
Research Article
Austin Food Sci. 2016; 1(6): 1028.
Epidemiological Investigation of a Powdered Infant Formula Product Batch Contaminated with Cronobacter in a Swiss Infant Formula Production Facility
Stoller A¹, Stephan R¹, Fricker-Feer C² and Lehner A¹*
¹Institute for Food Safety and Hygiene, University of Zurich, Switzerland
²Quality Assurance and Food Safety Department, Hochdorf Swiss Nutrition Ltd, Switzerland
*Corresponding author: Lehner A, Institute for Food Safety and Hygiene, University of Zurich, Switzerland
Received: November 03, 2016; Accepted: November 25, 2016; Published: November 30, 2016
Abstract
In this study, we report on the epidemiologic investigation in a Swiss powdered infant formula production facility after a batch of Powdered Infant Formula (PIF) was found to be contaminated with Cronobacter (C.) sakazakii during a routine testing of packed products of a Swiss PIF brand ready for distribution. Epidemiological investigation on isolates originating from PIF batches from the production unit by macro restriction typing quickly (PFGE) identified an isolate showing a pattern identical to the ones originating from the packed product, suggesting this strain being the source of contamination. To obtain an overview on the heterogeneity of strains isolated within this production unit 105 C. sakazakii isolates, which were routinely collected between August 2015 and September 2016, were characterized by PFGE and serotyping. In addition, Multilocus Sequence Typing (MLST) was performed on 11 selected isolates. Macro restriction analysis revealed the presence of two main clusters (C1,C2) containing multiple isolates from various sources and time points with the oldest isolates dating back to October and August 2015 respectively.
Of interest, within both clusters isolates were identified showing identical PFGE patterns but different serotypes. Moreover, MLST analysis on selected isolates revealed that isolates showing identical PFGE types may exhibit different MLSTs and/or serotypes. Our data suggest that application of one of the latter two typing methods bears the risk that strains may incorrectly be considered “epidemiologically unrelated”. Based on these results the application of PFGE using the primary (and the secondary) restriction enzyme is recommended for trace back studies.
Keywords: Cronobacter sakazakii; Contaminated powdered infant formula product; PFGE; MLST; Serotyping; Epidemiological trace back
Abbreviations
PFGE: Pulsed Field Gel Electrophoresis; MLST: Multilocus Sequence Typing; PIF: Powdered Infant Formula; ESIA: Enterobacter Sakazakii Isolation Agar; UV: Ultra Violet; CCD: Charged Coupled Device; TIFF: Tagged Imaged File Format; UPGMA: Unweighted Pair Group Method With Arithmetic Mean Alegorithm
Introduction
Cronobacter (C.) spp. are opportunistic foodborne pathogens that are gaining attention for their ability to cause Infections including meningitis, septicemia, necrotizing enterocolitis and pneumonia in neonates (infants of 28 days or younger) and premature babies but has also been known to cause disease in adults, most notably in elderly and immuno-compromised individuals [1-6]. Infections in infants have been epidemiologically linked to consumption of intrinsicallyand extrinsically-contaminated batches of temperature-abused and reconstituted Powdered Infant Formula (PIF) [7].
Cronobacter can survive under extreme desiccation (high osmotic stress) conditions and it is thought that this property contributes to its persistence in powdered infant formula factories, dried products and dry environments [8,9]. The genus comprises seven species-C. sakazakii, C. malonaticus, C. turicensis C. universalis, C. condimenti, C. muytjensii and C. dublinensis - all of which, except C. condimenti, have been reported to cause infections in humans [10,11]. C. sakazakii is by far the most frequently isolated species from patients as well as from PIF products and production environments [12,13].
The presence of these organisms in infant formula products represents a challenge for the PIF producing industry. Thus, the specific and accurate identification of the members of the pathogenic genus Cronobacter spp. and its discrimination from closely related, non-pathogenic organisms which may be present in the same habitat (products, environment) is critical. Inclusion of molecular identification methods into the cultural detection and identification procedure significantly improved the measures for the control of these organisms [14-17]. The risk posed by contaminated infant formula for consumption by neonates raises the question on the identification of the possible origin/routes of dissemination/transmission of these organisms into/within the infant formula processing environment and/or final products. Two possible routes have been described for dissemination of Cronobacter spp. into production lines and recontamination of pasteurized products. Organisms may either be attached to dust or to dry heat labile supplement ingredients [18-20].
Within the current study we report on the epidemiological investigation on C. sakazakii strains isolated from a PIF production facility after a batch of final products ready for distribution was found to be contaminated. Three different typing methods were applied in order to elucidate the source of contamination and to answer the question on the dissemination and persistence of strains within the production unit over a time period of 14 months. The study revealed that serotyping but also MLST is only of limited use and that PFGE using two different enzymes may be still the method of choice for epidemiological trace back studies within a plant and/or along the production process.
Materials and Methods
Strains
In June 2016, four presumptive Cronobacter spp. isolates (three from packed products, one out of vacuum cleaner bag) were identified after routine control of a packed product of PIF in the distribution unit of a Swiss PIF brand by a private laboratory. The bacteria, streaked on ESIA (Oxoid) [21] plates and showing the typical blue/ green colour were forwarded to the Institute for Food Safety and Hygiene for further investigation.
For trace back studies, cryo-preserved presumptive Cronobacter spp. isolates, collected in the production facility where the PIF batch in question was manufactured were included in the study. These isolates originated from the hygienic monitoring program performed in this facility between August 2015 and September 2016. The strain collection comprised isolates from production environment (walls, floors, vacuum cleaners, filters and drains) and finished products (powdered infant formula (stage 1), follow on formula (stage 2), growing up formula (stage 3)).
Genus and species identification
Strains from ESIA plates and cryo-preserved strains were streaked on blood agar plates and incubated for 24 h at 37 °C and colony material was used for further analysis. Isolates were genus and species identified by PCR according to the methods by Lehner, et al. [15], Stoop, et al. [16] and Lehner, et al. [17] respectively on bacterial lysates [13].
PFGE
PFGE analyses was carried out on all C. sakazakii isolates identified in this study (n=105) following the method described by Iversen, et al. [19]. The DNA of strains was digested with XbaI or SpeI and separated on a CHEF-DR III sytem (Bio-Rad) using the parameters described by Iversen, et al. [19] and Muller, et al. [13]. Macro-restriction patterns were photographed under UV light using a CCD photography system (Bio-RAD, Hercules, CA) from which Tagged Image (TIFF) files were imported into Gel Compar II software version 5.1 (Applied-Maths, Sint-Martens-Latem, Belgium). Dendrogram calculation and cluster analysis of the PFGE patterns was accomplished using the Jaccard index and the Unweighted Pair Group Method with Arithmetic mean (UPGMA). Optimization and band position tolerance of 3% were chosen. The relatedness of patterns was compared at 95% similarity.
Serotyping PCR
The PCR-based O-antigen serotyping scheme proposed by Sun, et al. [22] was applied to type C. sakazakii isolates as described previously [13].
MLST
Multi locus sequence typing was used as a molecular technique to further characterize selected C. sakazakii (n=11) following the protocol described by Joseph, et al. [12]. The strains included represented isolates from clusters containing multiple clonal pulso-types. Sequencing was outsourced (Microsynth, Balgach and Switzerland). Sequence types were determined by using the Cronobacter MLST website (https://pubmlst.org/cronobacter/) [23].
Results and Discussion
In June 2016, we received 3 samples with bacteria grown on Cronobacter selective (ESIA) plates which were isolated out of a batch of powdered infant formula during a routing testing for contaminations of packed products ready for distribution. The isolates were confirmed as C. sakazakii and PFGE typing using the primary enzyme XbaI revealed that the patterns of these isolates were undistinguishable. A fourth isolate which was obtained out of a vacuum cleaner bag used in the packaging unit of the distribution facility was sent to our laboratory a few weeks later and was also confirmed as C. sakazakii. However, the PFGE pattern was different from the ones obtained from the products. The results on this analysis are given in (Figure 1A).

Figure 1A: Results on the 4 C. sakazakii isolates obtained from final products and the environment in the packaging unit. Combined results on: PFGE (XbaI),
patterns sampling codes, sampling date, sampling source, serotype and MLST.

Figure 1B: Same isolates as A but including an isolate from a contaminated batch of PIF products obtained from the production unit and most probably being
responsible for the contamination of the final product. Combined results on: PFGE (XbaI), patterns sampling codes, sampling date, sampling source, serotype and
MLST.
In order to elucidate the contamination source isolates originating from several lots of PIF that were manufactured in the respective production unit within the questionable time period were analysed and confirmed C. sakazakii isolates were typed by PFGE. By this we were able to identify one isolate (HOSU_AS16_86) out of a batch showing an identical pulso-type to the ones from the isolates identified from the final product, thus strongly suggesting that the contamination most probably arose at some point during the production of the respective PIF batch (Figure 1B). In order to obtain an overview on the heterogeneity of strains isolated within this production unit over a longer period of time it was decided to perform a survey on isolates obtained from various sources (products and environmental samples) during a period of 14 months. Presumptive isolates (on ESIA) that had been collected between August 2015 and September 2016 during the routine hygiene monitoring implemented in this production unit were analysed and confirmed C. sakazakii isolates were further subtyped by PFGE and serotyping. Of 106 Cronobacter spp. isolates 105 were identified as C. sakazakii and one as C. malonaticus. Thus C. sakazakii was the most common species identified in this study (99%). This is in concordance to previous studies [13,19,20]. The C. sakazakii isolates were subjected to PFGE analysis using the primary enzyme XbaI and these clustered to form 28 distinguishable pulsotypes at a cut off value of 95% pattern similarity. The pulso-types of the isolates and their relationship between the sampling sources and the sampling dates are depicted in (Figure 2). Amongst several smaller clusters (containing 1-6 isolates) two dominant clusters (cluster 1 (C1), cluster 2 (C2)) both containing 31 isolates the pulso-types of which were undistinguishable from each other were determined. Moreover, the pulso-type of the isolates observed in C1 was identical to the one identified in the contaminated PIF sample from the packed product in the packaging/distribution facility (Figures 1A,1B and Figure 2). For both clusters, clonal isolates included samples from different time points and sampling sources (products and environmental samples). Most striking, the oldest isolates, both of a products, dated back to October (HOSU_AS16_102) and August 2015 (HOSU_AS16_94) suggesting a selection for two persistent strains within this production unit. Serotping was performed for all strains and these results are also included in (Figure 2). Interestingly, it was observed that isolates exhibiting undistinguishable pulsotypes may be of different serotype (Figure 1B). This applied e.g. for the isolates HOSU_AS16_103=O2, HOSU_AS16_104=O2, HOSU_ AS16_105=O2, HOSU_AS16_102=O2 and HOSU_AS16_86=O7. In order to shed light on this finding, MLST was applied on these strains. Surprisingly, MLST data were not consistent among isolates from this cluster either. Isolate HOSU_AS16_102 showed to be ST 4, clonal complex 4 whereas the other 3 isolates were ST 83 clonal complex 65 (Figure 3A). To substantiate this finding, we decided to perform MLST analysis also on 5 randomly selected isolates (HOSU_ AS16_47, HOSU_AS16_50, HOSU_AS16_81 HOSU_AS16_84, and HOSU_AS16_94) from pulso-type cluster 2. The results, shown in (Figure 3C), supported the previous finding. The MLST typing results suggested that isolates showing undistinguishable pulso-types may indeed be different from each other. In practice, in epidemiological investigations using PFGE typing it is suggested to PFGE type isolates showing identical patterns obtained with the primary restriction enzyme with a second enzyme e. g. SpeI. Thus, two sets of isolates from the two main clusters were subjected to PFGE typing using SpeI. The results are shown in (Figures 3B and 3D). In the case of the isolates from cluster 1 the PFGE profiles remained undistinguishable (Figure 3B) after application of the second restriction enzyme. Interestingly, after this analysis one of the isolates of the cluster 2 subset of isolates (HOSU_AS_84S) indeed exhibited a minor change in the pattern (Figure 3D). This pattern change was not seen in isolate HOSU_AS16_81S, which also exhibits ST 4. However, the minor (2 bands) change observed in the pattern of HOSU_AS_84S would not change the overall judgement on the “epidemiological relatedness” of the investigated strains (Figure 3D) [24]. PFGE is a well established mean of profiling bacterial strains for epidemiological purposes and it has been shown that PFGE may distinguish between strains within the same sequence type [25]. This is also the case in our study e.g. for HOSU_AS16_102, HOSU_AS16_81 and HOSU_AS16_84 which are of ST 4 but show distinctive pulsotypes. However, we also observed the opposite, namely isolates of undistinguishable pulsotype which exhibited different sequence types (Figures 3A and 3C). Interestingly, such data can also be found in the recent study by Fei, et al. [25]. However, this finding was not further discussed by the authors but instead ‘‘a stepwise analysis by MLST followed by PFGE may be suitable for comprehensive profiling of Cronobacter isolates’’ was suggested in this study.
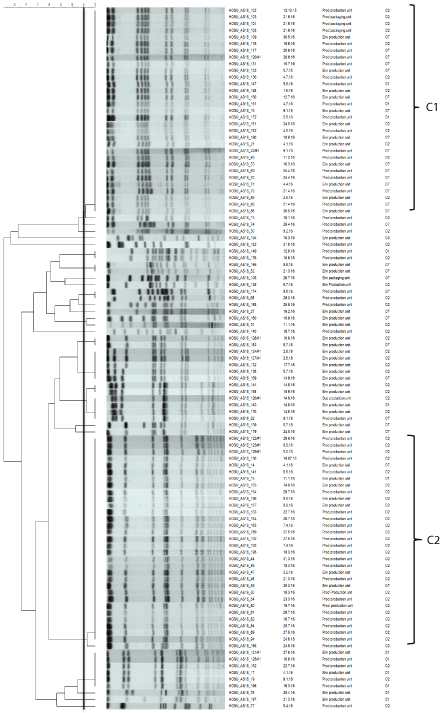
Figure 2: Results on all 105 C. sakazakii isolates included in the study. Dendrogram, PFGE (XbaI) patterns sampling codes, sampling date, sampling source,
serotype and MLST. Cluster C1 and C2 are marked. The line drawn marks the 95% pattern similarity cut-off.

Figure 3A: Combined results on the C. sakazakii isolates out of final product (packaging unit), the respective isolate from the production unit and the “oldest” isolate
identified in pulsotype cluster 1. PFGE; (XbaI) patterns sampling codes, sampling date, sampling source, serotype and MLST.

Figure 3B: Same strains as 3 A. PFGE patterns obtained with restriction enzyme SpeI.

Figure 3C: Combined results on 5 selected isolates from pulso-type cluster 2. PFGE; (XbaI) patterns sampling codes, sampling date, sampling source, serotype
and MLST.

Figure 3D: Same isolates as 3 C. PFGE patterns obtained with restriction enzyme SpeI. The profile of isolate HOSU_AS16_84S differs in 2 bands from the other
isolates.
One explanation for the discrepancies observed between the two typing results may lay in the fact that MLST was originally developed to obtain information on the phylogenetic relationship among strains while PFGE aims to answer the question on whether strains are “epidemiologically related” [24].
Thus, for source tracking purposes the application of PFGE is still recommended as the method of choice which will help to identify contamination routes and/or the nature of persisting strains which may help to limit the risk for contaminations in products.
References
- Yan QQ, Condell O, Power K, Butler F, Tall BD, Fanning S. Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: a review of our current understanding of the biology of this bacterium. J Appl Microbiol. 2012; 113: 1-15.
- Jaradat ZW, Al Mousa W, Elbetieha A, Al Nabulsi A, Tall BD. Cronobacter, an opportunistic food borne pathogen; a review of its virulence and environmental adaptive traits. J Med Microbiol. 2014; 63: 1023-1037.
- Tall BD, Chen Y, Yan QQ, Gopinath GR, Grim CJ, Jarvis KG, et al. Cronobacter: an emergent pathogen ausing meningitis to neonates through their feeds. Sci Prog. 2014; 97: 154-172.
- Patrick ME, Mahon BE, Greene SA, Rounds J, Cronquist A, Wymore K, et al. Incidence of Cronobacter spp. infections, United States. 2003-2009.
- Gosney MA, Martin MV, Wright AE, Gallagher M. Enterobacter sakazakii in the mouths of stroke patients and its association with aspiration pneumonia. Europ J Intern Med. 2006; 17: 185-188.
- Holy O, Petrzelova J, Hanulik V, Chroma M, Matouskova I, Forsythe SJ. Epidemiology of Cronobacter spp. Isolates from patients admitted to the Olomouc University Hospital (Czech Republic). Epidemiol Mikrobiol Imunol. 2014; 63: 69-72.
- Hunter CJ, Petrosyan M, Ford HR, Prasadarao NV. Enterobacter sakazakii: an emerging pathogen in infants and neonates. Surg Infect. 2008; 9: 533-539.
- Osaili TM, Shaker RR, Al-Haddaq MS, Al-Nabulsi AA, Holley RA. Heat resistance of Cronobacter species (Enterobacter sakazakii) in milk and special feeding formula. J Appl Microbiol. 2009; 107: 928-935.
- Osaili T, Forsythe S. Desiccation resistance and persistence of Cronobacter species in infant formula. Int J Food Microbiol. 2009; 136: 214-220.
- Iversen C, Mullane N, McCardell B, Tall BD, Lehner A, Fanning S, et al. Cronobacter gen. nov., a new genus to accommodate the biogroups of Enterobacter sakazakii and proposal of Cronobacter sakazakii gen. nov., comb. nov., Cronobacter malonaticus sp. nov., Cronobacter turicensis sp. nov., Cronobacter muytjensii sp. nov., Cronobacter dublinensis sp. nov., Cronobacter genomospecies 1 and of three subspecies, Cronobacter dublinensis subsp. dublinensis subsp. nov., Cronobacter dublinensis subsp. lausannensis subsp. nov. and Cronobacter dublinensis subsp. lactaridi subsp. nov. Int J Syst Evol Microbiol. 2008; 58: 1442-1447.
- Joseph S, Cetinkaya E, Drahovska H, Levican A, Figueras MJ, Forsythe SJ. Cronobacter condimenti sp. nov., isolated from spiced meat and Cronobacter universalis sp. nov., a species designation for Cronobacter sp. genomospecies 1, recovered from a leg infection, water and food ingredients. Int J Syst Evol Microbiol. 2012; 62: 1277-1283.
- Joseph S, Sonbol H, Hariri S, Desai P, MacClelland M, Forsythe SJ. Diversitiy of the Cronobacter genus as revealed by multi locus sequence typing. J Clin Microbiol. 2012; 50: 3031-3039.
- Muller A, Stephan R, Fricker-Feer C, Lehner A. Genetic diversity of Cronobacter sakazakii isolates collected from a Swiss infant formula production facility. J Food Prot. 2013; 76: 883-887.
- Iversen C, Lehner A, Mullane N, Marugg J, Fanning S, Stephan R, et al. Identification of “Cronobacter” spp. (Enterobacter sakazakii). J Cli Microbiol. 2007; 45: 3814-3816.
- Lehner A, Nitzsche S, Breeuwer P, Diep B, Thelen K, Stephan R. Comparison of two chromogenic media and evaluation of two molecular based identification systems for Enterobacter sakazakii detection. BMC Microbiol. 2006; 6: 15.
- Stoop B, Lehner A, Iversen C, Fanning S, Stephan R. Development and evaluation of rpoB based PCR systems to differentiate the six proposed species within the genus Cronobacter. Int J Food Microbiol. 2009; 136: 165- 168.
- Lehner A, Fricker-Feer C, Stephan R. Identification of the recently described Cronobacter condimenti by an rpoB-gene-based PCR system. J Med Microbiol. 2012; 61: 1034-1035.
- Mullane NR, Whyte P, Wall PG, Quinn T, Fanning S. Application of pulsed-field gel electrophoresis to characterize and trace the prevalence of Enterobacter sakazakii in infant formula processing facility. Int J Food Microbiol. 2007; 116: 73-81.
- Iversen C, Lehner A, Fricker-Feer C, Gschwend K, Stephan R. Genotyping of Cronobacter (Enterobacter sakazakii) strains from an infant formula processing plant. Arch Lebensmittelhyg. 2009; 60: 66-72.
- Lehner A, Fricker-Feer C, Gschwend K, Stephan R. Identification of Enterobacteriaceae and spp. in raw milk concentrate and milk powder: prevalence and genotyping. Arch Lebensmittelhyg. 2010; 61: 22-26.
- Anonymous. Milk and milk products-detection of Enterobacter sakazakii. Technical specification. 2006.
- Sun Y, Wang M, Wang Q, Cao B, He X, Li K, et al. Genetic analysis of the Cronobacter sakazakii O4 to O7 O-antigen gene clusters and development of a PCR assay for identification of all C. sakazakii O serotypes. Appl Environ Microbiol. 2012; 78: 3966-3974.
- Jolley KA, Chan M, Maiden MCJ. mlstdbNet-distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics. 2004; 5: 86.
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsedfield- gel-electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995; 33: 2233-2239.
- Fei P, Man C, Lou B, Forsythe SJ, Chai Y, Li R, et al. Genotyping and source tracking of Cronobacter sakazakii and C. malonaticus isolates from powdered infant formula and an infant formula production factory in China. Appl Environ Microbiol. 2015; 81: 5430-5439.