
Research Article
Austin Food Sci. 2019; 4(1): 1032.
Manipulation of Plasma Dehydroepiandrosteron (DHEA) by Truffle and the Cultured Mycelium: BRM like Function of Truffles
Nakajima K¹, Nakagawa S² and Konishi T¹*
¹Niigata University of Pharmacy & Applied Life Sciences (NUPALS), Office HALD Food Function Research, Niigata, Japan
²Department of Bio-analytical Chemistry, Niigata, Japan
*Corresponding author: Tetsuya Konishi, Niigata University of Pharmacy & Applied Life Sciences (NUPALS), Liaison R/D center, Office HALD Food Function Research, Niigata, Japan
Received: January 09, 2019; Accepted: February 06, 2019; Published: February 13, 2019
Abstract
The effect of cultured mycelium mass of Truffle (Tuber borchii) was studied on the plasma level of Dehydroepiandrosteron (DHEA) by gavage administration together with the fruiting bodies of Italian and Chinese Truffles (T mel and T tuber, respectively) in rats. Total Dehydroepiandrosteron (DHEA) was determined by ELISA method. All the truffle samples tested attenuated plasma Dehydroepiandrosteron (DHEA) level when determined at 2 and 6 hr after gavage administration. The modulation effect was slightly stronger in fruiting body of T mel than T tuber. The cultured mycelium mass also increased plasma Dehydroepiandrosteron (DHEA) almost as high as fruiting body of T mel.
Keywords: Truffle bortii; DHEA modulation; BRM; Anti-aging resource; Cultured mycelium
Introduction
Truffle is the edible subterranean fungus of genus Tuber and is known as precious cuisine material having special flavor. Traditional tale says that Truffle has anti-aging property and shows tonic and nourishing effects [1]. However, there is a limited number of basic studies on the physiological and pharmacological functions Truffles except some regarding the volatile component study [2], probably because of its limited supply for the basic research. Although semi-artificial cultivation has developed to grow fruiting body of truffle, submerged fermentation is currently challenged to produce mycelium mass or fruiting body [3]. The mycelium mass is not the substitute of fruiting body in terms of cuisine material but is an attractive resource for developing medicines or functional foods, which active ingredients will represent the physiological function of truffle as shown as in other mushrooms. For example, an anti-ulcer polysaccharide isolated primarily from the fruiting body of Helicium erinaceus (Lion mushroom) was produced by mycelium culture [4].
In the present study, we examined the feeding effect of cultured mycelium of Tuber borchii on plasma level of Dehydroepiandrosteron (DHEA) in rodent after gavage administration to evaluate possible anti-aging potential of Truffle.
Materials and Methods
Sample preparation
Truffle (Tuber borchii) mycelium mass was generously provided from Niigata Beer Brewery Co. Ltd., Niigata Japan where the T borchii was artificially cultured in glucose-based culture medium under the condition established (European patent No 2474221). The cultured mycelium mass was proved to be originated from planted T borchii by successive DNA amplification using universal and Tuber genus specific primers, respectively, according to the method reported by Zampieri et al [5]. The T borchii mycelium mass was granulated after freeze-dry treatment. The fruiting bodies of Chinese Truffle (T tuber) and Italian Truffle (T mel) as references were also provided by Niigata Beer Brewery Co. Ltd., and were treated as well. These dried Truffle samples were homogenized in saline to make suspensions with 10 and 100mg/mL concentrations for animal experiments.
Animal treatment
Rats at 7 weeks age were purchased from SLC and conditioned for one week before experiment. The rats were grouped into seven and each group consists of 6 rats. Group 1: saline control, Group 2: high dose of Chinese Truffle Group 3: low dose of Chinese Truffle, Group 4: high dose of Italian Truffle, Group 5: low dose of Italian Truffle, Group 6: high dose of T magnesium mycelium, Group 7: low dose of T magnesium mycelium. High dose and Low dose groups were given by gavage administration of 5 mL of respective Truffle suspension at the dose of 50mg/Kg and 500mg/Kg body weight, respectively.
At 2 and 6 hr after gavage administration of Truffle samples, 0.5 mL blood was sampled with heparinized syringe from the jugular vein of rat. The blood plasma was prepared by centrifugation at 4°C and kept in a freezer until use.
Determination of plasma level of DHEA by ELISA
DHEA ELISA kit (Catalogue No. ADI 900-093) was purchased from Enzo Life Science Co. Ltd. Plasma level of DHEA was measured according to the manual provided by the company. Briefly, 20 μL of plasma was pipetted into a well of 96 well Goat anti-Rabbit IgG microtiter plate and then diluted with 80 μL reaction buffer, followed by the addition of 50 μL of 10 times diluted blue conjugate (alkaline phosphatase conjugated DHEA) and DHEA EI antibody (rabbit polyclonal antibody to DHEA), and then incubated over night in a refrigerator at 4°C. Following addition and incubation with 200 μL of p-nitrophenyl phosphate in buffer, absorbance at 405 nm was measured for determining DHEA. Calibration of DHEA/mL plasma followed the company manual.
Statistical Treatment
Results of DHEA measurements are shown as the average of 6 specimens and expressed as mean ± SD values. Date were analyzed using Student’s t-test in Microsoft Excel 2007 and the differences between groups were tested for statistical significance using One-way ANOVA (P<0.05 : *, P<0.01 :†).
Result and Discussion
Plasma level of DHEA as total, both free and conjugated, was determined by ELISA method after 2 and 6 hours of gavage administration of Truffle suspension and the results are shown in Figure 1. The plasma DHEA levels were significantly increased at 2 and 6 hr after ingestion of all Truffle samples tested except the group with 50 mg/Kg of Chinese Truffle FB at 6 hr and 500 mg/Kg of Italian Truffle FB at 2hr after ingestion. The increased levels were dose dependent when observed at 6 hr after ingestion in both Chinese and Italian Truffle FB. Cultured mycelium suspension also significantly increased the plasma DHEA level after gavage administration both at 2 and 6 hr. The effect was almost same as that of Italian Truffle FB. The effect was more marked at 6 hr than at 2 hr after ingestion, however, the dose effect was not obvious such that there was no significant difference in the effect between the doses of 50 and 500 mg/Kg.
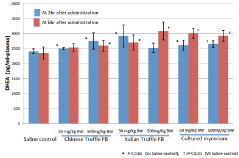
Figure 1: Plasma level of DHEA as total, both free and conjugated, was determined by ELISA method after 2 and 6 hours of gavage administration of Truffle
suspension results.
Since the fruiting bodies and cultured mycelium mass of Truffles do not contain measurable amount of DHEA when determined by LCMS analysis (data not shown), the present observation indicates the observed increase of plasma DHEA level is not due to the direct supply of DHEA from Truffle samples but the effect is indirect probably through the modulation of physiological process for DHEA biosynthesis.
DHEA is a precursor of series of steroid hormones and thus plays critical roles in maintaining many physiological reactions such as related to immune response, decreased libido, atherosclerosis, lipid metabolism and diabetic pathogenesis [6]. The plasma level of DHEA is reported to decrease depending on age and thus implicated as a marker of aging as same as Estrogens [7]. Therefore, supplementation of DHEA attracts attention to ameliorate the age dependent decline of physiological reactions including dementia and the benefits of DHEA supplementation are well discussed [8]. Present observation that Truffle intake manipulated the plasma level of DHEA indicated there is an alternate approach to indirectly modulate the physiological anti-aging factors like DHEA by food materials.
Although the mechanism and also the active principal ingredient involved is not yet understood, the DHEA modulating activity of Truffle reflects a part of Biological Reaction Modulating effects (BRM) implicated for the fungi or mushrooms function, and this suggests new approach to develop functional foods.
Acknowledgement
We thanks Kaneka Co. Ltd., Bio animal lab where Truffle sample administration to and sampling of blood from rats were carried out.
References
- El Enshasy H, Elasyed EA, Aziz R, Wadaan MA. Mushrooms and Truffles: Histrical Biofactories for Complimentary Medicine in Africa and in the Middle East. Evid Based Compliment Altern Med. 2013.
- Psionic G, Certain L, Provide G, Cicely A. Composition of commercial truffle flavored oils with GC-MS analysis and discrimination with an electronic nose. Food Chem. 2014; 146: 30-35.
- Tang YJ, Liu RS, Li HM. Current progress on truffle submerged fermentation: a promising alternative to its fruiting bodies. Apple Microbial Biotechnology. 2015; 99: 2041-2053.
- Wang M, Gao Y, Xu D, Konishi T, Gao Q. Hericium erinaceus (Yamabushitake): a unique resource for developing functional foods and medicines. Food Funct. 2014; 5: 3055-3064.
- Zampieri E, Mello A, Bonfnte P, Murat C. PCR primers specific for the genus Tuber reveal the presence of several truffle species in a truffle-ground. FEMS Microbiol Lett. 2009; 297: 67-72.
- Samaras N, Samaras D, Frangos E, Forster A, Philippe J. A review of Agerelated Dehydroepiandrosterone Decline and Its Association with Well-Known Geriatric Syndromes: Is Treatment Beneficial? Rejuv Res. 2013; 16: 285-294.
- Urbaanski HF, Mattson JA, Roth GS, Ingram DK. Dehydroepiandrostene Sulfate (DHEAS) as an endocrine marker of aging in calorie restriction studies. Exp Gerontol. 2013; 48: 1136-1139.
- Etienne-Emile B, Buy T, Sylvie L, Najiba L, Roger M, Debuire B, et al. Dhydroepiandrosterone (HEA), DHEA sulfate, and aging: Contribution of the DHEAge study to a sociobiomedical issue. Proc Nat Acad Sci US. 2000; 97: 4279-4284.