
Research Article
Austin Food Sci. 2017; 2(1): 1029.
Role of Steam Blanching and Vacuum Packaging on the Physical and Microbiological Quality of Fresh Vegetable Soybean (Edamame) During Storage
Kim C¹*, Torres A², Xu Y¹, Kaseloo P2, Nguyen L3, Awan Z3, Rutto L1, Sismour E1, Jiang GL1, Kering M1, Wynn C3, Stein R1 and Pao S4
1Agricultural Research Station, Virginia State University, USA
2Department of Biology, Virginia State University, USA
3Department of Family and Consumer Sciences, Virginia State University, USA
4Department of Food Science and Nutrition, California State University, Fresno, USA
*Corresponding author: Kim C, Agricultural Research Station, Virginia State University, USA
Received: November 16, 2016; Accepted: January 16, 2017; Published: January 23, 2017
Abstract
The large-seeded green vegetable soybean, edamame, is considered to be a super food due to its many health benefits and it has been gaining popularity among farmers as a promising cash crop. However, its high perishability presents challenges to marketing fresh edamame. This study investigated the use of the combination of steam blanching, vacuum packaging and cold storage for prolonging the physical and microbiological quality of edamame. Steam blanching for 30s at 90°C did not significantly change the hardness of whole pod. The green color intensity of whole edamame pods and beans had significantly increased by 35.5% and 28.3%, respectively. Steam blanching whole pods significantly reduced the total counts of aerobic mesophiles (>5.1 log CFU/g), yeasts and molds (>4.3 log CFU/g), psychrotrophs (>4.0 log CFU/g) and no significant increase in overall microbial counts was observed during 4°C refrigeration for <9 days. Residual aerobic mesophile and psychrotroph counts (=2.0 and =1.0 log CFU/g, respectively) on treated samples stored at room temperature (22°C) increased to >7.2 log CFU/g within 3 days. The current study demonstrated that cold storage at 4°C effectively prevented the proliferation of microorganisms on treated samples, but it also showed that microbial counts can rebound on treated samples at 22°C. The combination method used in this study is environmentally friendly and easily adaptable to a small farm setting, giving it a great deal of potential to benefit small farmers who wish to increase profitability by marketing their fresh edamame as an alternative crop.
Keywords: Edamame; Steam blanching; Vacuum packaging; Physical quality; Microbiological quality
Introduction
The 2013 market for edamame was valued at approximately $175 to $200 million [1]. Each year, Americans consume approximately 25,000 to 30,000 tons of edamame, most of which is imported from Asia frozen [2]. However, Kaiser and Ernst [3] reported that restaurants and wholesale outlets prefer to purchase fresh edamame. Most of the U.S. crop is produced in the West and Upper Midwest, but efforts have been made in eastern states to produce edamame to be sold fresh. Scientists [4-6] at Virginia State University have successfully developed three edamame cultivars that are suitable to Virginia growing conditions (Asmara, Randolph and Owens) these have been registered with USDA [7-9].
Edamame is a large-seeded green vegetable soybean Glycine max (L.) Merr. That is emerging as a promising cash crop for Virginia farmers, especially in the wake of declining tobacco production in the state. The reported health benefits of edamame and its characterization as a “super food” have contributed to its rise in popularity among American consumers, creating a potential niche market for small scale producers. However, issues related to the small harvest window and the short shelf life of the product present challenges in marketing edamame.
Edamame is highly perishable and the greatest challenge to its further commercialization is maintaining post-harvest shelf-life and minimizing the physicochemical and microbial deterioration that causes degradation of its color, texture and flavor [10,11]. Several studies have found no conventional washing methods capable of reducing microbial populations by more than 90 to 99 percent [12- 18]. Our previous study [19] found that blanching edamame in boiling water and storing at either 4°C or -20°C significantly preserved green color intensity and bean hardness. In addition, Gorris and Peppelenbos [20] found that vacuum packaging reduces oxidation and slows down the metabolic activity of microorganisms by limiting their oxygen supply, thus preserving nutritional value, flavor and overall quality. Because blanching in boiling water calls for high levels of water use, steam blanching may be an effective alternative that conserves both energy and water. There is no current research assessing the physical and microbiological quality of edamame that has been subjected to a regimen of steam blanching, vacuum packaging and storage at refrigeration temperature.
This study therefore aimed to evaluate the combination of steam blanching, vacuum packaging and cold storage in an effort to prolong the physical and microbiological quality of fresh edamame.
Materials and Methods
Sample preparation
Fresh edamame (var. ‘Asmara’) was harvested in the fall 2015 from Virginia State University’s Randolph Farm (Ettrick, VA). Harvest began when pods were approximately 85% full at the R6 growth stage and they were separated from the vines utilizing an edamame thresher (Model KE-6, Mitsuwa Co., Ltd., Niigata and Japan). Edamame pods were transported to the laboratory within 2h of harvest, stored at room temperature (22±2°C) until hand-sorted within 24h. Sterile gloves were used while removing debris and oneseed pods and selecting pods of uniform shape, color and size and that were free of defects.
Sample treatment
Several scientists [21-24] recognized washing as a significant postharvest treatment that may effectively reduce microbial counts on products. However, due to the unique characteristic of hairy surfaces on the edamame pods, our previous findings show that rinsing raw edamame in sterile tap water or 20ppm chlorine dioxide for 2min did not yield any significant reduction of naturallyoccurring microorganisms. Consequently, we decided to investigate blanching as a means to reduce the microbial load. Blanching was conducted using a commercially available household kitchen steamer (Rival Auto-Timer Food Steamer, Model CKRVSTLM21, Boca Raton, FL) fitted with a custom-made screen that was placed between upper and lower bowls (Figure 1). Approximately 600ml of tap water was added to the steamer’s base compartment before unit was heated to 90±2°C, as measured by a thermocouple connected to a thermometer (Traceable Infrared Dual Lasers Thermometer, Model S02273, Control Co., Friendswood, TX). Upon reaching the target temperature, each sample portion (50g per treatment) of edamame pods were placed on the screen in the unit and steamed for 30s. The Steamed Samples (S) were either immediately Vacuum Packaged (V) in heat-sealable Food Saver bags (17×28 cm in dimension, Jarden Consumer Solutions, Boca Raton, FL) using a commercially available household Food Saver vacuum sealing system (V2200 Series, Jarden Consumer Solutions) or Cooled (C) in a laminar flow hood (Purifier Class II Biosafety Cabinet, Lanconco, Kansas City, MI) on a sterilized mesh rack for 10min and then vacuum packaged as described above. Additionally, our prior study showed that vacuum packaging alone did not yield any significant reduction of the level of microorganisms; therefore, the treatment of vacuum packaging alone was not included in this study. Samples subjected to the combinations of steam blanching and vacuum packing and steam blanching, cooling and vacuum packaging are referred to as SV and SCV, respectively, for description purposes herein.

Figure 1: Treatment of steam blanching on edamame.
Sample storage
Samples were stored at either room temperature (22±2°C) or refrigerated (4°C). The physical qualities of refrigerated edamame whole-pod and edamame shelled bean samples were evaluated at day 9, 15 and 30, while samples stored at room temperature were evaluated at day 1 and 3. Microbiological qualities of wholepod and shelled bean samples were evaluated at day 1, 3, 9, 15 and 30. In addition, the immediate effects of steam treatment on the physical and microbiological quality were evaluated at day 0. At each evaluation point, the whole-pod sample and the shelled bean sample were each split in half (25g) and one half of each was used for physical quality analysis and the other half for microbial quality analysis. Raw samples stored in 4°C and 22°C without the vacuum sealing were used as controls.
Peroxidase assay
Peroxidase (POD) is one of the most heat stable enzymes and a popular blanching indicator present in vegetables. Therefore, the POD of edamame shelled beans was determined for the extent of heat transmission via steam blanching. The assay was conducted following the procedure reported in Xu [19]. In brief, crude enzyme extraction was carried out by grinding and homogenizing 2g of shelled beans with 10ml of chilled 1 M phosphate buffer solution at 4°C in a laboratory blender (Masticator Silver, IUL Instruments, Barcelona, Spain) for 2min. The homogenate was filtered through cheesecloth and centrifuged at 20,000×g for 30 min to obtain the crude enzyme extract. The POD activity was measured spectrophotometrically using the method as illustrated in Sheu and Chen [25].
Moisture analysis
The moisture content was determined using a moisture analyzer (Model MA35, Satorius, Goettingen, Germany) only for the edamame beans. Each homogenized sample (2g) was evenly placed on the sample pan inside the drying chamber and the analysis was conducted for 10min reaching final temperature at 130°C. The value of moisture contents (%) represents the average of three respective readings.
Hardness analysis
The hardness of whole edamamepods and edamameshelled beans was measured using a texture analyzer (TA.XT2 Texture Technologies, Scarsdale, NY) fitted with a 50kg load cell and using a 2mm diameter stainless steel punch probe (TA-52). Specimens were positioned on a perforated plate (5mm diameter) anchored to a raised platform to permit complete penetration by the probe. The probe was lowered automatically to a pre-set force of 15g (0.147 N) and the position and orientation of the specimen was adjusted so that the probe penetrated each specimen at approximately a perpendicular angle to the seam of whole pods or to the seam between cotyledons. Testing was conducted at a speed of 1mm/s over a distance of 10mm. The maximum peak height of the force-deformation curve was used as the hardness criterion. Ten respective readings were taken and the average of these readings was used to represent the respective sample bag.
Color measurement
The color of whole edamame pods and edamame shelled beans was determined using a Konica Minolta CR-400/410 Chromameter (Minolta Camera Co., Ltd, Osaka, Japan) referenced to the L*a*b* color space, where L* indicates lightness from 0 (black) to 100 (white), while a* and b* indicate chromaticity coordinates denoting the red-to-green color axis and the yellow-to-blue color, respectively. Color measurements were obtained for ten whole edamame pods and for ten shelled beans. The average of these measurements was used to represent the respective sample bag. The meter was standardized to a white calibration plate (L* = 93.6, a* = 0.6 and b* = 2.3) prior to initial use for each evaluation interval. The intensity of green color was calculated as -a/b [19].
Microbiological quality
Microbial testing procedures previously reported by Pao [26] were used. In brief, sealed bags were opened for sampling by spraying one corner with 70% ethanol and air-drying. The sanitized bag was then cut with flame-sterilized scissors. Sample portions (25g) were homogenized in 225ml of sterile peptone water (0.1%) using a laboratory blender (Masticator Silver) at high speed for 2min. Appropriate dilutions of the homogenate were surface-plated using Standard Method Agar (SMA; unless otherwise stated, all media were Bacto, from Becton Dickinson, Sparks, MD) for total aerobic mesophile counts after incubation at 36°C for 48h and psychrotroph plate counts after incubation at 7°C for 7 days. Yeast and mold counts were determined using Acidified Potato Dextrose agar after incubating at 25°C for 5 days. The detection limit for each microbial count was 10 CFU/g.
Statistical analysis
Each analysis was carried out induplicate. The data obtained from samples of each treatment were subjected to analysis of variance and Duncan’s multiple range tests (SAS Institute, Cary, NC) to determine if significant difference (P=0.05) existed between mean values of treatments.
Results and Discussion
Results obtained in our study on shelled edamame bean subjected to steam blanching for 30 s at 90°C demonstrated only a 36.7% loss (data not shown) in Peroxidase (POD) enzyme activity compared to 98% and 90% reduction after blanching edamame in boiling water for 2.5min [19] and 1.1min [25] indicating that steam blanching for a short time may be less detrimental to the product quality. In other words, steam blanching resulted in less enzyme deactivation indicating that the process is more favorable in terms of keeping the produce fresher and close to a raw stage than blanching the product in boiling water.
Physical quality
Results shown in (Figure 2) revealed that the combination treatments of both SV and SCV reduced the moisture content of shelled beans by 6.9% compared to that of the untreated samples (control); this may be due to the compounded influence of adding heat through steam blanching while also removing moisture through vacuum processing. While the moisture content of control samples stored at 4°C for 30 days decreased from 68.2% to 55.0%, or by 19.4%, each of the samples subjected to SV and SCV treatments decreased from 63.5% by 15.0% and by 4.3%, respectively. In addition, the moisture content of control samples stored at 22°C for 3 days increased by 3.4% while samples subjected to SV and SCV treatments showed a decrease by 2.2% and an increase by 2.2%, respectively. Overall variations and discrepancies in moisture content among control and treated samples, even during storage, could be due to several conditions including the relatively small sampling size (2g), the raw material quality and processing conditions such as manually peeling pods by hand; any of these factors independently or in combination, may have contributed to the difficulty in accurately and consistently measuring the moisture content in samples. Despite variations and discrepancies in the moisture content that appear in our study, treated samples stored at 4°C had equal to or higher moisture content than control samples for up to 30 days of storage.
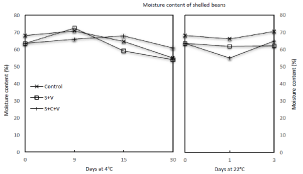
Figure 2: Effect of steam blanching and vacuum packaging on the moisture
content of shelled beans during storage at 4°C and 22°C.
Table 1 illustrates the compounded influence of treatments and storage temperatures on the hardness of whole pods and shelled beans during subsequent storage. For samples stored at 22°C, the experiment was ended after 3 days of storage due to the growth of molds on all samples. Steam blanching did not result in any significant (P=0.05) change in the hardness of whole pods but significantly (P<0.05) reduced the hardness of shelled beans. No significant (P=0.05) difference in the hardness of control or treated whole pods was observed during subsequent 30 and 3 days of storage at 4°C and 22°C, respectively. Although the hardness of the control shelled beans stored at 4°C for 30 days was significantly (P<0.05) reduced by 29.7%, no significant (P=0.05) difference in hardness for either SV-or SCV-treated beans was observed. It was noteworthy that SV-treated beans showed significantly (P<0.05) higher hardness than those of control and SCV-treated beans. In other words, whole pods subjected to additional cooling in a laminar hood were negatively affected in respect to the firmness of beans. Scientists [19,27,28] reported that textural changes in edamame beans during blanching and cooling/drying may be attributed to starch gelatinization, pectin solublization and the retrogradation of bean starch or water loss during refrigeration. In addition, Steinbuch [28] reported that textural changes in vegetables after blanching are affected by the inactivation of pectin esterase.
Sample
Treatment
Storage temp. (°C)
Storage (day)
0
1
3
9
15
30
Pods
C
4
A 1831.6±8.1 a
-
-
B 1557.7±88.6 ab
AB 1639.1±66.8 a
AB 1595.9±134.8 a
22
AB 1831.6±8.1 a
A 1900.3±14.3 a
B 1738.59±49.1 a
-
-
-
SV
4
A 2083.4±896.7 a
-
-
A 1801.5±109.1 a
A 1637.9±19.8 a
A1608.3±39.5 a
22
A 2083.4±896.7 a
A 1442.9±24.7 c
A 1420.1±140.9 b
-
-
-
SCV
4
A 2083.4±896.7 a
-
-
A 1457.1±89.4 b
A 1546.2±236.0 a
A 1526.8±45.4 a
22
A 2083.4±896.7 a
A 1497.7±2.2 b
A 1501.6±10.7 ab
-
-
-
Beans
C
4
A 979.6±27.8 a
-
-
A 876.8±58.8 a
A 878.3±69.5 a
B 688.5±23.7 b
22
A 979.6±27.8 a
A 1089.5±100.8 a
A 949.4±128.2 a
-
-
-
SV
4
AB 819.6±56.4 b
-
-
B 725.5±79.7 a
AB 854.9±0.2 a
A 885.35±1.7 a
22
A 819.6±56.4 b
A 929.6±18.3 ab
A 877.5±52.3 a
-
-
-
SCV
4
A 819.6±56.4 b
-
-
A854.3±173.9 a
A 870.38±3.7 a
A686.10±54.4 b
22
A 819.6±56.4 b
A 713.1±136.5 b
A 894.7±49.6 a
-
-
-
a Means preceded by the same uppercase letters in the same row are not significantly different (P>0.05); means followed by the same lowercase letters in the same column within same sample and same storage temperature (due to treatment) are not significantly different (P>0.05).
C: Control; SV: Steam Blanching and Vacuum Packing; SCV: Steam Blanching, Cooling and Vacuum Packaging; -: Not applicable.
Table 1: Effect of steam blanching and vacuum packaging on the hardness (force, gf) of edamame whole pods and shelled beans during 30 days of storage at 4°C and 22°C a.
Table 2 illustrates the compounded influence of treatments and storage temperatures on the green color intensity of whole pods and shelled beans during subsequent storage. Steam blanching resulted in a significant (P<0.05) increase in the green color intensity of whole pod edamame from 0.31±0.01 to 0.42±0.01, or by 35.5%. While no significant (P=0.05) change in the green color intensity was observed in edamame whole pods subjected to SCV treatment and stored at 4°C for =9days, all other pods subjected to either the treatment of SV or SCV and stored at 22°C showed significant (P<0.05) decreases in their green color intensity after storage day 1. A significantly (P<0.05) higher green color intensity was observed in the pods subjected to SCV treatment compared to control samples after 30 days of storage at 4°C. These findings are in agreement with other studies [19,29] that the green color intensity of edamame subjected to blanching increased compared to that of raw edamame, but the intensity decreased with an increase in storage time, due to degradation of chlorophyll. Physical information on control samples stored for >3 days at 22°C was not available due to sample spoilage. During storage for =3 days, the green color intensity of most pods subjected to treatments was not affected by storage temperature (data not shown).
Sample
Treatment
Storage temp. (°C)
Storage (day)
0
1
3
9
15
30
Pods
C
4
A 0.31±0.01 b
-
-
A 0.25±0.01 a
A 0.23±0.02 a
B -0.02±0.06 b
22
A 0.27±0.03 ab
B 0.06±0.01 a
-
-
-
SV
4
A 0.42±0.01 a
-
-
B 0.21±0.05 a
B 0.14±0.03 a
C 0.03±0.05 ab
22
B 0.12±0.08 b
B 0.05±0.01 a
-
-
-
SCV
4
A 0.42±0.01 a
-
-
AB 0.26±0.11 a
B 0.15±0.01 a
B 0.16±0.01 a
22
B 0.32±0.03 a
C 0.10±0.06 a
-
-
-
Beans
C
4
A 0.46±0.02 b
-
-
A 0.48±0.01 a
A 0.46±0.01 a
A 0.45±0.03 a
22
A 0.41±0.08 a
A 0.43±0.07 a
-
-
-
SV
4
A 0.59±0.03 a
-
-
B 0.49±0.01 a
B 0.43±0.03 a
C 0.32±0.05 b
22
B 0.43±0.08 a
B 0.30±0.01 a
-
-
-
SCV
4
A 0.59±0.03 a
-
-
AB 0.5±0.06 a
BC 0.44±0.04 a
C 0.33±0.04 b
22
A 0.53±0.03 a
B 0.31 0.04 a
-
-
-
a Means preceded by the same uppercase letters in the same row are not significantly different (P>0.05); means followed by the same lowercase letters in the same column within same sample and same storage temperature (due to treatment) are not significantly different (P>0.05).
Table 2: Effect of steam blanching and vacuum packaging on the green color intensity (-a/b) of edamame whole pods and shelled beans during 30 days of storage at 4°C and 22°C a.
Results shown in (Table 2) also illustrate that steam blanching significantly (P<0.05) increased the green color intensity of shelled bean edamame from 0.46 to 0.59 by 28.3%. No significant (P=0.05) differences in the green color intensity on control beans were observed during subsequent 30 and 3days of storage at 4°C and 22°C, respectively. The color intensity of beans subjected to either SV or SCV treatment and storage at 4°C was significantly reduced by 16.9% for = 9 days and by 25.4% for = 15 days, respectively. In addition, the green color intensity of beans subjected to either SV or SCV treatment and stored at 22°C was significantly reduced by 49.2% and 47.5%, respectively, which is about the same reduction as those of treated beans stored at 4°C for 30 days, indicating green color intensity in produce was significantly reduced with higher storage temperature. According to the study on the evaluation of the shelf-life of vegetabletype soybean pods, Santana [30] reported that the green color is an indication of the pod quality; to preserve the quality of the product, pods should be stored at 30°C and consumed within 24 h or stored at 7°C for up to 3 days.
Microbiological quality
The total aerobic mesophiles counts (7.1 log CFU/g) from freshly harvested raw edamame as shown in (Figure 3) was somewhat higher than those (6.20 and 6.8 log CFU/g, respectively) reported in our previous findings [19,26]. The results revealed that blanching whole edamame pod with steam at 90°C for 30s resulted in a =5.2 log CFU/g reduction of total aerobic mesophiles. The level (6.9–7.3 log CFU/g) of total aerobic mesophiles present on control samples at both 4°C and 22°C didn’t significantly (P=0.05) change during storage for up to 30 days and 6 days, respectively.
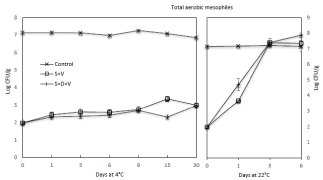
Figure 3: Effect of steam blanching and vacuum packaging on the growth of
total aerobic mesophiles on edamame during storage at 4°C and 22°C.
However, the levels on those edamame pods that were subjected to treatment and rendered to =2 log CFU/g, significantly (P<0.05) increased during 4°C storage at =15 days for SV treatment and at 30 days for SCV treatment. The significant increase of bacterial counts at the later stage of storage is due to some assumptive thermal tolerant bacteria or spores that survived during steam blanching [19]. It is also noteworthy that the level (=2.0 log CFU/g) of residual aerobic mesophiles multiplied to =6.5 log CFU/g at 22°C in 3 days and further reached =7.2 log CFU/g in 6 days. As a result of sample spoilage, microbial counts on blanched samples (either with SV or SCV treatments) stored at 22°C were not available after 6 days.
Due to concerns about microorganisms that may be able to propagate at cold temperatures during refrigerated storage of the samples, the level of psychrotrophs was investigated during subsequent storage. Results in (Figure 4) show that the level of psychrotroph on raw edamame pods was 5.1 log CFU/g and did not change significantly (P=0.05) through the rest of the 30 day storage. Blanching the samples with steam at 90°C for 30s significantly (P<0.05) rendered the level of psychrotrophs to a level below detection (=1.0 log CFU/g). However, a significant (P<0.05) rebound was observed on the SV-treated products stored at 4°C for >9 days and on the SCV-treated products stored for >15 days. At the end of 30 days of storage at 4°C, the level of psychrotrophs on the samples subjected to SV and SCV treatments reached 1.9 and 3.5 log CFU/g, respectively. Similar to the trend of aerobic mesophile counts, storage for =1 day at 22°C following SV and SCV treatments allowed the level of psychrotrophs to rebound to =3.6. It is also observed that the level of psychrotrophs on raw samples significantly increased to =6.6 log CFU/g at 22°C in 3 days and further reached =7.0 log CFU/g in 6 days.
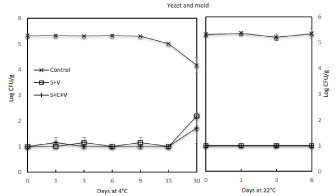
Figure 4: Effect of steam blanching and vacuum packaging on the growth of
psychrotrophs on edamame during storage at 4°C and 22°C.
Figure 5 illustrates the compounded influence of storage temperature and time on yeast and mold counts of whole pod edamame following SV and SCV treatments. Prior to the treatments, freshly harvested raw edamame had yeast and mold counts at 5.3 log CFU/g, which was higher than the 3.5 and 2.8 log CFU/g reported by Xu [19] and Pao [26] respectively. This discrepancy may be attributed to multiple factors including differences in raw material quality, edamame producers, locations where samples were grown, season and weather when samples were obtained. As found in similar results of aerobic mesophiles and psychrotrophs counts, blanching the samples with steam significantly (P<0.05) decreased the level of yeast and mold to =1.0 log CFU/g. No significant (P=0.05) change in yeast and mold levels was found on the samples subjected to SV or SCV treatments and stored at 4°C up to 15 days, but there was a significant (P<0.05) rebound observed on the samples after >15 days of storage. At the end of 30 days of storage at 4°C, their levels on SV and SCV-treated samples reached 2.2 and 1.7 log CFU/g, respectively. During storage at 22°C, the level of yeast and mold on raw and treated samples did not significantly (P=0.05) change by day 6 until visible molds showed on samples and further microbial evaluation was stopped.
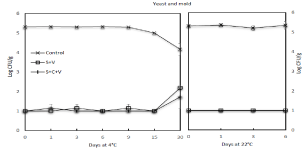
Figure 5: Effect of steam blanching and vacuum packaging on the growth of
yeast and mold on edamame during storage at 4°C and 22°C.
For samples stored at 22°C, the level of aerobic mesophiles and psychrotrophs rebounded to levels (7.4 and 6.5-6.7 log CFU/g, respectively) that were not significantly different (P=0.05) from pretreatment levels (7.1 and 5.1 log CFU/g) after 3 days following the treatments. In other words, the current study demonstrated that cold storage at 4°C is adequate for preventing naturally existing microorganisms from rebounding, whereas leaving treated edamame at ambient temperatures (22°C) may erase the decontamination impact from prior SV and SCV treatments. In summary, this study thus confirms our previous finding [31] that the benefits of unbroken cold-chain systems should be considered in implementing produce safety practices during long term storage, especially for products like edamame that have high water contents. Although findings in our study revealed that SV and SCV treatments resulted in decreased measurements in the green color intensity of pods with the progress of storage time, these decreases were visually imperceptible (Figure 6). Taking this into consideration, along with, the insignificant change in hardness (Table 1) and the improvement in microbial quality (Figures 3-5) between control and treated samples, consumer acceptance may need to be validated via sensory evaluation. Additionally, (Figure 6) shows the inflated package of control samples stored at 4°C for 30 days; this is probably caused by the production of gases associated with sample respiration and metabolic activity of microorganisms that are present on the samples [32,33]. Considering the fact that the current food code requires that refrigerated food items prepared in advance are to be discarded after 7 days [19], the findings in our study are encouraging. Blanching whole pod edamame with steam at 90°C for 30 sec before vacuum packaging can maintain the physical and microbiological quality of the samples without encountering an objectionable deterioration of quality, while also particularly preventing the rebound of microbial populations during subsequent storage at 4°C for =15 days. However, wide standard deviation of color and hardness were observed in edamame subjected to treatments. Additional research and extension efforts are therefore needed to determine the cause (s) of the observed deviations in physical quality and their occurrences before this environmentally friendly technology can be adaptable to a small farm setting; once these issues are addressed this has a great deal of potential to benefit small farmers who wish to increase profitability by marketing their fresh edamame as an alternative crop.

Figure 6: Effect of steam blanching and vacuum packaging on the visual quality of edamame after 30 days of storage at 4°C.
Conclusion
Despite differences in the color and hardness across the replications, use of SV and SCV treatments showed consistent and significant reduction (P<0.05) of microbial counts, indicating their robustness in terms of antimicrobial efficacy. To our knowledge, there is no published literature showing the antimicrobial efficacy of steam blanching and vacuum packaging with subsequent cold storage; these findings may therefore be of significant importance for extending the shelf life of fresh vegetable soybeans.
Acknowledgments
The authors acknowledge the technical advice and/or assistance from Ms. Catherine Baxley, Mr. Jackson Sismour, Ms. Krystal Jordan, and Classes 22 and 24 of the VSU Dietetic Internship program. This article is a contribution of Virginia State University (VSU), Agricultural Research Station (Journal Article Series Number 338) through the funding provided by the USDA Evans-Allen program.
References
- CBS News. Edamame: Is the Future of American soy farmers’ profits in edible soybeans for people. Associated Press. 2013.
- CBS News. Edamame production goes from Asia to Arkansas. CBS Interactive. 2014.
- Kaiser C, Ernst M. Edamame. University of Kentucky College of Agriculture. Food and Environment. 2013.
- Mebrahtu T, Mohamed A, Elmi A. Accumulation of phytate in vegetable-type soybean genotypes harvested at four developmental stages. Plant Foods Hum Nutr. 1997; 50: 179-187.
- Mebrahtu T, Mersie W. Genetic variability for ozone insensitivity in vegetabletype soybean. J New Seeds. 1999; 1: 11-23.
- Young G, Mebrahtu T, Johnson J. Acceptability of green soybeans as a vegetable entity. Plant Foods Human Nutr. 2000; 55: 323-333.
- Mebrahtu T, Devine T, Donald P, Abney T. Registration of ‘Asmara’ vegetable soybean. Crop Sci. 2005; 45: 408-409.
- Mebrahtu T, Devine T, Donald P, Abney T. Registration of ‘Randolph’ vegetable soybean. Crop Sci. 2005; 45: 2644-2645.
- Mebrahtu T, Devine TE, Donald P, Abney TS. Registration of ‘Owens’ vegetable soybean. J Plant Regist. 2007; 1: 95-96.
- Saldivar X, Wang YJ, Chen P, Mauromoustakos A. Effects of blanching and storage conditions on soluble sugar contents in vegetable soybean. LWTFood Sci Technol. 2010; 43: 1368-1372.
- Sugimoto M, Goto H, Otomo K, Ito M, Onuma H, Suzuki A, et al. Metabolomic profiles and sensory attributes of edamame under various storage duration and temperature conditions. J Agric Food Chem. 2010; 58: 8418-8425.
- Bai JH, Saftner RA, Watana AE, Lee YS. Modified atmosphere maintains quality of fresh-cut cantaloupe (Cucumis melo L.). J Food Sci. 2001; 66: 1207-1211.
- Emmambux MN, Taylor JRN. Sorghum ka?rin interaction with various phenolic compounds. J Sci Food Agric. 2003; 83: 402-407.
- Lanciotti R, Corbo MR, Gardini F, Sinigaglia M, Geerzoni ME. Effect of hexanal on the shelf life of fresh apple slices. J Agric Food Chem. 1999; 47: 4769-4776.
- Hadjok C, Mittal GS, Warriner K. Inactivation of human pathogens and spoilage bacteria on the surface and internalized within fresh produce by using a combination of ultraviolet light and hydrogen peroxide. J Appl Microbiol. 2008; 104: 1014-1024.
- Sapers GM. Efficacy of Washing and sanitizing methods for disinfection of fresh fruit and vegetable products. Food Technol Biotech. 2001; 39: 305-311.
- Sapers GM, Simmons GF. Hydrogen peroxide disinfection of minimally processed fruits and vegetables. Food Technol. 1998; 52: 48-52.
- Chapter V. Methods to Reduce/Eliminate Pathogens from Produce and Fresh-Cut Produce. Food and Drug Administration. 2014.
- Xu Y, Sismour E, Pao S, Rutto L, Grizzard C. Ren S. Textural and Microbiological Qualities of Vegetable Soybean (Edamame) Affected by Blanching and Storage Conditions. J Food Process Technol. 2012; 23: 1-6.
- Gorris LGM, Peppelenbos HW. Modified atmosphere and vacuum packaging to extend the shelf life of respiring food products. Hort Technol. 1992; 2: 303- 309.
- Beuchat LR, Nail BV, Adler BB, Clavero MRS. Efficacy of spray application of chlorinated water in killing pathogenic bacteria on raw apples, tomatoes and lettuce. J Food Prot. 1998; 61: 1305-1311.
- Ukuku DO, Davis J. Effects of indigenous surface microflora of cantaloupe and washing treatment on survival and transfer of inoculated Listeria monocytogenes to fresh-cut pieces. IFT Annual Meeting Book of Abstracts. June 23-27; New Orleans (LA). 2001.
- Parnell TL, Harris LJ, Suslow TV. Reducing Salmonella on cantaloupes and honeydew melons using wash practices applicable to postharvest handling, foodservice and consumer preparation. Int J Food Microbiol. 2005; 99: 59-70.
- Pao S, Kelsey DF, Long W. Spray wshing of tomatoes with chlorine dioxide to minimize Salmonella on inoculated fruit surfaces and cross-contamination from revolving brushes. J Food Prot. 2009; 72: 2448-2452.
- Sheu SC, Chen AO. Lipoxygenase as blanching index for frozen vegetable soybeans. J Food Sci. 1991; 56: 448-451.
- Pao S, Ettinger MR, Khalid MF, Mebrahtu T, Mullins C. Microbiological quality of frozen “edamame” (vegetable soybean). J Food Safety. 2008; 28: 300-313.
- Snyder HE, Kwon TW. Soybean utilization. AVI Books, Van Nostrand Reinhold, New York. 1987.
- Steinbuch E. Technical note: improvement of texture of frozen vegetables by stepwise blanching treatments. Int J Food Sci & Technol. 1976; 12: 435-436
- Mozzoni LA, Chen P, Morawicki RO, Hettiarachchy NS, Brye KR, Mauromoustakos A. Quality attributes of vegetable soybean as a function of boiling time and condition. Int J Food Sci Tech. 2009; 44: 2089-2099.
- Santana AC, Carrao-Panizzi MC, Mandarino JMG, Leite RS, Silva, JB, et al. Evaluation of the shelf-life of vegetable-type soybean pods. Braz arch biol technol. 2012; 55: 591-595.
- Pao S, Long W, Kim C, Rafie AR. Salmonella population rebound and its prevention on spray washed and non-washed Jalapeño peppers and roma tomatoes in humid storage. Foodborne Pathog Dis. 2012; 9: 361-366.
- Anonymous. How come plants produce oxygen even though they need oxygen for respiration? 2015.
- Bactest. How can I measure and monitor bacterial gas production. Bactest Limited. London. UK.