- Rayan AM, Abbott LC. Compositional analysis of genetically modified corn events (NK603, MON88017 × MON810 and MON89034 × MON88017) compared to conventional corn. Food Chem. 2015; 176: 99-105.
- Flachowsky G, Chesson A, Aulich K. Animal nutrition with feeds from genetically modi?ed plants. Animal Feed Sci Technol. 2005; 133: 2-30.
- Reuter T, Aulrich K. Investigations on genetically modified maize (Bt-maize) in pig nutrition: fate of feed-ingested foreign DNA in pig bodies. Eur Food Res Technol. 2002; 216: 185-192.
- Osei SA, Dei HK, Tuah AK. Evaluation of quality protein maize as a feed ingredient for layer pullet. J Animal Food Sci. 1999; 8: 181-189.
- ILSI. ILSI Crop composition database Version 5.1 International Life Sciences Institute. Washington DC. 2013.
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 3rd Edition Wordsworth Publishers. 2003; 134-156.
- Rudan-Tasic D, Klofutar C. Characteristics of vegetable oils of some Slovene manufacturers. Acta Chim Slov. 1999; 46: 511-521.
- Nasir M. Characterization of maize germ from various hybrids and use of its components for value added baked products. Thesis Faisalabad. 2009.
- Hsu SY, Yu SH. Comparisons on 11 plant oil fat substitutes for low-fat kung-wans. J Food Eng. 2002; 51: 215-220.
- CRA (Corn Refiners Association). Corn oil 5th Edition. Corn refiners association. Washington D.C., USA. 2006.
- Nasir M, Butt MS, Anjum FM, Jamil A, Ahmad I. Physical and sensory properties of maize germ oil fortified cakes. Int J Agric Biol. 2009; 11: 311-315.
- Codex standard for named vegetable oils. CX-STAN 210-1999. 2001; 8: 11-25.
- Onyeike EN, Juliana UO. Influence of heat processing methods on the nutrient composition and lipid characterization of groundnut (Arachis hypogaea) seed pastes. Biokemistri. 2003; 15: 34-43.
- Leibovitz Z, Ruckenstein C. Our experiences in processing maize (Corn) germ oil. J Am Oil Chem Soc. 1983; 60: 347-351.
- Sabah EL-Kheir MK, Alamin AA, Sulafa HN, Ali AK. Composition and quality of six refined edible oils in Khartoum state, Sudan. ARPN J Sci Technol. 2012; 2: 177-181.
- Katade S, Deshmukh M, Phalgune U, Biswas S, Deshpande N. Screening of Sterculia guttata seeds along with extraction and characterization of seed Oil. Asi J Chem. 2008; 20: 357-360.
- Abdulkadir M, Abubakar GI. Production and refining of corn oil from hominy feed a by-product of dehulling operation. ARPN J Eng App Sci. 2011; 6: 22-28.
- Mohamed MI, Hamza ZU. Physicochemical properties of oil extracts from Sesamum indicum L seeds grown in Jigawa state-Nigeria. J Appl Sci Environ Manage. 2008; 12: 99-101.
- Diaz MF, Hernandez R, Martinez G, Vidal G, Gomez M, Fernandez H, et al. Comparative study of ozonized olive oil and ozonized sunflower oil. J Braz Chem Soc. 2006; 17: 403-407.
- Zeb A, Mehmood S. Carotenoids contents from various sources and their potential health applications. Pakistan J Nutr. 2004; 3: 199-204.
- Watson SA. Corn: Amazing maize General properties in CRC Handbook of processing and utilisation in agriculture, Volume II: Part 1 Plant Products. I.A. Wolffe (Ed). CRC Press, Inc. Florida. 1982.
- Juyoung K, Deok NK, Sung HL, Sang-Ho Y, Suyong L. Correlation of fatty acid composition of vegetable oils with rheological behavior and oil uptake. Food Chem. 2010; 118: 398-402.
- Carlsson AS, Clayton D, Salentijn E, Toonen M. Oil crop platforms for industrial uses: outputs from the EPOBIO project. 2007.
- Murphy DJ. Designer oil crops. VCH Press, Weinheim. 1994.
- Murphy DJ. Manipulation of oil crops for industrial applications. CABI Press UK. 2010; 183-206.
- Murphy DJ. Plant Breeding and Biotechnology: Societal Context and the Future of Agriculture. Cambridge University Press. 2007.
- Voelker TA, Hayes TR, Cranmer AM, Turner JC, Davies HM. Genetic engineering of a quantitative trait: metabolic and genetic parameters influencing the accumulation of laurate in rapeseed. Plant J. 1996; 9: 229-241.
- Crevel RWR, Kerkhoff MAT, Koning MMG. Review: Allergenicity of refined vegetable oils. Food Chem Toxicol. 2000; 38: 385-393.
- Herman EM. Plant culture: Searching questions; genetically modified soybeans and food allergies. J Exp Bot. 2003; 54: 1317-1319.